1. Introduction
According to the World Health Organization (WHO), health is a “state of complete physical, mental, and social well-being, and not merely the absence of disease or infirmity”. Therefore, mental health, defined by the WHO as a “state of mental well-being that enables people to cope with the stresses of life, realize their abilities, learn well and work well, and contribute to their community”, is as fundamental as physical health in the achievement of positive overall wellness in an individual [
1].
Stress, anxiety, compromised mental well-being, and sleep quality are fundamental and interconnected aspects of mental health. They can impact each other and together contribute to a general state of emotional and mental wellness. Mental stress can be medically understood as the ‘individual’s perception of a stimulus as overwhelming’ which results in a response and a transformed state [
2]. Anxiety is defined by the American Psychological Association as “an emotion characterized by feelings of tension, worried thoughts, and physical changes like increased blood pressure.” Both stress and anxiety are emotional responses. Stress is usually precipitated by an external factor, whereas anxiety is defined by the persistence of excessive worries even in the absence of a stressor. Well-being is broadly defined as ‘the quality and state of a person’s life’ [
3] and consists of two components: feeling healthy and relatively robust and being able to carry out one’s job and other tasks satisfactorily [
4]. Finally, sleep quality is defined as an individual’s level of satisfaction with all aspects of the sleep experience [
5]. Sleep quality is highly dependent on the person’s general well-being.
Maternal mental stress, anxiety, compromised well-being, and sleep quality have been associated with several adverse pregnancy outcomes such as preterm birth (PTB) [
6,
7,
8,
9,
10,
11,
12], low birthweight (LBW) [
7,
13,
14,
15], gestational diabetes (GD) [
16,
17], labor complications [
12,
18,
19,
20,
21], or hypertension and preeclampsia (PE) [
22,
23]. Moreover, maternal stress has been demonstrated to be a prenatal programming factor that affects the fetal neurodevelopment [
24] and could compromise the socioemotional competencies in childhood that are the foundation for future well-being [
24].
Mental stress, anxiety, compromised well-being, and sleep disturbances are common during pregnancy. Around 20% of pregnant women could experience excessive concern regarding future events in pregnancy under normal circumstances [
4]. Up to 70% of pregnant women report symptoms of stress and anxiety during pregnancy, with between 10% and 16% of them fulfilling the criteria for a major depressive disorder [
25,
26]. While the real prevalence of antenatal psychosocial stress is still unclear [
27], in a 2003 study, Rondó et al. found high stress in 22–25% of pregnant women during the three trimesters of pregnancy [
7]. In a meta-analysis of 102 studies involving 221,974 women, Dennis et al. found that the prevalence rate for self-reported anxiety symptoms in the first trimester was 18.2% and 24.6% in the third trimester [
28]. These percentages decreased when employing diagnostic interviews: the prevalence rate for any anxiety disorder during the first trimester was 18% and 15% in the final two trimesters of pregnancy [
28]. However, we can speculate that the symptoms of depression can overlap with some normal feelings during pregnancy, which could explain such high percentages and the disparity found among studies [
26]. There is no clear evidence of the prevalence of compromised well-being during pregnancy. A highly variable prevalence of poor sleep quality in pregnant women has also been reported, ranging from 17% to 76% [
29]. This disparity could be due to dissimilar sample compositions and different methods and timings of assessments [
30]. Moreover, some authors have even postulated the possibility that the previously validated cut-off values for sleep questionnaires in the general population may not be valid in pregnancy, thus requiring a higher score [
30].
Different risk factors for antenatal mood disorders have been postulated in the previously published literature. Sociodemographic variables such as age have been considered in multiple studies with inconsistent findings among them [
31,
32]. Other sociodemographic variables considered in the previous literature are maternal socioeconomic status and educational level: in a 2010 systematic review, Lancaster et al. found a small association between low educational level and depression symptoms that could not be demonstrated in the multivariate analyses [
33]. Later, Biaggi et al. found low maternal educational level to be associated with anxiety and depressive symptoms [
32]. As for ethnicity, socioeconomic status, employment, an unfavorable socioeconomic situation, unemployment, and belonging to a minority ethnic group are associated with depression in several studies [
31,
32,
34] but inconsistent results are described in others [
32,
33]. On the other hand, other factors such as smoking, alcohol intake, and drug abuse showed inconsistent findings in their association with depression and sleep quality [
29,
32,
33,
34]. A personal medical history of anxiety and depression has strongly been associated with perinatal depression [
31,
32,
33,
34]. Other studies suggest an association between previous obstetric history, like previous abortions or pregnancy complications, with depressive symptoms and poor sleep quality [
29,
31,
32] but also with inconsistent findings [
33]. A complex multifactorial origin for the etiology of these conditions could be a possible explanation for such different results reported in the literature [
33].
Despite the high prevalence of these antenatal negative affective states and their impact on pregnancy, it is still unclear if they worsened during pregnancy and what the potential risk factors for these conditions are during pregnancy.
The aim of this study was to determine maternal stress, anxiety, well-being, and sleep quality across different stages of pregnancy and to identify related risk factors.
4. Discussion
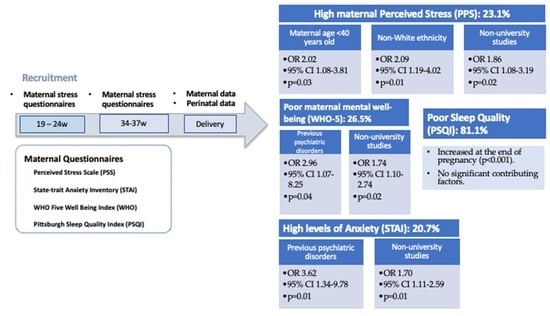
Our study reveals the potential importance of assessing antenatal negative affective states in a pregnant population. Stress, anxiety, compromised well-being, and sleep disorders have been reported by a significant number of pregnant participants in our cohort. There is a possible underassessment of these conditions by obstetric-care providers in daily clinical practice and our results stress the importance of actively evaluating signs and symptoms of negative affective states and sleep quality throughout gestation.
Perceived stress and STAI-T did not change throughout pregnancy; however, STAI-S increased in the third trimester of pregnancy. Previously published studies have shown that anxiety and depressive symptoms are not homogeneous during the perinatal period [
32,
43,
44]. Thus, nearly one quarter of participants scored as high stress and anxiety in the third trimester of pregnancy. Such percentages of perceived stress and anxiety highlight the importance of targeting these patients with clinically validated questionnaires in routine pregnancy follow-ups, with the aim of offering support interventions to these patients. Moreover, previous evidence has suggested that pregnancy-related anxiety constitutes a different concept from general anxiety. This fact could be a possible explanation for a limited measurement and assessment of anxiety in pregnancies and could also encourage the need for research in pregnancy-adapted measurement tools [
45].
To the best of our knowledge, there are no data regarding the prevalence of compromised well-being in the pregnant population with which to compare our results. However, in a study conducted by Sattler et al. in a group of overweight and obese women in Europe, a prevalence of low well-being of 27% before 20 weeks of pregnancy is reported [
46]. Similarly, during the COVID-19 pandemic Mortazavi et al. reported a prevalence of compromised wellbeing of 24.4% pregnant women during gestation [
4]. Around 26% of our population had compromised well-being, which is a similar percentage. The WHO-5 questionnaire is considered a good screening questionnaire with high sensitivity and specificity for clinical depression [
46]. It has the advantage of being a relatively easy and quick instrument to use in daily clinical practice allowing a first detection of women with a negative affective state who could benefit from a further mental health assessment.
The prevalence of sleep disturbances in our cohort was very high: more than 80% of participants were found to have compromised sleep at 34–36 weeks of gestation. Our prevalence results are higher than expected according to the literature, ranging from 17% to 76% [
29]. As suggested in previous studies, this fact could highlight the possibility that the validated cut-off for sleep questionnaires in the general population may not be valid in pregnancies, the latter requiring a higher score [
30]. Moreover, we found that the results of sleep quality questionnaires worsened in the third-trimester assessment as compared to the results found in the previous weeks of gestation. The worsening of sleep quality throughout gestation identified in our cohort is in line with previous evidence: according to a meta-analysis of 24 studies, it was found that sleep disturbances tend to increase during pregnancy and clinicians should be aware that complaints of very poor sleep could require intervention [
30].
Diagnosis and screening of maternal mental health have long been recommended by scientific societies. For instance, the American College of Obstetricians and Gynecologists recommends the use of a validated and standardized tool to screen pregnant women at least once during the perinatal period for symptoms of depression and anxiety [
47]. However, the use of multiple questionnaires to assess maternal mental health and sleep quality can be challenging in daily clinical practice, especially in an environment with a high healthcare workload. Therefore, we believe that understanding the associated risk factors may help to target those patients at higher risk and thus facilitate daily clinical practice as they can be identified at the beginning of pregnancy. Various risk factors for antenatal negative mood states have been postulated in the previous literature [
29,
31,
32,
33,
34].
In our cohort, we found that a main risk factor for maternal perceived stress, a higher level of state anxiety, and poorer well-being in the third trimester was non-university studies. In line with these results, some previous research in the pregnant population had already postulated a low educational profile as a risk factor for antenatal depression [
31,
32]. However, in contrast to our findings, Lancaster et al. described only a small association of lower educational levels with depressive symptoms in a systematic review [
33]. In general, among the non-pregnant population, a low educational level has also been associated with anxiety and depression [
48]. Our results could be explained by the fact that, as previously suggested in the literature, normally, education is likely to result in good mental health rather than come from good mental health and, in turn, education may also provide success in pursuing personal ends that include emotional well-being [
48,
49].
For the STAI-T personality questionnaire, we found preeclampsia in a previous pregnancy to be a potential risk factor. In a systematic review, Grigoriadis et al. found that prenatal maternal anxiety was not significantly associated with preeclampsia, although there was a significant heterogeneity across studies [
50]. However, we did not find any data regarding the association between previous preeclampsia and compromised mental health in subsequent pregnancies in the previous literature. A prior history of adverse obstetric events has already been related to the symptoms of anxiety and depression [
31,
32,
51], which could be in line with our results regarding the occurrence of preeclampsia in a previous pregnancy.
Perceived stress was also influenced in our cohort by non-white ethnicity and a maternal age of <40 years, and the latter was also found to be a risk factor for a higher score in trait anxiety among our participants. The literature also provides inconsistent findings as far as maternal age and ethnicity are concerned, as reported in the systematic reviews by Lancaster et al. [
33] and Biaggi et al. [
32]. In their review, Biaggi et al. described 13 studies where young age was posited as a risk factor, in contrast with 10 studies where advanced maternal age was described as a risk factor for antenatal depression and anxiety [
32].
A higher level of anxiety in the third trimester and poorer maternal well-being in the third-trimester assessment were provided by the presence of a previous psychiatric disorder. These results are in line with previously published evidence, as previous mental health disorders have been strongly related to higher anxiety in the past, in particular a history of anxiety and depression and a history of psychiatric treatment [
32]. Lancaster et al. also reported an association between a personal history of depression and an increased risk of antepartum depressive symptoms [
33]. Multiple studies conducted during the COVID-19 pandemic on maternal mental status proposed the presence of a previous psychiatric disorder as a risk factor for negative maternal affective states [
52,
53,
54,
55].
As for poor maternal sleep quality, no significant contributing factors were found. These findings are in contrast with those found in previous research where some risk factors could be postulated as contributors to sleep disturbances during pregnancy, such as a history of stillbirth, general health-related quality of life, or insufficient physical activity [
29]. Christian et al. found that African-Americans’ ethnicity and multiparity were related to poor sleep during pregnancy [
56]. Other studies reported gestational age [
30] or previous maternal BMI to be contributing factors [
57]. Our univariate analysis also suggested ethnicity and obesity to be contributing factors; however, we could not demonstrate it in the multivariate analysis.
Finally, previous research has a well-documented association between anxiety, life stress, sleep quality, and maternal mental well-being [
29,
32,
33]. Our results are in line with previous evidence as we found a correlation between anxiety, stress, and poorer mental well-being. In contrast, we found a low correlation between sleep quality and stress, anxiety, and mental well-being.
On the other hand, despite these associations, we believe the use of four validated questionnaires assessing different dimensions of maternal mental health may provide a more integrative approach to overall mental health, as the absence of problems in one dimension does not necessarily guarantee the same results in other aspects of mental health.
The strengths of this study were the use of various validated questionnaires with potential clinical applicability to assess different aspects of mental health: mental stress, anxiety, well-being, and sleep quality; and that they were assessed in the second and third trimester of pregnancy, which allowed an analysis of the experimented changes throughout pregnancy.
Among the study’s limitations is the fact that our population was a high socioeconomic cohort, with a high education profile, and most of the participants were between 30 and 40 years of age, with a low level of ethnical variety and a low proportion of obesity and gestational diabetes. This might explain some of the findings, especially in sleep disturbances, where we could not demonstrate the contribution of these factors in multivariate analysis.
We have no data regarding the first trimester of pregnancy nor the influence that these negative affective states had on perinatal results. Moreover, the neurocognitive function was not assessed, despite its potential influence on mental health [
58]. In interpreting the results, it is important to understand that the use of self-reporting instruments may potentially overestimate prevalence, but it is also important to state that they also have high clinical applicability in public health and daily obstetric-care practice. Our study confirms the importance of promoting good mental health [
59], especially during pregnancy.