Delivery of Topical Drugs to the Olfactory Cleft
Abstract
:1. Introduction
2. Nasal Anatomy
3. Different Methods of Topical Administration
3.1. Nasal Sprays
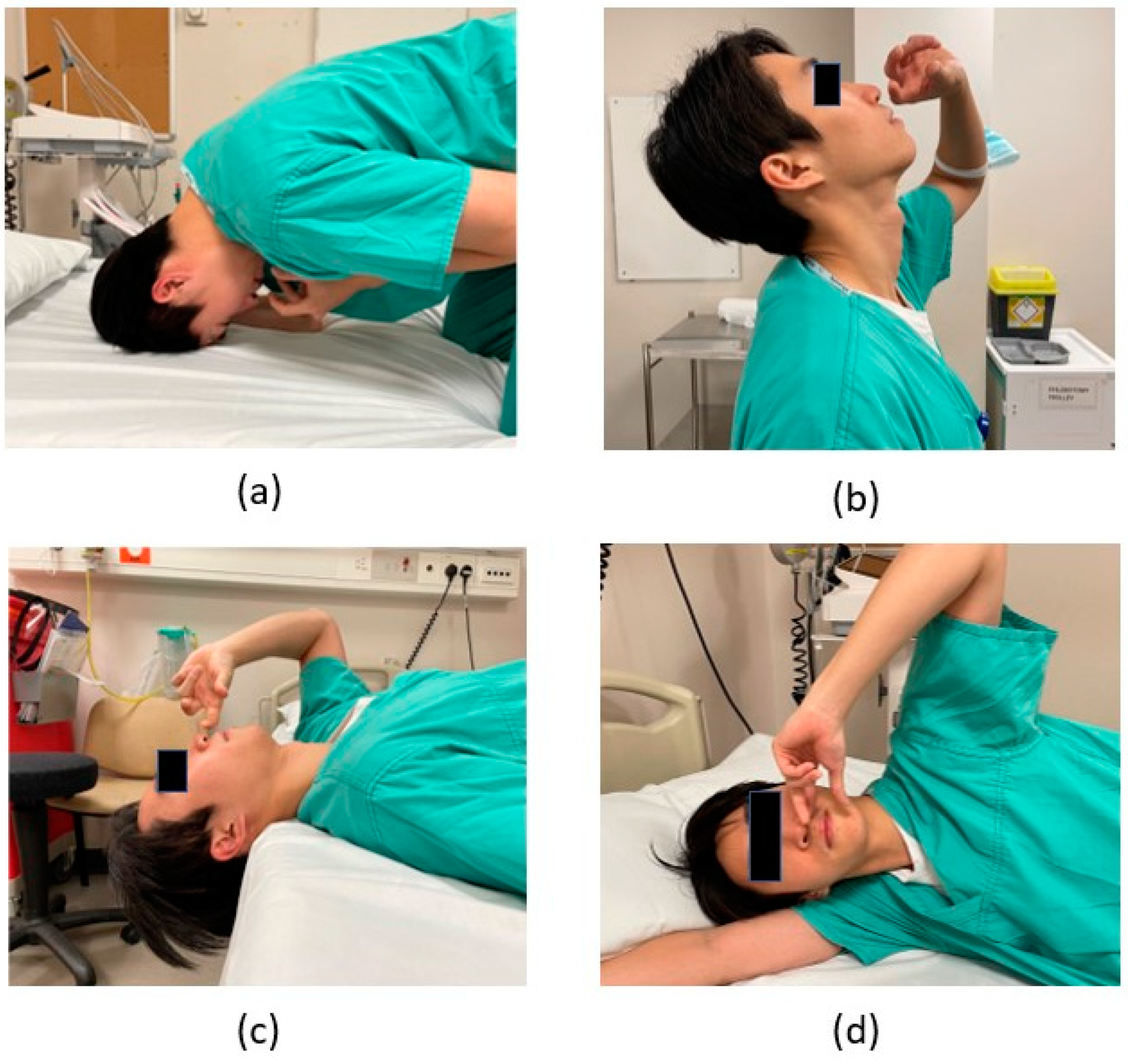
3.2. Nasal Drops with Various Head Positions
3.3. Rinses/Irrigation
3.4. Atomisers
3.5. The Exhalation Delivery System (OptiNose)
3.6. Direct Administration under Endoscopic Guidance
4. Different Topical Agents
4.1. Intranasal Corticosteroids
4.2. Intranasal Insulin
4.3. Intranasal Theophylline
4.4. Intranasal Tetra Sodium Pyrophosphate/Sodium Citrate
4.5. Intranasal Platelet-Rich Plasma
4.6. Vitamin A
4.7. Omega-3
5. Discussion
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Philpott, C.M.; Boak, D. The Impact of Olfactory Disorders in the United Kingdom. Chem. Senses 2014, 39, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Whitcroft, K.L.; Altundag, A.; Balungwe, P.; Boscolo-Rizzo, P.; Douglas, R.; Enecilla, M.L.B.; Fjaeldstad, A.W.; Fornazieri, M.A.; Frasnelli, J.; Gane, S.; et al. Position paper on olfactory dysfunction: 2023. Rhinology, 2023; ahead of print. [Google Scholar] [CrossRef]
- Desiato, V.M.; Levy, D.A.; Byun, Y.J.; Nguyen, S.A.; Soler, Z.M.; Schlosser, R.J. The Prevalence of Olfactory Dysfunction in the General Population: A Systematic Review and Meta-analysis. Am. J. Rhinol. Allergy 2021, 35, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.G. Nasal Anatomy and Function. Facial Plast Surg. 2017, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, B.B.; Leopold, D.A. Smell and taste disorders. Facial Plast Surg. Clin. N. Am. 2004, 12, 459–468, vii. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, M.; Bethge, C.; Witt, M.; Hummel, T. Intranasal Administration of Drugs. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Holmes, T.; Gao, J.; Guilmette, R.; Li, S.; Surakitbanharn, Y.; Rowlings, C. Characterization of Nasal Spray Pumps and Deposition Pattern in a Replica of the Human Nasal Airway. J. Aerosol Med. 2001, 14, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Benninger, M.S.; Hadley, J.A.; Osguthorpe, J.D.; Marple, B.F.; Leopold, D.A.; Derebery, M.J.; Hannley, M. Techniques of Intranasal Steroid Use. Otolaryngol.-Head Neck Surg. 2004, 130, 5–24. [Google Scholar] [CrossRef]
- Hardy, J.G.; Lee, S.W.; Wilson, C.G. Intranasal drug delivery by spray and drops. J. Pharm. Pharmacol. 2011, 37, 294–297. [Google Scholar] [CrossRef]
- Newman, S.P.; Morén, F.; Clarke, S.W. Deposition pattern of nasal sprays in man. Rhinology 1988, 26, 112–120. [Google Scholar]
- Weber, R.; Keerl, R.; Radziwill, R.; Schick, B.; Jaspersen, D.; Dshambazov, K.; Mlynski, G.; Draf, W. Videoendoscopic analysis of nasal steroid distribution. Rhinology 1999, 37, 69–73. [Google Scholar]
- Heilmann, S.; Huettenbrink, K.-B.; Hummel, T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am. J. Rhinol. 2018, 18, 29–33. [Google Scholar] [CrossRef]
- Muenkaew, Y.; Tangbumrungtham, N.; Roongpuvapaht, B.; Tanjararak, K. Comparison of sinus distribution between nasal irrigation and nasal spray using fluorescein-labelled in patients with chronic rhinosinusitis: A randomised clinical trial. Clin. Otolaryngol. 2023, 48, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kayarkar, R.; Clifton, N.; Woolford, T. An evaluation of the best head position for instillation of steroid nose drops. Clin. Otolaryngol. Allied Sci. 2002, 27, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Trabut, S.; Friedrich, H.; Caversaccio, M.; Negoias, S. Challenges in topical therapy of chronic rhinosinusitis: The case of nasal drops application—A systematic review. Auris Nasus Larynx 2020, 47, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Morén, F.; Björnek, K.; Klint, T.; Wagner, Z.G. A comparative distribution study of two procedures for administration of nose drops. Acta Oto-Laryngol. 2009, 106, 286–290. [Google Scholar] [CrossRef]
- Kubba, H.; Spinou, E.; Robertson, A. The Effect of Head Position on the Distribution of Drops within the Nose. Am. J. Rhinol. 2018, 14, 83–86. [Google Scholar] [CrossRef]
- Mori, E.; Merkonidis, C.; Cuevas, M.; Gudziol, V.; Matsuwaki, Y.; Hummel, T. The administration of nasal drops in the “Kaiteki” position allows for delivery of the drug to the olfactory cleft: A pilot study in healthy subjects. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 939–943. [Google Scholar] [CrossRef]
- Milk, D.G.; Khong, G.C.; Çam, O.H.; Alfaro-Iraheta, F.; Tierney, C.; Kassem, F.; Leong, S.C. A Comparison between Mygind and Kaiteki positions in administration of drops to the olfactory cleft. Clin. Otolaryngol. 2021, 46, 406–411. [Google Scholar] [CrossRef]
- Rawal, R.B.; Deal, A.M.; Ebert, C.S.; Dhandha, V.H.; Mitchell, C.A.; Hang, A.X.; Gore, M.R.; Senior, B.A.; Zanation, A.M. Post-operative budesonide irrigations for patients with polyposis: A blinded, randomized controlled trial. Rhinology 2015, 53, 227–234. [Google Scholar] [CrossRef]
- Rotenberg, B.W.; Zhang, I.; Arra, I.; Payton, K.B. Postoperative care for Samter’s triad patients undergoing endoscopic sinus surgery: A double-blinded, randomized controlled trial. Laryngoscope 2011, 121, 2702–2705. [Google Scholar] [CrossRef]
- Harvey, R.J.; Snidvongs, K.; Kalish, L.H.; Oakley, G.M.; Sacks, R. Corticosteroid nasal irrigations are more effective than simple sprays in a randomized double-blinded placebo-controlled trial for chronic rhinosinusitis after sinus surgery. Int. Forum Allergy Rhinol. 2018, 8, 461–470. [Google Scholar] [CrossRef]
- Soudry, E.; Wang, J.; Vaezeafshar, R.; Katznelson, L.; Hwang, P.H. Safety analysis of long-term budesonide nasal irrigations in patients with chronic rhinosinusitis post endoscopic sinus surgery. Int. Forum Allergy Rhinol. 2016, 6, 568–572. [Google Scholar] [CrossRef]
- Thamboo, A.; Manji, J.; Szeitz, A.; Santos, R.D.; Hathorn, I.; Gan, E.C.; Alsaleh, S.; Javer, A.R. The safety and efficacy of short-term budesonide delivered via mucosal atomization device for chronic rhinosinusitis without nasal polyposis. Int. Forum Allergy Rhinol. 2014, 4, 397–402. [Google Scholar] [CrossRef]
- Habib, A.-R.R.; Thamboo, A.; Manji, J.; Santos, R.C.D.; Gan, E.C.; Anstead, A.; Javer, A.R. The effect of head position on the distribution of topical nasal medication using the Mucosal Atomization Device: A cadaver study. Int. Forum Allergy Rhinol. 2013, 3, 958–962. [Google Scholar] [CrossRef]
- Cannady, S.B.; Batra, P.S.; Citardi, M.J.; Lanza, D.C. Comparison of Delivery of Topical Medications to the Paranasal Sinuses via “Vertex-to-floor” Position and Atomizer Spray after FESS. Otolaryngol.-Head Neck Surg. 2005, 133, 735–740. [Google Scholar] [CrossRef]
- Exhalation Delivery Systems. Optinose. Available online: https://www.optinose.com/exhalation-delivery-systems/technical-overview/ (accessed on 1 December 2022).
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef]
- Sher, M.R.; Mair, E.A.; Messina, J.; Carothers, J.; Mahmoud, R.; Djupesland, P.G. EXHANCE-3: A Phase 3, Three-Month Study of Safety and Efficacy of Fluticasone Propionate Exhalation Delivery System (FLU-EDS) in Patients with Chronic Rhinosinusitis with (CRSwNP) and without Nasal Polyps (CRSsNP). J. Allergy Clin. Immunol. 2017, 139, AB66. [Google Scholar] [CrossRef]
- Vlckova, I.; Navratil, P.; Kana, R.; Pavlicek, P.; Chrbolka, P.; Djupesland, P.G. Effective treatment of mild-to-moderate nasal polyposis with fluticasone delivered by a novel device. Rhinol. J. 2009, 47, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Leopold, D.A.; Elkayam, D.; Messina, J.C.; Kosik-Gonzalez, C.; Djupesland, P.G.; Mahmoud, R.A. Navigate II: Randomized, double-blind trial of the exhalation delivery system with fluticasone for nasal polyposis. J. Allergy Clin. Immunol. 2019, 143, 126–134.e5. [Google Scholar] [CrossRef] [PubMed]
- Kuan, E.C.; Kovacs, A.J.; Workman, A.D.; Bosso, J.V.; Adappa, N.D. Efficacy of fluticasone exhalation delivery system in the management of chronic rhinosinusitis: What is the evidence? Int. Forum Allergy Rhinol. 2019, 9, S16–S21. [Google Scholar] [CrossRef]
- Rezaeian, A. Effect of Intranasal Insulin on Olfactory Recovery in Patients with Hyposmia: A Randomized Clinical Trial. Otolaryngol.-Head Neck Surg. 2018, 158, 1134–1139. [Google Scholar] [CrossRef]
- Chong, L.Y.; Head, K.; Hopkins, C.; Philpott, C.; Schilder, A.G.; Burton, M.J. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst. Rev. 2016, 2016, CD011996. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Kang, M.; Hwang, S.H. Efficacy of topical steroids for the treatment of olfactory disorders caused by COVID-19: A systematic review and meta-analysis. Clin. Otolaryngol. 2022, 47, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Huart, C.; Philpott, C.M.; Altundag, A.; Fjaeldstad, A.W.; Frasnelli, J.; Gane, S.; Hsieh, J.W.; Holbrook, E.H.; Konstantinidis, I.; Landis, B.N.; et al. Systemic corticosteroids in coronavirus disease 2019 (COVID-19)-related smell dysfunction: An international view. Int. Forum Allergy Rhinol. 2021, 11, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Patel, Z.M. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int. Forum Allergy Rhinol. 2018, 8, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, M.; Singh, A.; Jain, N.; Avanthika, C.; Jhaveri, S.; De la Hoz, I.; Sanka, S.; Goli, S.R. A Comprehensive Review of Intranasal Insulin and Its Effect on the Cognitive Function of Diabetics. Cureus 2021, 13, e17219. [Google Scholar] [CrossRef] [PubMed]
- Schmid, V.; Kullmann, S.; Gfrörer, W.; Hund, V.; Hallschmid, M.; Lipp, H.; Häring, H.; Preissl, H.; Fritsche, A.; Heni, M. Safety of intranasal human insulin: A review. Diabetes Obes. Metab. 2018, 20, 1563–1577. [Google Scholar] [CrossRef] [PubMed]
- Brünner, Y.F.; Benedict, C.; Freiherr, J. Intranasal Insulin Reduces Olfactory Sensitivity in Normosmic Humans. J. Clin. Endocrinol. Metab. 2013, 98, E1626–E1630. [Google Scholar] [CrossRef]
- Rodriguez-Raecke, R.; Brünner, Y.F.; Kofoet, A.; Mutic, S.; Benedict, C.; Freiherr, J. Odor Sensitivity After Intranasal Insulin Application Is Modulated by Gender. Front. Endocrinol. 2018, 9, 580. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Badawi, A.M.; Mansour, H.F. Insulin fast-dissolving film for intranasal delivery via olfactory region, a promising approach for the treatment of anosmia in COVID-19 patients: Design, in-vitro characterization and clinical evaluation. Int. J. Pharm. 2021, 601, 120600. [Google Scholar] [CrossRef]
- Thanarajah, S.E.; Hoffstall, V.; Rigoux, L.; Hanssen, R.; Brüning, J.C.; Tittgemeyer, M. The role of insulin sensitivity and intranasally applied insulin on olfactory perception. Sci. Rep. 2019, 9, 7222. [Google Scholar] [CrossRef] [PubMed]
- Helman, S.N.; Adler, J.; Jafari, A.; Bennett, S.; Vuncannon, J.R.; Cozart, A.C.; Wise, S.K.; Kuruvilla, M.E.; Levy, J.M. Treatment strategies for postviral olfactory dysfunction: A systematic review. Allergy Asthma Proc. 2022, 43, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Lee, J.J.; Perrin, A.; Khan, A.; Smith, H.J.; Farrell, N.; Kallogjeri, D.; Piccirillo, J.F. Efficacy and Safety of Saline Nasal Irrigation Plus Theophylline for Treatment of COVID-19–Related Olfactory Dysfunction. JAMA Otolaryngol.–Head Neck Surg. 2022, 148, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Hosein, W.; Henkin, R.I. Therapeutic diminution of Interleukin-10 with intranasal theophylline administration in hyposmic patients. Am. J. Otolaryngol. 2022, 43, 103375. [Google Scholar] [CrossRef] [PubMed]
- Menini, A. Calcium signalling and regulation in olfactory neurons. Curr. Opin. Neurobiol. 1999, 9, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-Y.; Yau, K.-W. Direct modulation by Ca2+–calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature 1994, 368, 545–548. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, A.Z.; Chan, G.C.; Baker, L.P.; Impey, S.; Beavo, J.A.; Storm, D.R. Phosphorylation and Inhibition of Olfactory Adenylyl Cyclase by CaM Kinase II in Neurons. Neuron 1998, 21, 495–504. [Google Scholar] [CrossRef]
- Panagiotopoulos, G.; Naxakis, S.; Papavasiliou, A.; Filipakis, K.; Papatheodorou, G.; Goumas, P. Decreasing nasal mucus Ca++ improves hyposmia. Rhinology 2005, 43, 130–134. [Google Scholar]
- Philpott, C.; Erskine, S.; Clark, A.; Leeper, A.; Salam, M.; Sharma, R.; Murty, G.; Hummel, T. A randomised controlled trial of sodium citrate spray for non-conductive olfactory disorders. Clin. Otolaryngol. 2017, 42, 1295–1302. [Google Scholar] [CrossRef]
- Whitcroft, K.L.; Merkonidis, C.; Cuevas, M.; Haehner, A.; Philpott, C.; Hummel, T. Intranasal sodium citrate solution improves olfaction in post-viral hyposmia. Rhinol. J. 2016, 54, 368–374. [Google Scholar] [CrossRef]
- Abdelazim, M.H.; Abdelazim, A.H. Effect of Sodium Gluconate on Decreasing Elevated Nasal Calcium and Improving Olfactory Function Post COVID-19 Infection. Am. J. Rhinol. Allergy 2022, 36, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, M.H.; Abdelazim, A.H.; Moneir, W. The effect of intra-nasal tetra sodium pyrophosphate on decreasing elevated nasal calcium and improving olfactory function post COVID-19: A randomized controlled trial. Allergy Asthma Clin. Immunol. 2022, 18, 67. [Google Scholar] [CrossRef]
- Abdelazim, M.H.; Abdelazim, A.H.; Ismaiel, W.F.; Alsobky, M.E.; Younes, A.; Hadeya, A.M.; Ramzy, S.; Shahin, M. Effect of intra-nasal nitrilotriacetic acid trisodium salt in lowering elevated calcium cations and improving olfactory dysfunction in COVID-19 patients. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4623–4628. [Google Scholar] [CrossRef]
- Mavrogeni, P.; Kanakopoulos, A.; Maihoub, S.; Maihoub, S.; Krasznai, M.; Szirmai, A. Anosmia treatment by platelet rich plasma injection. Int. Tinnitus J. 2016, 20, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Mundy, D.C.; Patel, Z.M. The use of platelet-rich plasma in treatment of olfactory dysfunction: A pilot study. Laryngoscope Investig. Otolaryngol. 2020, 5, 187–193. [Google Scholar] [CrossRef]
- Balmer, J.E.; Blomhoff, R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002, 43, 1773–1808. [Google Scholar] [CrossRef]
- Rawson, N.; LaMantia, A.-S. A speculative essay on retinoic acid regulation of neural stem cells in the developing and aging olfactory system. Exp. Gerontol. 2007, 42, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.B.; Briggs, M. Treatment of Uncomplicated Anosmia by Vitamin A. Arch. Otolaryngol.-Head Neck Surg. 1962, 75, 116–124. [Google Scholar] [CrossRef]
- Hummel, T.; Whitcroft, K.L.; Rueter, G.; Haehner, A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2819–2825. [Google Scholar] [CrossRef]
- Kumaresan, K.; Bengtsson, S.; Sami, S.; Clark, A.; Hummel, T.; Boardman, J.; High, J.; Sobhan, R.; Philpott, C. A double-blinded randomised controlled trial of vitamin A drops to treat post-viral olfactory loss: Study protocol for a proof-of-concept study for vitamin A nasal drops in post-viral olfactory loss (APOLLO). Pilot Feasibility Stud. 2023, 9, 174. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Greiner, R.S.; Moriguchi, T.; Slotnick, B.M.; Hutton, A.; Salem, N. Olfactory discrimination deficits in n−3 fatty acid-deficient rats. Physiol. Behav. 2001, 72, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Rathor, A.; Krook, K.; Ma, Y.; Rotella, M.R.; Dodd, R.L.; Hwang, P.H.; Nayak, J.V.; Oyesiku, N.M.; DelGaudio, J.M.; et al. Effect of Omega-3 Supplementation in Patients With Smell Dysfunction Following Endoscopic Sellar and Parasellar Tumor Resection: A Multicenter Prospective Randomized Controlled Trial. Neurosurgery 2020, 87, E91–E98. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Tan, B.K.; Lavin, J.M.; Meen, E.; Conley, D.B. Comparison of nasal sprays and irrigations in the delivery of topical agents to the olfactory mucosa. Laryngoscope 2013, 123, 2950–2957. [Google Scholar] [CrossRef]
- Hura, N.; Xie, D.X.; Choby, G.W.; Schlosser, R.J.; Orlov, C.P.; Seal, S.M.; Rowan, N.R. Treatment of post-viral olfactory dysfunction: An evidence-based review with recommendations. Int. Forum Allergy Rhinol. 2020, 10, 1065–1086. [Google Scholar] [CrossRef]
- COMET Initiative. Developing a Core Outcome Set for Clinical Trials in Olfactory Disorders. Available online: https://www.comet-initiative.org/Studies/Details/1957 (accessed on 29 March 2023).
- Luke, L.; Lee, L.; Jegatheeswaran, L.; Philpott, C. Investigations and Outcomes for Olfactory Disorders. Curr. Otorhinolaryngol. Rep. 2022, 10, 377–384. [Google Scholar] [CrossRef]
| Treatment Method | Drug | Mechanism of Action | Clinical Application | Method of Application | References |
|---|---|---|---|---|---|
| Intranasal Corticosteroids | Anti-inflammatory agents | Reduces inflammation in the olfactory epithelium | Chronic rhinosinusitis | Sprays/aerosols Drops Irrigations | [2,23,24] |
| Intranasal Insulin | Hormone involved in glucose metabolism and cell growth | Stimulates olfactory stem cell proliferation and differentiation | Postviral olfactory dysfunction, general olfactory sensitivity | Sprays Gelfoam | [39,40] |
| Intranasal Theophylline | Phosphodiesterase inhibitor | Increases cyclic adenosine monophosphate and cyclic guanosine monophosphate levels in nasal secretions | General olfactory sensitivity | Irrigation sprays | [45,46] |
| Intranasal Tetra Sodium Pyrophosphate/Sodium Citrate | Calcium chelating agent | Lowers intranasal calcium concentration to improve olfactory signalling | Postviral olfactory dysfunction, general olfactory sensitivity | Drops Sprays | [28,47,50,51] |
| Intranasal Platelet-Rich Plasma | Contains growth factors important in tissue repair | Aids regeneration of damaged olfactory neuroepithelial cells or basal stem cells | Postviral olfactory dysfunction, general olfactory sensitivity | Injection | [56,57] |
| Vitamin A | Metabolite of vitamin A | Induces neuronal regeneration in the olfactory epithelium | Postviral olfactory dysfunction, general olfactory sensitivity | Drops | [58,59,60,61] |
| Omega-3 | Polyunsaturated fatty acid | Enhances membrane fluidity and synaptic function | General olfactory sensitivity | Irrigation | [63,64,65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espehana, A.; Lee, L.; Garden, E.M.; Klyvyte, G.; Gokani, S.; Jegatheeswaran, L.; Wong, J.J.; Philpott, C. Delivery of Topical Drugs to the Olfactory Cleft. J. Clin. Med. 2023, 12, 7387. https://doi.org/10.3390/jcm12237387
Espehana A, Lee L, Garden EM, Klyvyte G, Gokani S, Jegatheeswaran L, Wong JJ, Philpott C. Delivery of Topical Drugs to the Olfactory Cleft. Journal of Clinical Medicine. 2023; 12(23):7387. https://doi.org/10.3390/jcm12237387
Chicago/Turabian StyleEspehana, Andreas, Liam Lee, Elizabeth Mairenn Garden, Gabija Klyvyte, Shyam Gokani, Lavandan Jegatheeswaran, Jeremy Jonathan Wong, and Carl Philpott. 2023. "Delivery of Topical Drugs to the Olfactory Cleft" Journal of Clinical Medicine 12, no. 23: 7387. https://doi.org/10.3390/jcm12237387





