What Should We Know about Drug Levels and Therapeutic Drug Monitoring during Pregnancy and Breastfeeding in Inflammatory Bowel Disease under Biologic Therapy?
Abstract
:1. Introduction
1.1. Search Strategy
1.2. Data Extraction
1.3. Mechanisms of Drug Transport for Antibody Therapies across the Human Placenta
1.4. Transfer of Maternal Biologics and Drugs from Breast Tissue into Breast Milk
1.5. Pharmacokinetics of Anti-TNF Agents in IBD during Pregnancy
Study Selection
1.6. Maternal Infliximab Trough Concentrations during Pregnancy
1.7. IFX and Maternal Trough Concentration before, during and after Pregnancy
1.8. Placenta Drug Transfer in Pregnant Women Treated with Infliximab
1.9. IFX Drug Levels during Breastfeeding
1.10. Duration of IFX Detection in Newborns
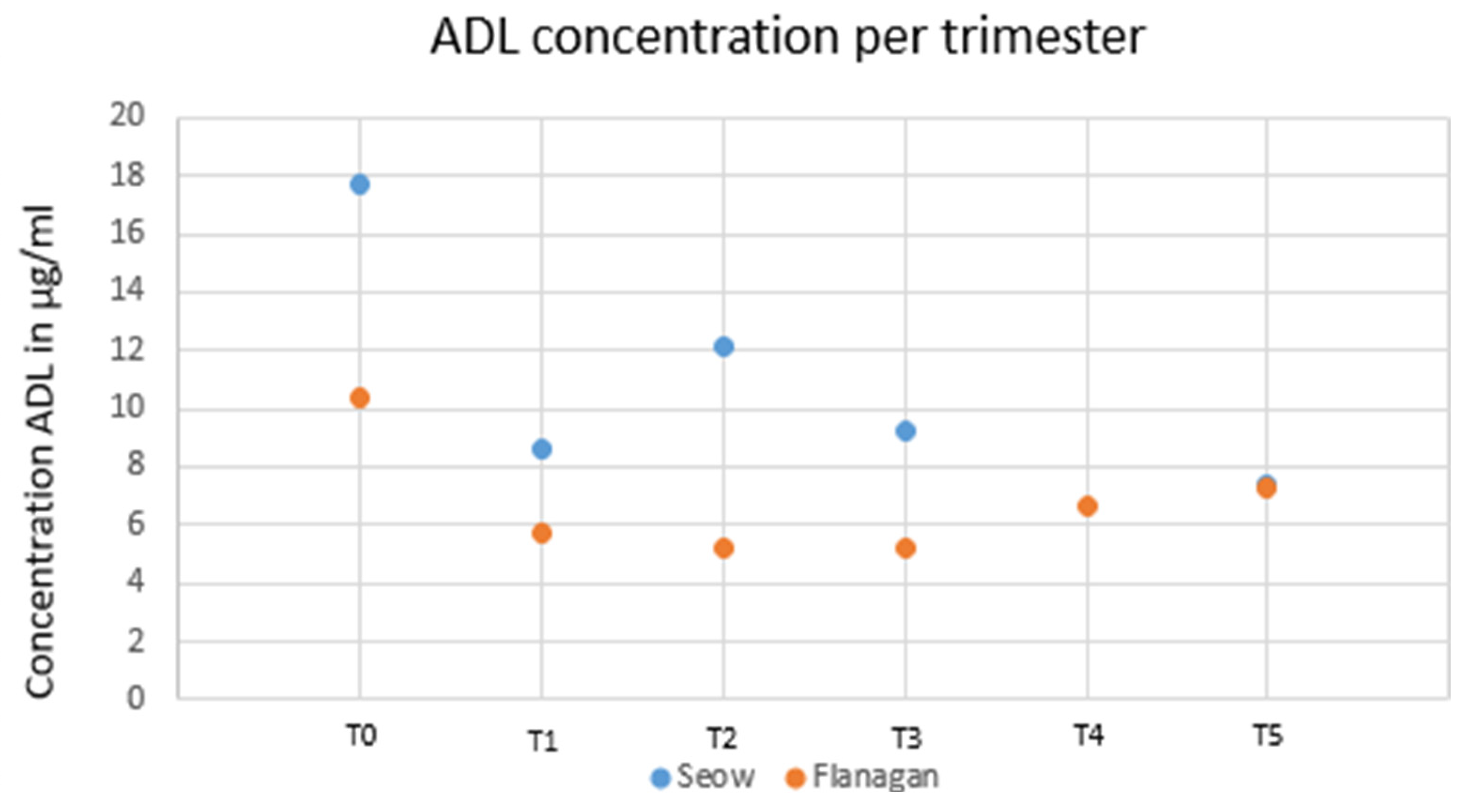
1.11. TNF-α Inhibitors—ADA and Maternal Trough Concentration during Pregnancy
1.12. Placental Transfer of ADA
1.13. ADA and Breast Milk (Table 3)
| Drug | Total Patients | Total Patients with a Detectable Level, n (%) | Peak Time Range, h | Peak (Range), μg/mL |
|---|---|---|---|---|
| Adalimumab | 21 | 2 (9.5) | 12–24 | 0.71 (0.45–0.71) |
| Infliximab | 29 | 19 (66.0) | 24–48 | 0.74 (0.15–0.74) |
| Golimumab | 1 | 0 (0) | NA | NA |
| Certolizumab | 13 | 3 (23.0) | 24–48 | 0.29 (0.27–0.29) |
| Ustekinumab | 6 | 4 (66.7) | 12–24 | 1.57 (0.72–1.57) |
| Natalizumab | 2 | 1 (50.0) | 24 | 0.46 |
1.14. Duration of ADA in Newborns
1.15. TNF-α Inhibitors—GLM Maternal Trough Concentration during Pregnancy, in Breast Milk and in Children
1.16. Outcomes of Pregnancy and Children When Using Anti-TNF during Pregnancy
1.17. Serum Ustekinumab Levels during Pregnancy and Breastfeeding (Figure 4)

1.18. Ustekinumab Levels during Breastfeeding (Table 3)
1.19. Serum Vedolizumab Trough Levels during Pregnancy (Figure 4)
1.20. Serum Vedolizumab Trough Levels during Breastfeeding (Table 3)
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kane, S.V.; Acquah, L.A. Placental transport of immunoglobulins: A clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am. J. Gastroenterol. 2009, 104, 228–233. [Google Scholar] [CrossRef]
- Gusdon, J.P. Fetal and maternal immunoglobulin levels during pregnancy. Am. J. Obstet. Gynecol. 1969, 103, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Garty, B.Z.; Ludomirsky, A.; Danon, Y.L.; Peter, J.B.; Douglas, S.D. Placental transfer of immunoglobulin G subclasses. Clin. Diagn. Lab. Immunol. 1994, 1, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.; Sager, R.; Kuhn, P.; Nicolaides, K.H.; Schneider, H. Evolution of Maternofetal Transport of Immunoglobulins During Human Pregnancy. Am. J. Reprod. Immunol. 1996, 36, 248–255. [Google Scholar] [CrossRef]
- Julsgaard, M.; Christensen, L.A.; Gibson, P.R.; Gearry, R.B.; Fallingborg, J.; Hvas, C.L.; Bibby, B.M.; Uldbjerg, N.; Connell, W.R.; Rosella, O.; et al. Concentrations of Adalimumab and Infliximab in Mothers and Newborns, and Effects on Infection. Gastroenterology 2016, 151, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Long, M.D.; Kane, S.V.; Roy, A.; Dubinsky, M.C.; Sands, B.E.; Cohen, R.D.; Chambers, C.D.; Sandborn, W.J.; Crohn’s Colitis Foundation Clinical Research Alliance. Pregnancy and Neonatal Outcomes after Fetal Exposure to Biologics and Thiopurines among Women with Inflammatory Bowel Disease. Gastroenterology 2021, 160, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef]
- Fritzsche, J.; Pilch, A.B.; Mury, D.; Schaefer, C.; Weber-Schoendorfer, C. Infliximab and Adalimumab Use During Breastfeeding. J. Clin. Gastroenterol. 2012, 46, 718–719. [Google Scholar] [CrossRef]
- Bortlik, M.; Machkova, N.; Duricova, D.; Malickova, K.; Hrdlicka, L.; Lukas, M.; Kohout, P.; Shonova, O.; Lukas, M. Pregnancy and newborn outcome of mothers with inflammatory bowel diseases exposed to anti-TNF-α therapy during pregnancy: Three-center study. Scand. J. Gastroenterol. 2013, 48, 951–958. [Google Scholar] [CrossRef]
- Kane, S.; Ford, J.; Cohen, R.; Wagner, C. Absence of infliximab in infants and breast milk from nursing mothers receiving therapy for crohn’s disease before and after delivery. J. Clin. Gastroenterol. 2009, 43, 613–616. [Google Scholar] [CrossRef]
- Seow, C.H.; Leung, Y.; Casteele, N.V.; Afshar, E.E.; Tanyingoh, D.; Bindra, G.; Stewart, M.J.; Beck, P.L.; Kaplan, G.G.; Ghosh, S.; et al. The effects of pregnancy on the pharmacokinetics of infliximab and adalimumab in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 1329–1338. [Google Scholar] [CrossRef]
- Flanagan, E.; Gibson, P.R.; Wright, E.K.; Moore, G.T.; Sparrow, M.P.; Connell, W.; Kamm, M.A.; Begun, J.; Christensen, B.; De Cruz, P.; et al. Infliximab, adalimumab and vedolizumab concentrations across pregnancy and vedolizumab concentrations in infants following intrauterine exposure. Aliment. Pharmacol. Ther. 2020, 52, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Eliesen, G.A.M.; van Drongelen, J.; van Hove, H.; Kooijman, N.I.; van den Broek, P.; de Vries, A.; Roeleveld, N.; Russel, F.G.M.; Greupink, R. Assessment of Placental Disposition of Infliximab and Etanercept in Women with Autoimmune Diseases and in the Ex Vivo Perfused Placenta. Clin. Pharmacol. Ther. 2020, 108, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Grišić, A.M.; Dorn-Rasmussen, M.; Ungar, B.; Brynskov, J.; Ilvemark, J.F.K.F.; Bolstad, N.; Warren, D.J.; Ainsworth, M.A.; Huisinga, W.; Ben-Horin, S.; et al. Infliximab clearance decreases in the second and third trimesters of pregnancy in inflammatory bowel disease. United Eur. Gastroenterol. J. 2021, 9, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskas, E.A.; Church, J.A.; Silverman, N.; Barry, M.; Targan, S.R.; Dubinsky, M.C. Case Report: Evidence for Transplacental Transfer of Maternally Administered Infliximab to the Newborn. Clin. Gastroenterol. Hepatol. 2006, 4, 1255–1258. [Google Scholar] [CrossRef]
- Steenholdt, C.; Al-Khalaf, M.; Ainsworth, M.A.; Brynskov, J. Therapeutic infliximab drug level in a child born to a woman with ulcerative colitis treated until gestation week 31. J. Crohn’s Colitis 2012, 6, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Kanis, S.L.; de Lima-Karagiannis, A.; van der Ent, C.; Rizopoulos, D.; van der Woude, C.J. Anti-TNF Levels in Cord Blood at Birth are Associated with Anti-TNF Type. J. Crohn’s Colitis 2018, 12, 939–947. [Google Scholar] [CrossRef]
- Matro, R.; Martin, C.F.; Wolf, D.; Shah, S.A.; Mahadevan, U. Exposure Concentrations of Infants Breastfed by Women Receiving Biologic Therapies for Inflammatory Bowel Diseases and Effects of Breastfeeding on Infections and Development. Gastroenterology 2018, 155, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Julsgaard, M.; Zhu, X.; Martin, J.; Barclay, M.L.; Cranswick, N.; Gibson, P.R.; Gearry, R.B.; van der Giessen, J.; Connor, S.J.; et al. Timing of Live Attenuated Vaccination in Infants Exposed to Infliximab or Adalimumab in Utero: A Prospective Cohort Study in 107 Children. J. Crohns. Colitis 2022, 16, 1835–1844. [Google Scholar] [CrossRef]
- Chen, J.; Lin, R.; Guo, G.; Wu, W.; Ke, M.; Ke, C.; Huang, P.; Lin, C. Physiologically-Based Pharmacokinetic Modeling of Anti-Tumor Necrosis Factor Agents for Inflammatory Bowel Disease Patients to Predict the Withdrawal Time in Pregnancy and Vaccine Time in Infants. Clin. Pharmacol. Ther. 2023, 114, 1254–1263. [Google Scholar] [CrossRef]
- Papamichael, K.; Vogelzang, E.H.; Lambert, J.; Wolbink, G.; Cheifetz, A.S. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev. Clin. Immunol. 2019, 15, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Bejan-Angoulvant, T.; Ternant, D.; Daoued, F.; Medina, F.; Bernard, L.; Mammou, S.; Paintaud, G.; Mulleman, D. Relationship Between Serum Infliximab Concentrations and Risk of Infections in Patients Treated for Spondyloarthritis. Arthritis Rheumatol. 2017, 69, 108–113. [Google Scholar] [CrossRef]
- Mahadevan, U.; Robinson, C.; Bernasko, N.; Boland, B.; Chambers, C.; Dubinsky, M.; Friedman, S.; Kane, S.; Manthey, J.; Sauberan, J.; et al. Inflammatory Bowel Disease in Pregnancy Clinical Care Pathway: A Report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology 2019, 156, 1508–1524. [Google Scholar] [CrossRef] [PubMed]
- Benoit, L.; Mir, O.; Berveiller, P. Treating Ulcerative Colitis During Pregnancy: Evidence of Materno–Fetal Transfer of Golimumab. J. Crohn’s Colitis 2019, 13, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Y.; Chen, F.; Shen, M. Comparative safety of infliximab and adalimumab on pregnancy outcomes of women with inflammatory bowel diseases: A systematic review & meta-analysis. BMC Pregnancy Childbirth 2022, 22, 854. [Google Scholar]
- Shihab, Z.; Yeomans, N.D.; De Cruz, P. Anti-Tumour Necrosis Factor α Therapies and Inflammatory Bowel Disease Pregnancy Outcomes: A Meta-analysis. J. Crohn’s Colitis 2016, 10, 979–988. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Gubatan, J.M.; Juhl, C.B.; Streett, S.E.; Maxwell, C. Biologics for Inflammatory Bowel Disease and Their Safety in Pregnancy: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 74–87. [Google Scholar] [CrossRef]
- Beaulieu, D.B.; Ananthakrishnan, A.N.; Martin, C.; Cohen, R.D.; Kane, S.V.; Mahadevan, U. Use of Biologic Therapy by Pregnant Women with Inflammatory Bowel Disease Does Not Affect Infant Response to Vaccines. Clin. Gastroenterol. Hepatol. 2017, 16, 99–105. [Google Scholar] [CrossRef]
- Fitzpatrick, T.; Alsager, K.; Sadarangani, M.; Pham-Huy, A.; Murguía-Favela, L.; Morris, S.K.; Seow, C.H.; Piché-Renaud, P.-P.; Jadavji, T.; Vanderkooi, O.G.; et al. Immunological effects and safety of live rotavirus vaccination after antenatal exposure to immunomodulatory biologic agents: A prospective cohort study from the Canadian Immunization Research Network. Lancet Child Adolesc. Health 2023, 7, 648–656. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Chaparro, M. Vaccines in Children Exposed to Biological Agents In Utero and/or During Breastfeeding: Are They Effective and Safe? J. Crohn’s Colitis 2023, 17, 995–1009. [Google Scholar] [CrossRef]
- Selinger, C.P.; Nelson-Piercy, C.; Fraser, A.; Hall, V.; Limdi, J.; Smith, L.; Smith, M.; Nasur, R.; Gunn, M.; King, A.; et al. IBD in pregnancy: Recent advances, practical management. Frontline Gastroenterol. 2020, 12, 214–224. [Google Scholar] [CrossRef]
- Lédée-Bataille, N.; Dubanchet, S.; Coulomb-L’Hermine, A.; Durand-Gasselin, I.; Frydman, R.; Chaouat, G. A new role for natural killer cells, interleukin (IL)-12, and IL-18 in repeated implantation failure after in vitro fertilization. Fertil. Steril. 2004, 81, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-Y.; Li, M.-J. Interleukin 23 regulates the functions of human decidual immune cells during early pregnancy. Biochem. Biophys. Res. Commun. 2016, 469, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Sako, M.; Yoshimura, N.; Sonoda, A.; Okano, S.; Ueda, M.; Tezuka, M.; Mine, M.; Yamanishi, S.; Hashimoto, K.; Kobayashi, K.; et al. Safety Prediction of Infants Born to Mothers with Crohn’s Disease Treated with Biological Agents in the Late Gestation Period. J. Anus Rectum Colon 2021, 5, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Klenske, E.; Osaba, L.; Nagore, D.; Rath, T.; Neurath, M.F.; Atreya, R. Drug Levels in the Maternal Serum, Cord Blood and Breast Milk of a Ustekinumab-Treated Patient with Crohn’s Disease. J. Crohns. Colitis 2019, 13, 267–269. [Google Scholar] [CrossRef]
- Flanagan, E.; Prentice, R.; Wright, E.K.; Gibson, P.R.; Ross, A.L.; Begun, J.; Sparrow, M.P.; Goldberg, R.; Rosella, O.; Burns, M.; et al. Ustekinumab levels in pregnant women with inflammatory bowel disease and infants exposed in utero. Aliment. Pharmacol. Ther. 2022, 55, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Saito, J.; Kaneko, K.; Kawasaki, H.; Hayakawa, T.; Yakuwa, N.; Suzuki, T.; Sago, H.; Yamatani, A.; Murashima, A. Ustekinumab during pregnancy and lactation: Drug levels in maternal serum, cord blood, breast milk, and infant serum. J. Pharm. Health Care Sci. 2022, 8, 18. [Google Scholar] [CrossRef]
- Rowan, C.R.; Cullen, G.; Mulcahy, H.E.; Keegan, D.; Byrne, K.; Murphy, D.J.; Sheridan, J.; Doherty, G.A. Ustekinumab Drug Levels in Maternal and Cord Blood in a Woman with Crohn’s Disease Treated Until 33 Weeks of Gestation. J. Crohn’s Colitis 2017, 12, 376–378. [Google Scholar] [CrossRef]
- Mitrova, K.; Pipek, B.; Bortlik, M.; Bouchner, L.; Brezina, J.; Douda, T.; Drasar, T.; Drastich, P.; Falt, P.; Klvana, P.; et al. Differences in the placental pharmacokinetics of vedolizumab and ustekinumab during pregnancy in women with inflammatory bowel disease: A prospective multicentre study. Therap. Adv. Gastroenterol. 2021, 14, 17562848211032790. [Google Scholar] [CrossRef]
- Prentice, R.; Flanagan, E.; Wright, E.K.; Gibson, P.R.; Rosella, S.; Godberg, R.; Prideaux, L.; Kiburg, K.; Ross, A.L.; Burns, M.; et al. Pharmacokinetics of vedolizumab and ustekinumab in pregnant womeen with inflammatory bowel disease and their infants exposed in utero. J. Crohn’s Colitis 2023, 17 (Suppl. S1), 508–510. [Google Scholar] [CrossRef]
- Avni-Biron, I.; Mishael, T.; Zittan, E.; Livne-Margolin, M.; Zinger, A.; Tzadok, R.; Goldenberg, R.; Kopylov, U.; Ron, Y.; Hadar, E.; et al. Ustekinumab during pregnancy in patients with inflammatory bowel disease: A prospective multicentre cohort study. Aliment. Pharmacol. Ther. 2022, 56, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Wils, P.; Seksik, P.; Stefanescu, C.; Nancey, S.; Allez, M.; de Chambrun, G.P.; Altwegg, R.; Gilletta, C.; Vuitton, L.; Viennot, S.; et al. Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: A multicentre cohort study. Aliment. Pharmacol. Ther. 2020, 53, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Moens, A.; van der Woude, C.J.; Julsgaard, M.; Humblet, E.; Sheridan, J.; Baumgart, D.C.; Gilletta De Saint-Joseph, C.; Nancey, S.; Rahier, J.F.; Bossuyt, P.; et al. Pregnancy outcomes in inflammatory bowel disease patients treated with vedolizumab, anti-TNF or conventional therapy: Results of the European CONCEIVE study. Aliment. Pharmacol. Ther. 2020, 51, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Chaparro, M.; Julsgaard, M.; Katsanos, K.; Zelinkova, Z.; Agrawal, M.; Ardizzone, S.; Campmans-Kuijpers, M.; Dragoni, G.; Ferrante, M.; et al. European Crohn’s and Colitis Guidelines on Sexuality, Fertility, Pregnancy, and Lactation. J. Crohn’s Colitis 2023, 17, 1–27. [Google Scholar] [CrossRef]


| Drug | Dose and Frequency | Timing of Last Dose (Weeks before Delivery) |
|---|---|---|
| Adalimumab | 40 mg/2 W | 0–2 |
| 40 mg/W | 0–3 | |
| 80 mg/2 W | 0–4 | |
| Infliximab | 5 mg/kg/8 W | 5–11 |
| 5 mg/kg/6 W | 6–13 | |
| 7.5 mg/kg/8 W | 8–13 | |
| 8 mg/kg/8 W | 8–13 |
| Drug | Dose and Frequency | Timing of Vaccination (Months after Delivery) |
|---|---|---|
| Adalimumab | 40 mg/2 W | 8 |
| 40 mg/W | 9 | |
| 80 mg/2 W | 9 | |
| Infliximab | 5 mg/kg/8 W | 11 |
| 5 mg/kg/6 W | 12 | |
| 7.5 mg/kg/8 W | 12 | |
| 8 mg/kg/8 W | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrau, M.; Roblin, X.; Andromaque, L.; Rozieres, A.; Faure, M.; Paul, S.; Nancey, S. What Should We Know about Drug Levels and Therapeutic Drug Monitoring during Pregnancy and Breastfeeding in Inflammatory Bowel Disease under Biologic Therapy? J. Clin. Med. 2023, 12, 7495. https://doi.org/10.3390/jcm12237495
Barrau M, Roblin X, Andromaque L, Rozieres A, Faure M, Paul S, Nancey S. What Should We Know about Drug Levels and Therapeutic Drug Monitoring during Pregnancy and Breastfeeding in Inflammatory Bowel Disease under Biologic Therapy? Journal of Clinical Medicine. 2023; 12(23):7495. https://doi.org/10.3390/jcm12237495
Chicago/Turabian StyleBarrau, Mathilde, Xavier Roblin, Leslie Andromaque, Aurore Rozieres, Mathias Faure, Stéphane Paul, and Stéphane Nancey. 2023. "What Should We Know about Drug Levels and Therapeutic Drug Monitoring during Pregnancy and Breastfeeding in Inflammatory Bowel Disease under Biologic Therapy?" Journal of Clinical Medicine 12, no. 23: 7495. https://doi.org/10.3390/jcm12237495





