Intraoperative Blood Flow Analysis of Free Flaps with Arteriovenous Loops for Autologous Microsurgical Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.2. Transit-Time Flowmetry (TTFM)
2.3. Arterial Vascular Resistance (aVR)
2.4. Microvascular Indocyanine Green Angiography (mICG-A)
2.5. Postoperative Hemodynamic Complications
2.6. Statistical Analysis
3. Results
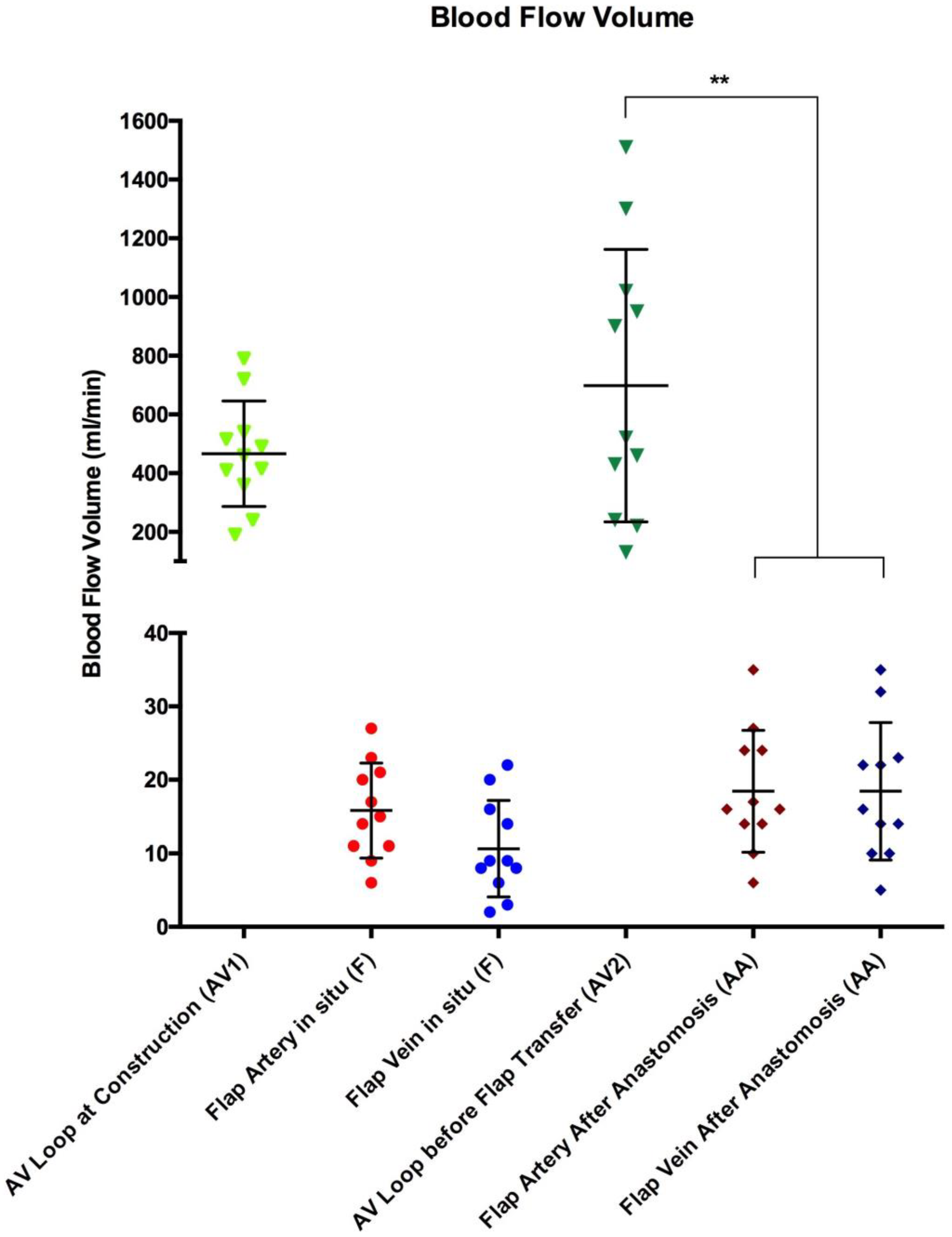
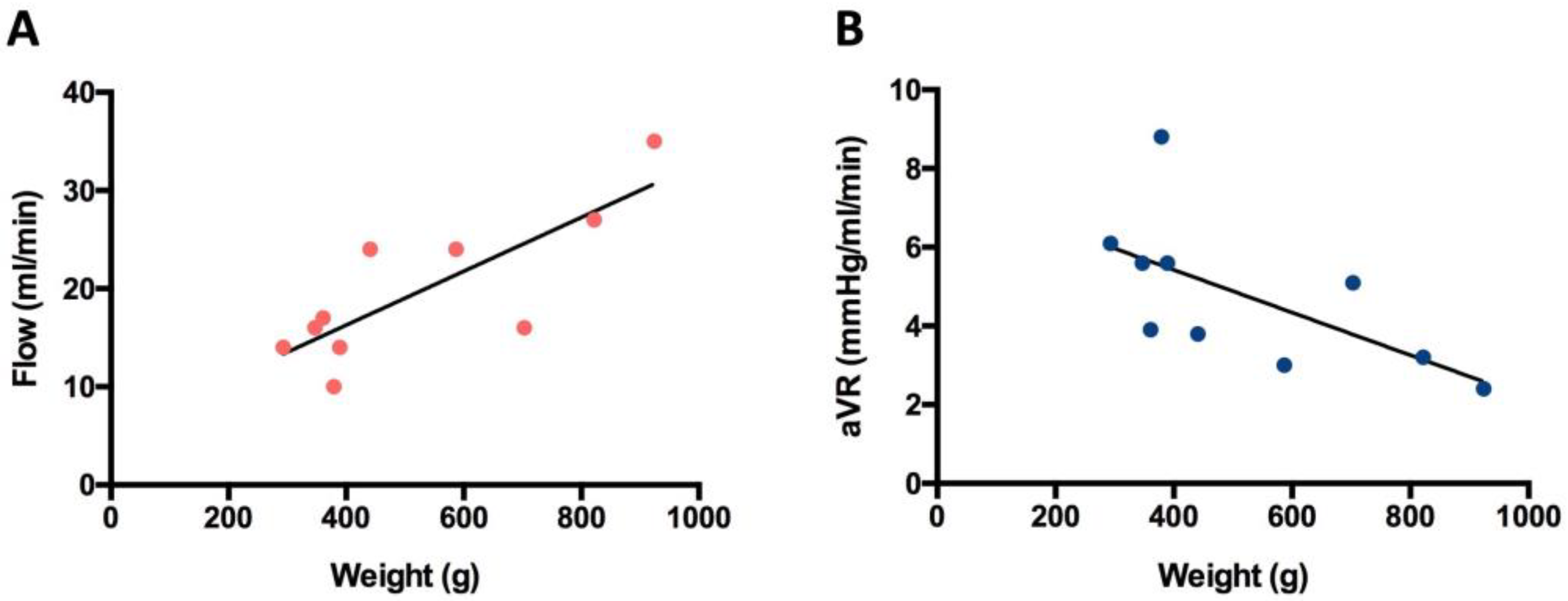
3.1. Blood Flow Volume (mL/min)
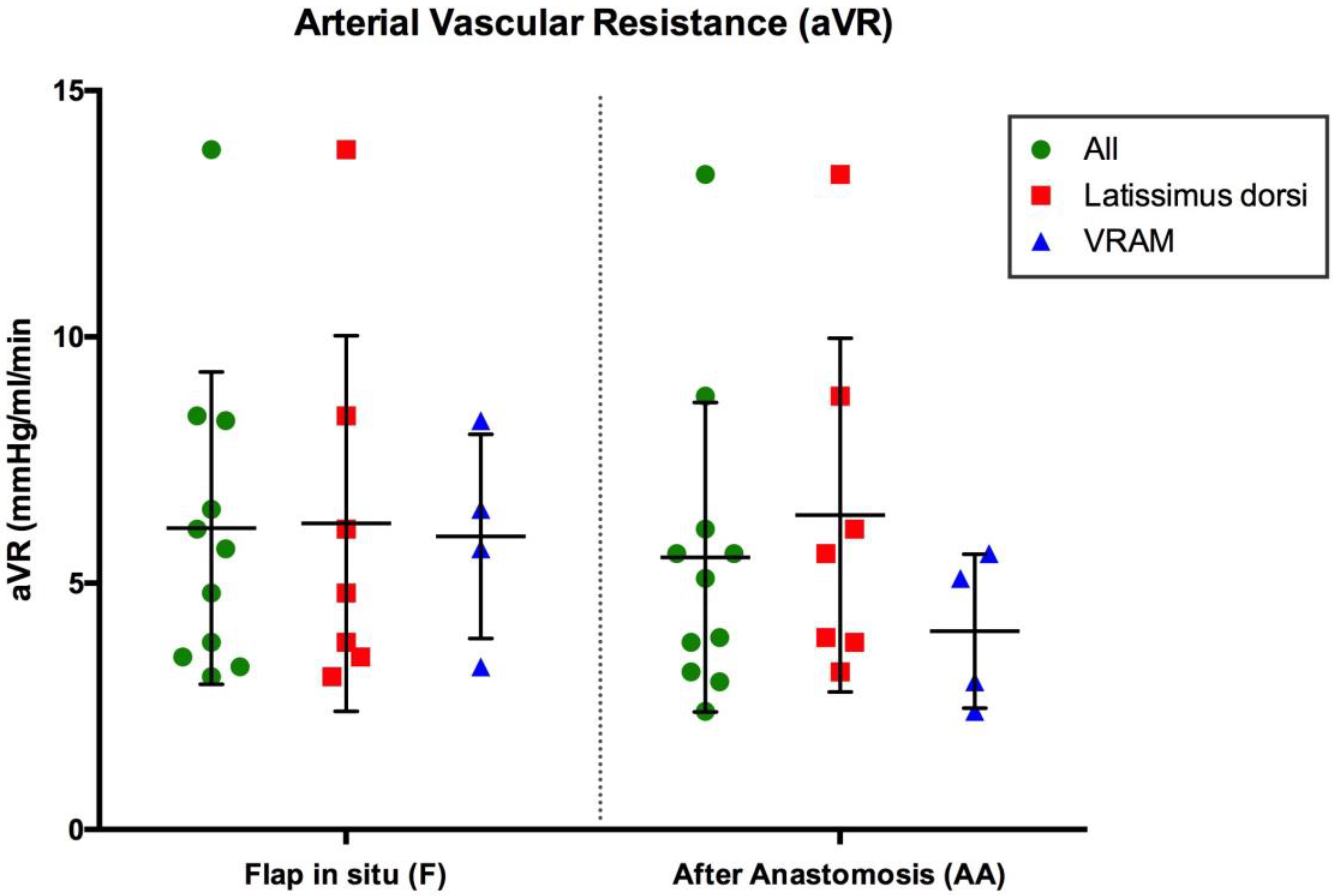
3.2. Arterial Vascular Resistance (mmHg/mL/min)
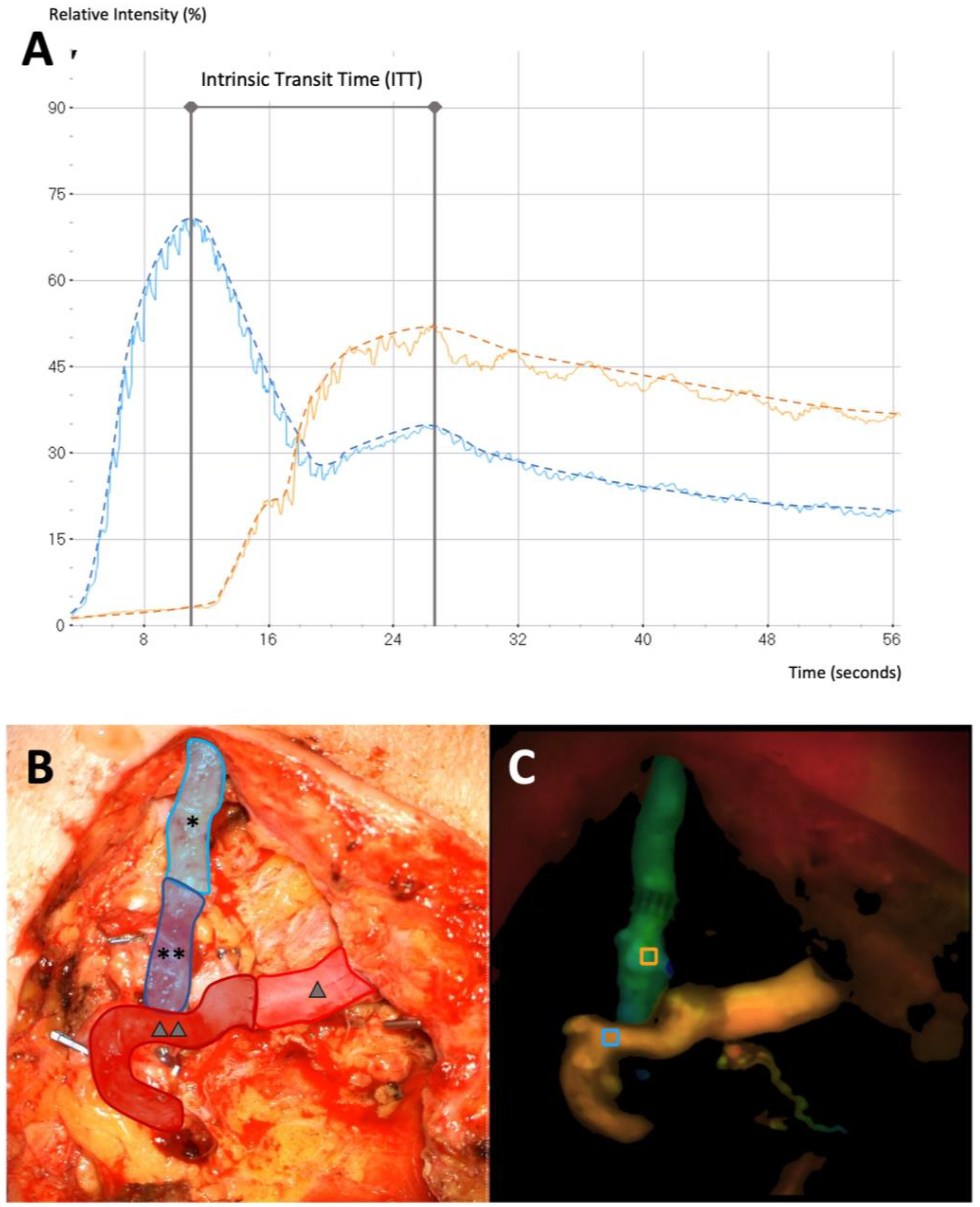
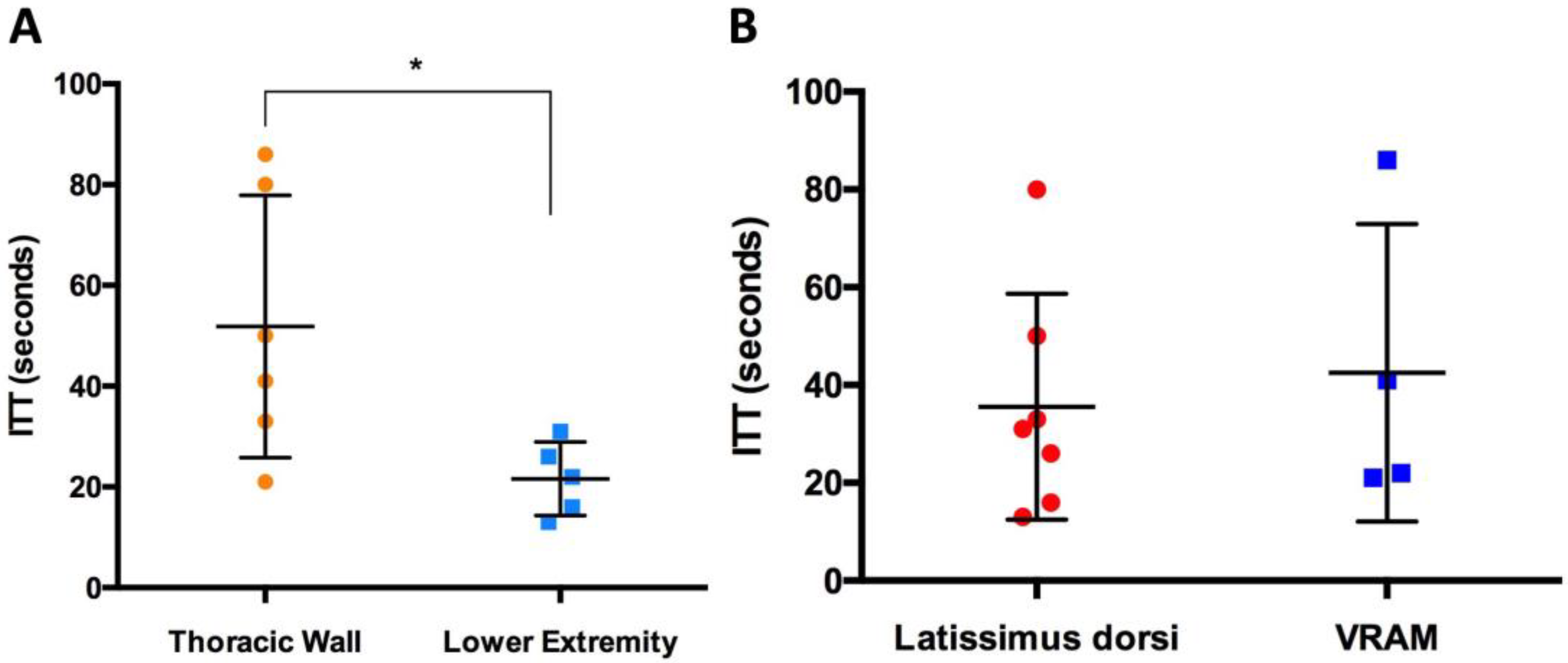
3.3. Intrinsic Transit Time
3.4. Postoperative Hemodynamic Complications
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geierlehner, A.; Horch, R.E.; Muller-Seubert, W.; Arkudas, A.; Ludolph, I. Limb salvage procedure in immunocompromised patients with therapy-resistant leg ulcers-The value of ultra-radical debridement and instillation negative-pressure wound therapy. Int. Wound J. 2020, 17, 1496–1507. [Google Scholar] [CrossRef]
- Gierek, M.; Klama-Baryla, A.; Labus, W.; Bergler-Czop, B.; Pietrauszka, K.; Niemiec, P. Platelet-Rich Plasma and Acellular Dermal Matrix in the Surgical Treatment of Hidradenitis Suppurativa: A Comparative Retrospective Study. J. Clin. Med. 2023, 12, 2112. [Google Scholar] [CrossRef] [PubMed]
- Simman, R. Wound closure and the reconstructive ladder in plastic surgery. J. Am. Coll. Certif. Wound Spec. 2009, 1, 6–11. [Google Scholar] [CrossRef]
- Lese, I.; Biedermann, R.; Constantinescu, M.; Grobbelaar, A.O.; Olariu, R. Predicting risk factors that lead to free flap failure and vascular compromise: A single unit experience with 565 free tissue transfers. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 512–522. [Google Scholar] [CrossRef]
- Wang, C.; Liufu, N.; Ji, F.; Han, Z.; Liu, Z.; Cao, M. Risk factors associated with postoperative complications following free flap reconstruction of head and neck defects. J. Stomatol. Oral. Maxillofac. Surg. 2022, 123, e894–e898. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Creff, G.; Mazoue, V.; Coudert, P.; De Crouy Chanel, O.; Jegoux, F. Risk factors associated with early and late free flap complications in head and neck osseous reconstruction. Eur. Arch. Otorhinolaryngol. 2023, 280, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Machens, H.G.; Mailander, P.; Pasel, J.; Lutz, B.S.; Funke, M.; Siemers, F.; Berger, A.C. Flap perfusion after free musculocutaneous tissue transfer: The impact of postoperative complications. Plast. Reconstr. Surg. 2000, 105, 2395–2399. [Google Scholar] [CrossRef]
- Threlfall, G.N.; Little, J.M.; Cummine, J. Free flap transfer--preliminary establishment of an arteriovenous fistula: A case report. Aust. N. Z. J. Surg. 1982, 52, 182–184. [Google Scholar] [CrossRef]
- Bruner, S.; Bickert, B.; Sauerbier, M.; Germann, G. Concept of arteriovenous loupes in high-risk free-tissue transfer: History and clinical experiences. Microsurgery 2004, 24, 104–113. [Google Scholar] [CrossRef]
- Matschke, J.; Armbruster, R.; Reeps, C.; Weitz, J.; Dragu, A. AV loop free flap: An interdisciplinary approach for perineal and sacral defect reconstruction after radical oncological exenteration and radiation in a colorectal cancer patient. World J. Surg. Oncol. 2019, 17, 154. [Google Scholar] [CrossRef]
- Knackstedt, R.; Aliotta, R.; Gatherwright, J.; Djohan, R.; Gastman, B.; Schwarz, G.; Hendrickson, M.; Gurunluoglu, R. Single-stage versus two-stage arteriovenous loop microsurgical reconstruction: A meta-analysis of the literature. Microsurgery 2018, 38, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Arkudas, A.; Horch, R.E.; Regus, S.; Meyer, A.; Lang, W.; Schmitz, M.; Boos, A.M.; Ludolph, I.; Beier, J.P. Retrospective cohort study of combined approach for trunk reconstruction using arteriovenous loops and free flaps. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 394–401. [Google Scholar] [CrossRef]
- Marchesini, A.; Senesi, L.; De Francesco, F.; Pangrazi, P.P.; Campodonico, A.; Politano, R.; Riccio, M. Efficacy of the Arteriovenous Loop for Free Flap Reconstruction in Patients with Complex Limb Trauma: Case Series and Literature Review. Medicina 2020, 56, 632. [Google Scholar] [CrossRef]
- Rother, U.; Muller-Mohnssen, H.; Lang, W.; Ludolph, I.; Arkudas, A.; Horch, R.E.; Regus, S.; Meyer, A. Wound closure by means of free flap and arteriovenous loop: Development of flap autonomy in the long-term follow-up. Int. Wound J. 2020, 17, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.S.; Barakat, A.Z.; Jaber, M.M.; Mashal, A.A. Free Flap Transfer with Arteriovenous Loop Establishment for Upper Limb Salvage in a Crush Injury. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1913. [Google Scholar] [CrossRef] [PubMed]
- Parra, L.; Andres, J.; Robustillo, M.; Garcia, C.; Iglesias, I.; Diaz, A. Ipsilateral Arteriovenous Loop and Latissimus Dorsi Free Flap for Knee Reconstruction in an Elderly Patient: A Case Report. World J. Plast. Surg. 2018, 7, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, I.; Horch, R.E.; Arkudas, A.; Schmitz, M. Enhancing Safety in Reconstructive Microsurgery Using Intraoperative Indocyanine Green Angiography. Front. Surg. 2019, 6, 39. [Google Scholar] [CrossRef]
- Akita, S.; Mitsukawa, N.; Tokumoto, H.; Kubota, Y.; Kuriyama, M.; Sasahara, Y.; Yamaji, Y.; Satoh, K. Regional Oxygen Saturation Index: A Novel Criterion for Free Flap Assessment Using Tissue Oximetry. Plast. Reconstr. Surg. 2016, 138, 510e–518e. [Google Scholar] [CrossRef]
- Wechselberger, G.; Rumer, A.; Schoeller, T.; Schwabegger, A.; Ninkovic, M.; Anderl, H. Free-flap monitoring with tissue-oxygen measurement. J. Reconstr. Microsurg. 1997, 13, 125–130. [Google Scholar] [CrossRef]
- Udesen, A.; Lontoft, E.; Kristensen, S.R. Monitoring of free TRAM flaps with microdialysis. J. Reconstr. Microsurg. 2000, 16, 101–106. [Google Scholar] [CrossRef]
- Schrogendorfer, K.F.; Nickl, S.; Keck, M.; Lumenta, D.B.; Loewe, C.; Gschwandtner, M.; Haslik, W.; Nedomansky, J. Viability of five different pre- and intraoperative imaging methods for autologous breast reconstruction. Eur. Surg. 2016, 48, 326–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gierek, M.; Bergler-Czop, B.; Slabon, A.; Labus, W.; Ochala-Gierek, G. Laser speckle contrast analysis (LASCA): A new device in the diagnosis and monitoring of surgical treatment of hidradenitis suppurativa. Postepy Dermatol. Alergol. 2023, 40, 253–258. [Google Scholar] [CrossRef]
- Hembd, A.S.; Yan, J.; Zhu, H.; Haddock, N.T.; Teotia, S.S. Intraoperative Assessment of DIEP Flap Breast Reconstruction Using Indocyanine Green Angiography: Reduction of Fat Necrosis, Resection Volumes, and Postoperative Surveillance. Plast. Reconstr. Surg. 2020, 146, 1e–10e. [Google Scholar] [CrossRef]
- Geierlehner, A.; Horch, R.E.; Ludolph, I.; Arkudas, A. Intraoperative Blood Flow Analysis of DIEP vs. ms-TRAM Flap Breast Reconstruction Combining Transit-Time Flowmetry and Microvascular Indocyanine Green Angiography. J. Pers. Med. 2022, 12, 482. [Google Scholar] [CrossRef]
- Beldi, G.; Bosshard, A.; Hess, O.M.; Althaus, U.; Walpoth, B.H. Transit time flow measurement: Experimental validation and comparison of three different systems. Ann. Thorac. Surg. 2000, 70, 212–217. [Google Scholar] [CrossRef]
- Warren, J.V.; Gorlin, R. Calculation of vascular resistance. Methods Med. Res. 1958, 7, 98–99. [Google Scholar]
- Mahabir, R.C.; Williamson, J.S.; Carr, N.J.; Courtemanche, D.J. Vascular resistance in human muscle flaps. Ann. Plast. Surg. 2001, 47, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kawaguchi, K.; Basugi, A.; Kanai, I.; Hamada, Y. Intraoperative real-time assessment of blood flow using indocyanine green angiography after anastomoses in free-flap reconstructions. Br. J. Oral. Maxillofac. Surg. 2017, 55, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Preidl, R.H.; Schlittenbauer, T.; Weber, M.; Neukam, F.W.; Wehrhan, F. Assessment of free microvascular flap perfusion by intraoperative fluorescence angiography in craniomaxillofacial surgery. J. Craniomaxillofac. Surg. 2015, 43, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Hitier, M.; Cracowski, J.L.; Hamou, C.; Righini, C.; Bettega, G. Indocyanine green fluorescence angiography for free flap monitoring: A pilot study. J. Craniomaxillofac. Surg. 2016, 44, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Geierlehner, A.; Ludolph, I.; Arkudas, A.; Horch, R.E. A Myocutaneous Latissimus Dorsi Propeller Flap Based on a Single Dorsal Intercostal Perforator. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3881. [Google Scholar] [CrossRef]
- Karanas, Y.L.; Yim, K.K.; Johannet, P.; Hui, K.; Lineaweaver, W.C. Use of 20 cm or longer interposition vein grafts in free flap reconstruction of the trunk. Plast. Reconstr. Surg. 1998, 101, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Mucke, T.; Hapfelmeier, A.; Schmidt, L.H.; Fichter, A.M.; Kanatas, A.; Wolff, K.D.; Ritschl, L.M. A comparative analysis using flowmeter, laser-Doppler |spectrophotometry, and indocyanine green-videoangiography for detection of vascular stenosis in free flaps. Sci. Rep. 2020, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Malagon, P.; Carrasco, C.; Vila, J.; Priego, D.; Higueras, C. Intraoperative hemodynamic changes in the arterial blood flow measured by transit time flowmetry (TTFM) during breast reconstruction with free diep flap. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1775–1784. [Google Scholar] [CrossRef]
- Lorenzetti, F.; Giordano, S.; Tukiainen, E. Intraoperative hemodynamic evaluation of the latissimus dorsi muscle flap: A prospective study. J. Reconstr. Microsurg. 2012, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, F.; Kuokkanen, H.; von Smitten, K.; Asko-Seljavaara, S. Intraoperative evaluation of blood flow in the internal mammary or thoracodorsal artery as a recipient vessel for a free TRAM flap. Ann. Plast. Surg. 2001, 46, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, F.; Suominen, S.; Tukiainen, E.; Kuokkanen, H.; Suominen, E.; Vuola, J.; Asko-Seljavaara, S. Evaluation of blood flow in free microvascular flaps. J. Reconstr. Microsurg. 2001, 17, 163–167. [Google Scholar] [CrossRef]
- Sasmor, M.T.; Reus, W.F.; Straker, D.J.; Colen, L.B. Vascular resistance considerations in free-tissue transfer. J. Reconstr. Microsurg. 1992, 8, 195–200. [Google Scholar] [CrossRef]
- Takanari, K.; Kamei, Y.; Toriyama, K.; Yagi, S.; Torii, S. Differences in blood flow volume and vascular resistance between free flaps: Assessment in 58 cases. J. Reconstr. Microsurg. 2009, 25, 39–45. [Google Scholar] [CrossRef]
- Holm, C.; Mayr, M.; Hofter, E.; Dornseifer, U.; Ninkovic, M. Assessment of the patency of microvascular anastomoses using microscope-integrated near-infrared angiography: A preliminary study. Microsurgery 2009, 29, 509–514. [Google Scholar] [CrossRef]
- Holzbach, T.; Artunian, N.; Spanholtz, T.A.; Volkmer, E.; Engelhardt, T.O.; Giunta, R.E. Microscope-integrated intraoperative indocyanine green angiography in plastic surgery. Handchir. Mikrochir. Plast. Chir. 2012, 44, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Dornseifer, U.; Sturtz, G.; Basso, G.; Schuster, T.; Ninkovic, M. The intrinsic transit time of free microvascular flaps: Clinical and prognostic implications. Microsurgery 2010, 30, 91–96. [Google Scholar] [CrossRef] [PubMed]
| Stage | Measurement | TTFM | aVR | mICG-A |
|---|---|---|---|---|
| 1. AV Loop Construction | AV1 | x | x | |
| 2. Free Flap Transfer | F | x | x | |
| AV2 | x | x | ||
| AA | x | x | x |
| Flow in mL/in (Mean ± SD) | ITT in Seconds (Mean ± SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Flap Pedicle in Situ (F) | AV Loop before Flap Transfer (AV2) | After Anastomosis (AA) | After Anastomosis (AA) | p-Value | |||||
| Type of Flap | No. (%) | Artery | Vein | Artery | Vein | Artery F vs. AA | Artery AV vs. AA | ||
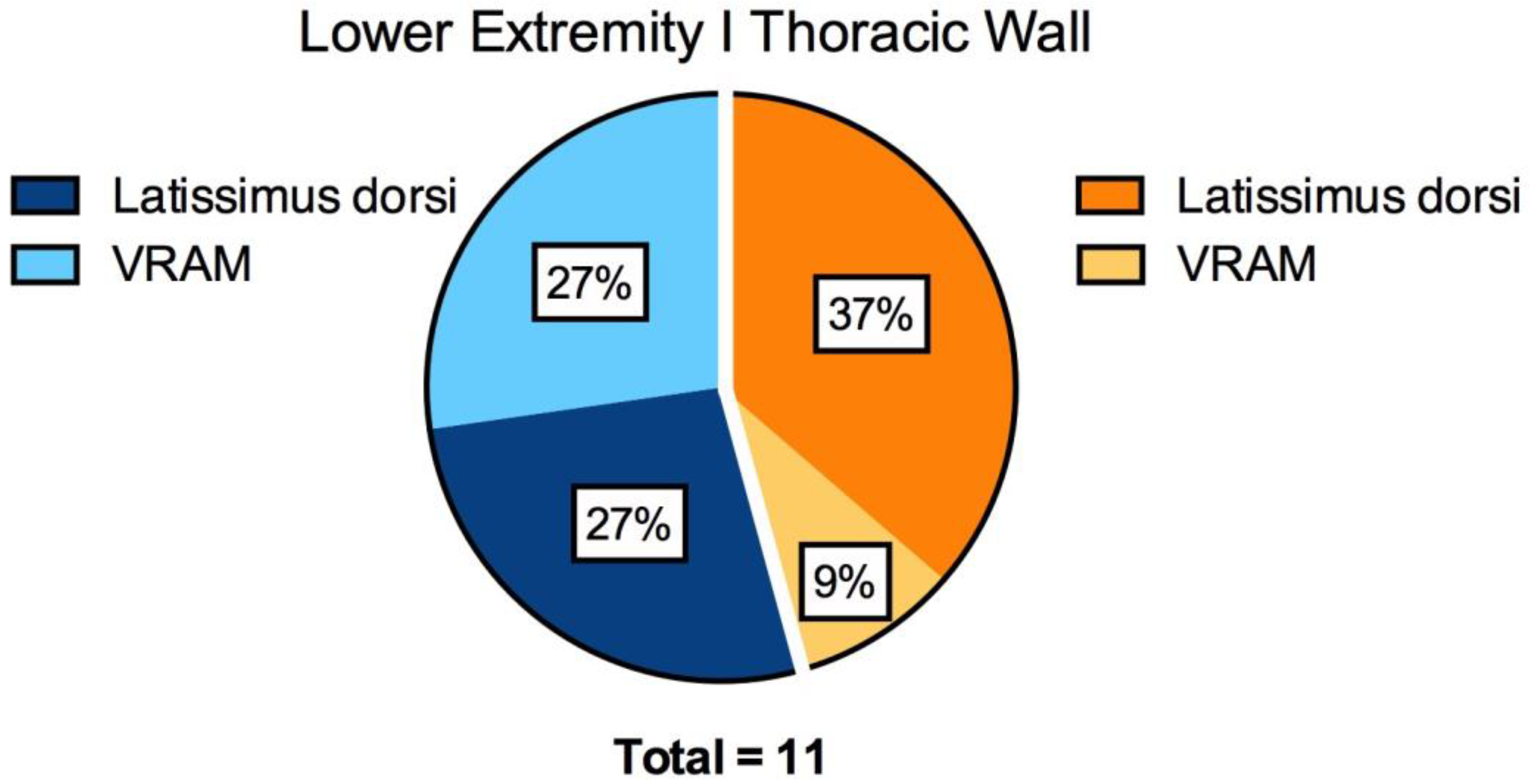
| All | 11 | 15.8 ± 6.4 | 10.6 ± 6.6 | 698 ± 464 | 18.5 ± 8.3 | 18.5 ± 9.3 | 38 ± 25 | 0.3 | <0.0001 |
| Latissimus | 7 | 16.6 ± 6.9 | 13 ± 7.1 | 780 ± 495 | 16.3 ± 7.4 | 17 ± 10.3 | 36 ± 23 | 0.8 | <0.01 |
| VRAM | 4 | 14.5 ± 6.2 | 6.5 ± 2.6 | 555 ± 430 | 22.3 ± 9.5 | 21 ± 8.1 | 43 ± 30 | 0.1 | <0.01 |
| p-value (Latissimus vs. VRAM) | 0.6 | 0.5 | 0.3 | 0.8 | |||||
| Flow (Mean ± SD) | ITT (Mean ± SD) | p-Value | ||||
|---|---|---|---|---|---|---|
| AV Loop Site | mL/min | Seconds | ||||
| AV Loop at Construction (AV1) | AV Loop before Flap Transfer (AV2) | After Anastomosis (AA) | After Anastomosis (AA) | Flow AV1 vs. AV2 | Flow AV2 vs. AA | |
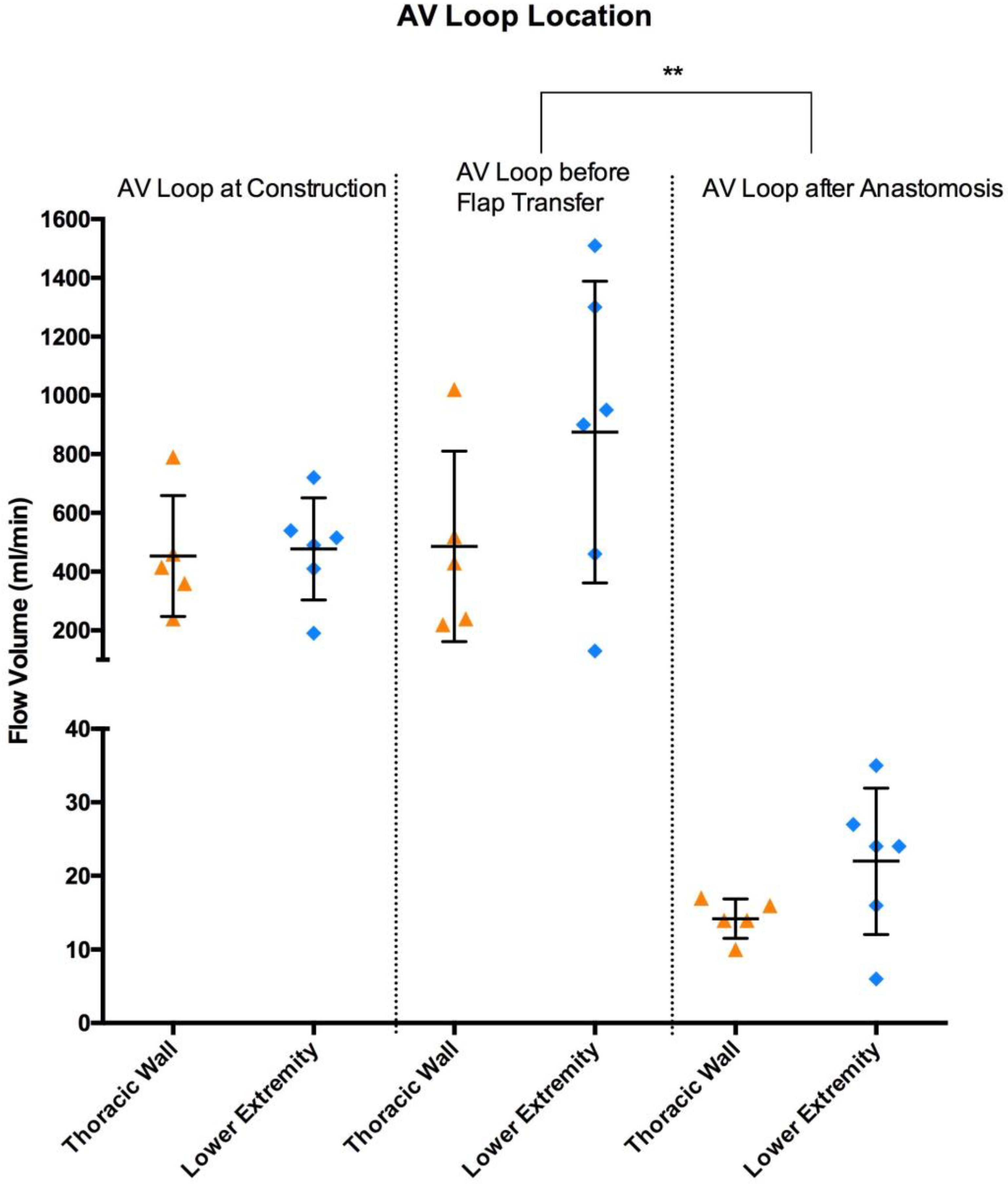
| All | 466 ± 180 | 698 ± 464 | 18.5 ± 8.3 | 38 ± 25 | 0.1 | <0.0001 |
| Lower Extremity | 478 ± 174 | 875 ± 513 | 22 ± 9.9 | 52 ± 26 | 0.2 | 0.001 |
| Thoracic Wall | 453 ± 206 | 486 ± 324 | 14.2 ± 2.7 | 22 ± 7 | 0.8 | 0.0002 |
| p-value (Lower Extremity vs. Thoracic Wall) | 0.6 | 0.3 | 0.1 | 0.03 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geierlehner, A.; Horch, R.E.; Ludolph, I.; Lang, W.; Rother, U.; Meyer, A.; Arkudas, A. Intraoperative Blood Flow Analysis of Free Flaps with Arteriovenous Loops for Autologous Microsurgical Reconstruction. J. Clin. Med. 2023, 12, 7477. https://doi.org/10.3390/jcm12237477
Geierlehner A, Horch RE, Ludolph I, Lang W, Rother U, Meyer A, Arkudas A. Intraoperative Blood Flow Analysis of Free Flaps with Arteriovenous Loops for Autologous Microsurgical Reconstruction. Journal of Clinical Medicine. 2023; 12(23):7477. https://doi.org/10.3390/jcm12237477
Chicago/Turabian StyleGeierlehner, Alexander, Raymund E. Horch, Ingo Ludolph, Werner Lang, Ulrich Rother, Alexander Meyer, and Andreas Arkudas. 2023. "Intraoperative Blood Flow Analysis of Free Flaps with Arteriovenous Loops for Autologous Microsurgical Reconstruction" Journal of Clinical Medicine 12, no. 23: 7477. https://doi.org/10.3390/jcm12237477
APA StyleGeierlehner, A., Horch, R. E., Ludolph, I., Lang, W., Rother, U., Meyer, A., & Arkudas, A. (2023). Intraoperative Blood Flow Analysis of Free Flaps with Arteriovenous Loops for Autologous Microsurgical Reconstruction. Journal of Clinical Medicine, 12(23), 7477. https://doi.org/10.3390/jcm12237477








