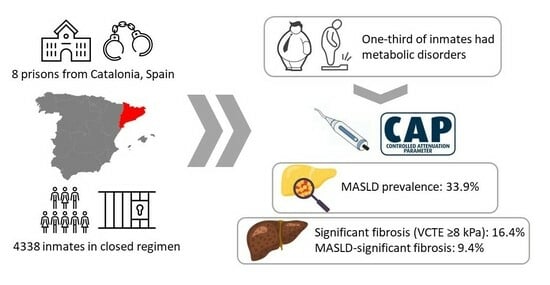
Prevalence and Risk Factors of MASLD and Liver Fibrosis amongst the Penitentiary Population in Catalonia: The PRISONAFLD Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients and Setting
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Outcomes
2.5. Definitions
2.6. Procedures
2.7. Statistical Analysis
3. Results
3.1. Sample
3.2. Study Population
3.3. Liver Fibrosis Distribution and Relationship with MASLD
3.4. Predictors of MASLD and MASLD-Associated Liver Fibrosis among the Prison Population
3.5. Genetic Single Nucleotide Polymorphism Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Allen, L.; Williams, J.; Townsend, N.; Mikkelsen, B.; Roberts, N.; Foster, C.; Wickramasinghe, K. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: A systematic review. Lancet Glob. Health 2017, 5, e277–e289. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.L.S.; Barbalho, S.M.; de Araujo, R.R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2021, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.W.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023. [Google Scholar] [CrossRef]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cuevas, J.; Lucano-Landeros, S.; López-Cifuentes, D.; Santos, A.; Armendariz-Borunda, J. Epidemiologic, Genetic, Pathogenic, Metabolic, Epigenetic Aspects Involved in NASH-HCC: Current Therapeutic Strategies. Cancers 2022, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Calleja, J.L.; Rivera-Esteban, J.; Aller, R.; Hernández-Conde, M.; Abad, J.; Pericàs, J.M.; Benito, H.G.; Serra, M.A.; Escudero, A.; Ampuero, J.; et al. Prevalence estimation of significant fibrosis because of NASH in Spain combining transient elastography and histology. Liver Int. 2022, 42, 1783–1792. [Google Scholar] [CrossRef]
- Kjaergaard, M.; Lindvig, K.P.; Thorhauge, K.H.; Andersen, P.; Hansen, J.K.; Kastrup, N.; Jensen, J.M.; Hansen, C.D.; Johansen, S.; Israelsen, M.; et al. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. J. Hepatol. 2023, 79, 277–286. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guideline Panel; EASL Governing Board Representative. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Fazel, S.; Baillargeon, J. The health of prisoners. Lancet 2011, 377, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.A. Chronic medical conditions among jail detainees in residencial psychiatric treatment: A latent class analysis. J. Urban Health 2011, 4, 700–717. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, W.; Elger, B.; Wangmo, T. Ageing Prisoners’ Health Care: Analysing the Legal Settings in Europe and the United States. Gerontology 2012, 59, 267–275. [Google Scholar] [CrossRef]
- García-Guerrero, J.; Vera-Remartinez, E.; Planelles, M.V. Causas y tendencia de la mortalidad en una prisión española (1994–2009). Rev. Esp. Salud Pública 2011, 85, 245–255. [Google Scholar] [CrossRef]
- Wobeser, W.L.; Datema, J.; Bechard, B.; Ford, P. Causes of death among people in custody in Ontario, 1990–1999. CMAJ 2002, 167, 1109–1113. [Google Scholar]
- Fazel, S.; Benning, R. Natural deaths in male prisoners: A 20-year mortality study. Eur. J. Public Health 2006, 16, 441–444. [Google Scholar] [CrossRef]
- Lagarrigue, A.; Ajana, S.; Capuron, L.; Féart, C.; Moisan, M.-P. Obesity in French Inmates: Gender Differences and Relationship with Mood, Eating Behavior and Physical Activity. PLoS ONE 2017, 12, e0170413. [Google Scholar] [CrossRef] [PubMed]
- Gates, M.L.; Bradford, R.K. The impact of incarceration on obesity: Are prisoners with chronic diseases becoming overweight and obese during their confinement? J. Obes. 2015, 2015, 532468. [Google Scholar] [CrossRef] [PubMed]
- Talens, M.; Tumas, N.; Lazarus, J.V.; Benach, J.; Pericàs, J.M. What Do We Know about Inequalities in NAFLD Distribution and Outcomes? A Scoping Review. J. Clin. Med. 2021, 10, 5019. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Day, C.P. The genetics of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.J.; Kronfli, N.; Cabezas, J.; Sheehan, Y.; Thurairajah, P.H.; Lines, R.; Lloyd, A.R. Hepatitis C elimination among people incarcerated in prisons: Challenges and recommendations for action within a health systems framework. Lancet Gastroenterol. Hepatol. 2021, 6, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.; Rossow, I.; Bukten, A. Alcohol Problems among Prisoners: Subgroup Variations, Concurrent Drug Problems, and Treatment Needs. Eur. Addict. Res. 2020, 27, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Yela, E.; Sole, N.; Quintero, S. Substantial concordance between transient elastography and APRI and FIB-4 combination amongst hepatitis C inmates with non advanced liver fibrosis. Rev. Esp. Sanid Penit. 2022, 24, 33–37. [Google Scholar] [CrossRef]
- Papaluca, T.; Craigie, A.; McDonald, L.; Edwards, A.; MacIsaac, M.; Holmes, J.A.; Jarman, M.; Lee, T.; Huang, H.; Chan, A.; et al. Non-invasive fibrosis algorithms are clinically useful for excluding cirrhosis in prisoners living with hepatitis C. PLoS ONE 2020, 15, e0242101. [Google Scholar] [CrossRef]
- Merchante, N.; Mena, Á.; Pascasio, J.-M.; Marco, A.; Rodriguez, M.; Hernandez-Guerra, M.; Simón, M.-A. Prediction of liver stiffness by serum indexes in HCV-infected patients with or without HIV coinfection. Medicine 2021, 100, e27838. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Coste, P.; Llop, E.; Perelló, C.; Hernández, M.; López, M.; Abad, J.; Ferre, C.; Martínez, J.L.; Fernández, N.; Calleja, J.L. Comparison of non-invasive fibrosis scores to predict increased liver stiffness in the general population with unknown liver disease: Searching for the primary physician’s best friend. Dig. Liver Dis. 2022, 54, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Zarski, J.; de Ledinghen, V.; Rousselet, M.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2012, 57, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Enea, M.; Romero-Gomez, M.; Viganò, M.; Bugianesi, E.; Wong, V.W.; Fracanzani, A.L.; Sebastiani, G.; Boursier, J.; Berzigotti, A.; et al. Liver-related and extrahepatic events in patients with non-alcoholic fatty liver disease: A retrospective competing risks analysis. Aliment. Pharmacol. Ther. 2022, 55, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Marco Mouriño, A.; Rivera-Esteban, J.; Augustin, S.; Turu Santigosa, E.; Pericàs, J.M.; FatPrison Study Group. Morbilidad metabólica en la población penitenciaria de Cataluña. Aten. Primaria 2023, 55, 102620. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K.; Gerhard, G.S. Metabolic dysfunction and nonalcoholic fatty liver disease risk in individuals with a normal body mass index. Curr. Opin. Gastroenterol. 2023, 39, 156–162. [Google Scholar] [CrossRef]
- Pons, M.; Rivera-Esteban, J.; Ma, M.M.; Davyduke, T.; Delamarre, A.; Hermabessière, P.; Dupuy, J.; Wong, G.L.; Yip, T.C.; Pennisi, G.; et al. Point-of-Care Noninvasive Prediction of Liver-Related Events in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef]
- Navarro, J. HIV and liver disease. Aids Rev. 2023, 24, 87–96. [Google Scholar] [CrossRef]
- Maurice, J.B.; Patel, A.; Scott, A.J.; Patel, K.; Thursz, M.; Lemoine, M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017, 31, 1621–1632. [Google Scholar] [CrossRef]
- Manzano-Nunez, R.; Rivera-Esteban, J.; Navarro, J.; Bañares, J.; Sena, E.; Schattenberg, J.M.; Lazarus, J.V.; Curran, A.; Pericàs, J.M. Uncovering the NAFLD burden in people living with HIV from high- and middle-income nations: A meta-analysis with a data gap from Subsaharan Africa. J. Int. AIDS Soc. 2023, 26, e26072. [Google Scholar] [CrossRef]
- Ginès, P.; Castera, L.; Lammert, F.; Graupera, I.; Serra-Burriel, M.; Allen, A.M.; Wong, V.W.; Hartmann, P.; Thiele, M.; Caballeria, L.; et al. Population screening for liver fibrosis: Toward early diagnosis and intervention for chronic liver diseases. Hepatology 2021, 75, 219–228. [Google Scholar] [CrossRef]
- Caballería, L.; Pera, G.; Arteaga, I.; Rodríguez, L.; Alumà, A.; Morillas, R.M.; de la Ossa, N.; Díaz, A.; Expósito, C.; Miranda, D.; et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2018, 16, 1138–1145.e5. [Google Scholar] [CrossRef]


| Variables | Overall n = 646 | MASLD n = 219 | Non-MASLD n = 427 | p-Value |
|---|---|---|---|---|
| Age (years) | 48.0 ± 12.1 | 49.5 ± 12.1 | 47.3 ± 12.1 | 0.027 |
| Male sex, n (%) | 578 (89.5) | 198 (90.4) | 380 (89.0) | 0.57 |
| Origin, n (%) | ||||
| Europe | 410 (63.5) | 137 (62.6) | 273 (63.9) | 0.73 |
| Latin-America | 123 (19.0) | 39 (17.8) | 84 (19.7) | 0.56 |
| Maghreb-Arab | 66 (10.2) | 26 (11.9) | 40 (9.4) | 0.32 |
| Sub-Saharan Africa | 23 (3.6) | 6 (2.7) | 17 (4.0) | 0.42 |
| Asia | 26 (4.0) | 11 (5.0) | 15 (3.5) | 0.35 |
| Body mass index (kg/m2) | 28.3 (25.6–31.7) | 30.4 (27.0–32.5) | 28.3 (25.1–31.2) | <0.001 |
| Obesity (≥30 kg/m2), n (%) | 239 (38.4) | 117 (57.1) | 151 (36.1) | <0.001 |
| Waist circumference (cm) | 100 (92–109) | 106 (97–115) | 97 (90–106) | <0.001 |
| Type 2 diabetes, n (%) | 140 (21.7) | 63 (28.8) | 77 (18.0) | 0.002 |
| High blood pressure, n (%) | 265 (41.0) | 98 (44.7) | 167 (39.1) | 0.16 |
| Dyslipidemia, n (%) | 416 (64.5) | 148 (67.6) | 268 (62.9) | 0.24 |
| Metabolic syndrome, n (%) | 196 (31.9) | 92 (44.0) | 104 (25.6) | <0.001 |
| HIV, n (%) | 34 (6.1) | 17 (9.2) | 17 (4.5) | 0.029 |
| AST (U/L) | 22 ± 8 | 23 ± 9 | 21 ± 6 | 0.017 |
| ALT (U/L) | 24 ± 14 | 27 ± 16 | 22 ± 11 | <0.001 |
| AP (UI/L) | 82 ± 25 | 84 ± 25 | 81 ± 25 | 0.27 |
| GGT (UI/L) | 32 ± 27 | 36 ± 30 | 30 ± 24 | 0.007 |
| Fasting glucose (mg/dL) | 105 ± 59 | 114 ± 58 | 100 ± 59 | 0.004 |
| Cholesterol (mg/dL) | 187 ± 44 | 190 ± 45 | 185 ± 43 | 0.16 |
| Triglycerides (mg/dL) | 147 ± 89 | 168 ± 109 | 136 ± 74 | <0.001 |
| Creatinine (mg/dL) | 0.90 ± 0.21 | 0.91 ± 0.44 | 0.89 ± 0.17 | 0.19 |
| Platelet count (109/L) | 250 ± 64 | 251 ± 62 | 249 ± 65 | 0.79 |
| Liver stiffness (kPa) | 6.4 ± 5.6 | 7.9 ± 8.7 | 5.7 ± 2.5 | <0.001 |
| XL probe, n (%) | 325 (50.3) | 159 (72.6) | 166 (38.9) | <0.001 |
| CAP (dB/m) | 253 ± 63 | 324 ± 37 | 216 ± 36 | <0.001 |
| FIB-4 index | 1.00 ± 0.55 | 1.00 ± 0.54 | 1.00 ± 0.55 | 0.99 |
| Fatty Liver Index | 59 ± 26 | 71 ± 25 | 54 ± 26 | <0.001 |
| ELF score * | 9.3 ± 0.8 | 9.4 ± 0.8 | 9.3 ± 0.8 | 0.44 |
| PNPLA3 risk alleles ** | 34 (43.0) | 14 (50.0) | 20 (39.2) | 0.35 |
| TM6SF2 risk alleles ** | 8 (10.1) | 2 (7.1) | 6 (11.8) | 0.51 |
| VCTE Ranges | MASLD | Non-MASLD | Total |
|---|---|---|---|
| <5 kPa, n (%) | 58 (26.4) | 188 (44.0) | 246 (38.1) |
| 5–10 kPa, n (%) | 131 (59.8) | 217 (50.7) | 348 (53.9) |
| 10–15 kPa, n (%) | 16 (7.4) | 16 (3.7) | 32 (4.9) |
| ≥15 kPa, n (%) | 14 (6.4) | 7 (1.6) | 20 (3.1) |
| Total | 219 (33.9) | 427 (66.1) | 646 (100) |
| Variables | MASLD | MASLD-Significant Fibrosis | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Model 1 | ||||
| T2D | 1.70 (1.08–2.67) | 0.02 | 1.99 (1.00–3.99) | 0.050 |
| Waist circumference * | 2.91 (1.97–4.30) | <0.001 | 3.58 (1.74–7.31) | 0.001 |
| ALT (40 UI/L) | 2.42 (1.30–4.49) | 0.005 | 4.06 (1.87–8.80) | <0.001 |
| HIV infection | 1.18 (0.51–2.72) | 0.69 | 2.02 (0.67–3.04) | 0.20 |
| Model 2 | ||||
| MetS | 2.18 (1.47–3.22) | <0.001 | 2.14 (1.14–4.03) | 0.018 |
| ALT (40 UI/L) | 2.52 (1.35–4.47) | 0.003 | 4.20 (1.98–8.89) | <0.001 |
| HIV infection | 1.67 (0.76–3.62) | 0.19 | 2.25 (0.79–6.44) | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Esteban, J.; Jiménez-Masip, A.; Muñoz-Martínez, S.; Augustin, S.; Guerrero, R.A.; Gabriel-Medina, P.; Ferrer-Costa, R.; Rodríguez-Frías, F.; Turu, E.; Marco, A.; et al. Prevalence and Risk Factors of MASLD and Liver Fibrosis amongst the Penitentiary Population in Catalonia: The PRISONAFLD Study. J. Clin. Med. 2023, 12, 7276. https://doi.org/10.3390/jcm12237276
Rivera-Esteban J, Jiménez-Masip A, Muñoz-Martínez S, Augustin S, Guerrero RA, Gabriel-Medina P, Ferrer-Costa R, Rodríguez-Frías F, Turu E, Marco A, et al. Prevalence and Risk Factors of MASLD and Liver Fibrosis amongst the Penitentiary Population in Catalonia: The PRISONAFLD Study. Journal of Clinical Medicine. 2023; 12(23):7276. https://doi.org/10.3390/jcm12237276
Chicago/Turabian StyleRivera-Esteban, Jesús, Alba Jiménez-Masip, Sergio Muñoz-Martínez, Salvador Augustin, Rafael A. Guerrero, Pablo Gabriel-Medina, Roser Ferrer-Costa, Francisco Rodríguez-Frías, Elisabet Turu, Andrés Marco, and et al. 2023. "Prevalence and Risk Factors of MASLD and Liver Fibrosis amongst the Penitentiary Population in Catalonia: The PRISONAFLD Study" Journal of Clinical Medicine 12, no. 23: 7276. https://doi.org/10.3390/jcm12237276