Ischemic Stroke in the Course of COVID-19 in a 16-Year-Old Boy
Abstract
:1. Introduction
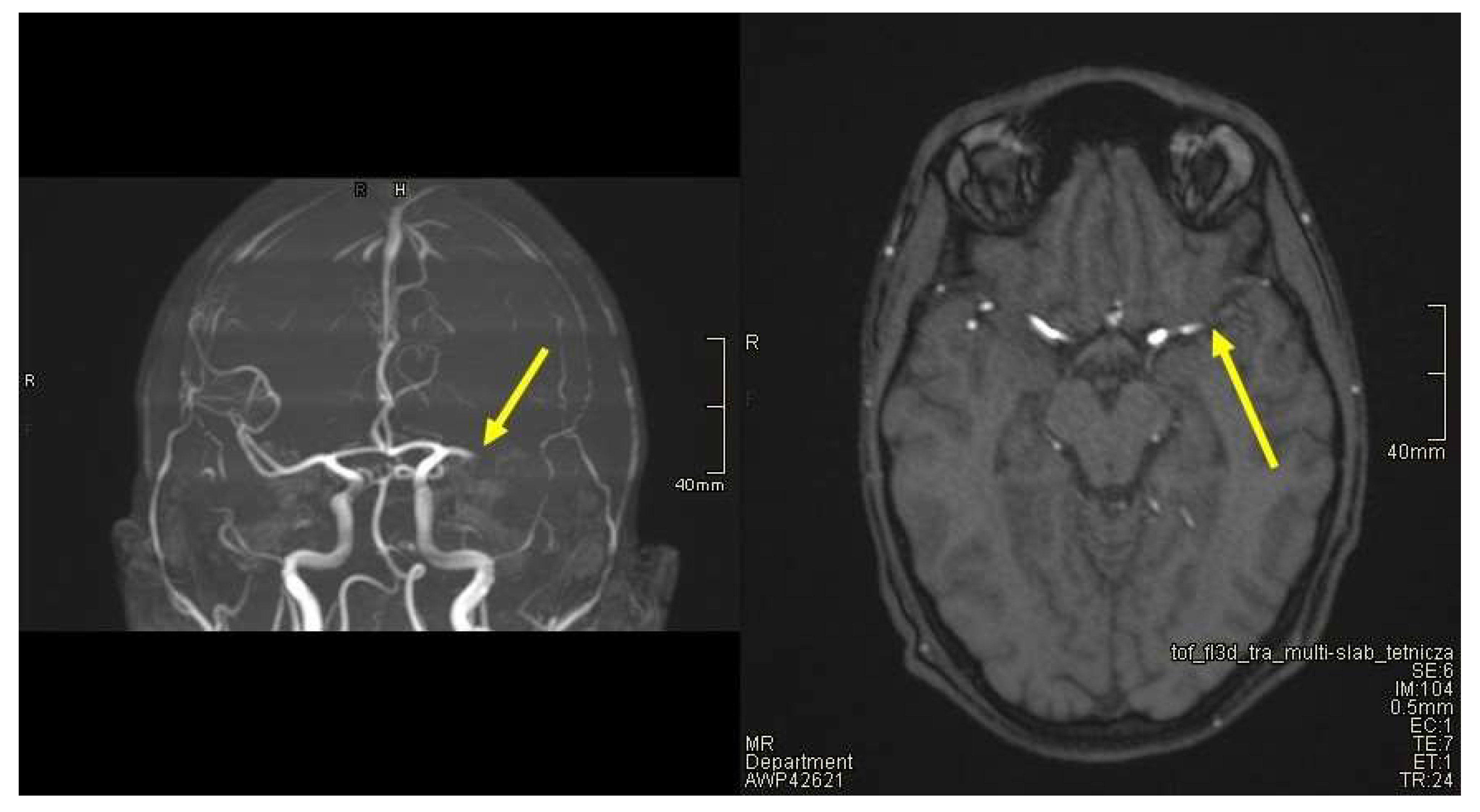
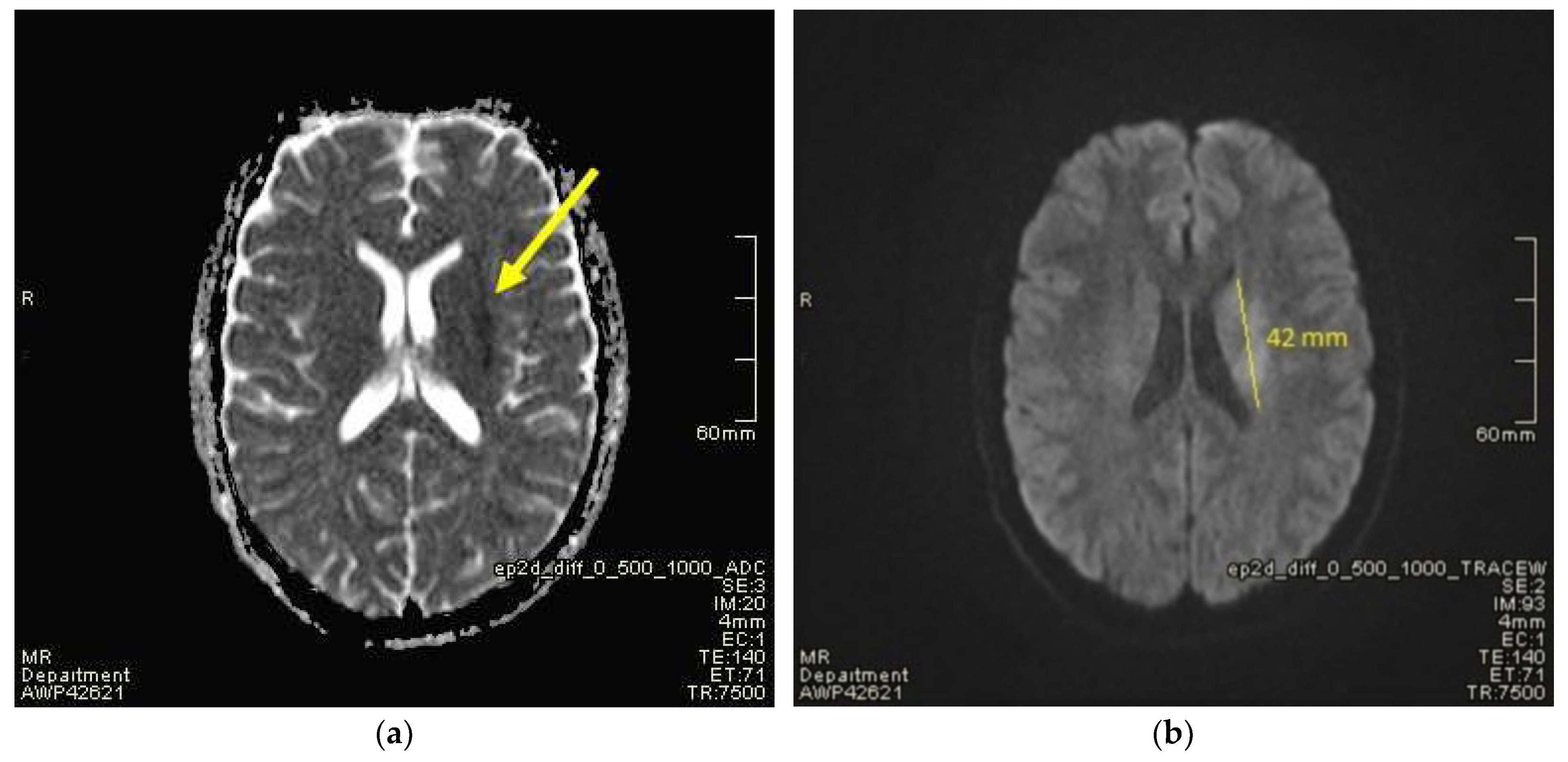
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Hobbs, C.V. COVID-19: A Pediatric Update in Epidemiology, Management, Prevention, and Long-term Effects. Pediatr. Rev. 2023, 44, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauvé, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 103, 246–256. [Google Scholar] [CrossRef]
- World Health Organization. WHO Issues Guidelines on the Treatment of Children with Multisystem Inflammatory Syndrome Associated with COVID-19. Available online: https://www.who.int/news/item/23-11-2021-who-issues-guidelines-on-the-treatment-of-children-with-multisystem-inflammatory-syndrome-associated-with-COVID-19 (accessed on 19 August 2023).
- Okarska-Napierała, M.; Ludwikowska, K.; Jackowska, T.; Książyk, J.; Buda, P.; Mazur, A.; Szenborn, L.; Werner, B.; Wysocki, J.; Kuchar, E. Approach to a child with Multisystem Inflammatory Syndrome associated with COVID19. Recommendations by the Polish Paediatric Society Expert Group. Update—February 2021. Pediatr. Polska 2021, 96, 121–128. [Google Scholar] [CrossRef]
- Thiriard, A.; Meyer, B.; Eberhardt, C.S.; Loevy, N.; Grazioli, S.; Adouan, W.; Fontannaz, P.; Marechal, F.; L’huillier, A.G.; Siegrist, C.-A.; et al. Antibody response in children with multisystem inflammatory syndrome related to COVID-19 (MIS-C) compared to children with uncomplicated COVID-19. Front. Immunol. 2023, 14, 1107156. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.; Gavade, V.; Patil, N.; Mali, V.; Girwalkar, A.; Tarkasband, V.; Loya, S.; Chavan, A.; Nanivadekar, N.; Shinde, R.; et al. Neonatal Multisystem Inflammatory Syndrome (MIS-N) Associated with Prenatal Maternal SARS-CoV-2: A Case Series. Children 2021, 8, 572. [Google Scholar] [CrossRef]
- Chang, J.; Bulwa, Z.; Breit, H.; Cherian, L.J.; Conners, J.J.; Song, S.Y.; Dafer, R.M. Acute Large Vessel Ischemic Stroke in Patients With COVID-19–Related Multisystem Inflammatory Syndrome. Pediatr. Neurol. 2022, 126, 104–107. [Google Scholar] [CrossRef]
- World Health Organisation. Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. 2020. Available online: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-COVID-19 (accessed on 19 August 2021).
- McArdle, A.J.; Vito, O.; Patel, H.; Seaby, E.G.; Shah, P.; Wilson, C.; Broderick, C.; Nijman, R.; Tremoulet, A.H.; Munblit, D.; et al. Treatment of Multisystem Inflammatory Syndrome in Children. N. Engl. J. Med. 2021, 385, 11–22. [Google Scholar] [CrossRef]
- Guimarães, D.; Pissarra, R.; Reis-Melo, A.; Guimarães, H. Multisystem inflammatory syndrome in children (MISC): A systematic review. Int. J. Clin. Pract. 2021, 75, e14450. [Google Scholar] [CrossRef]
- Menon, N.M.; Srivaths, L.V. Thromboembolism in children with multisystem inflammatory syndrome: A literature review. Pediatr. Res. 2022, 92, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Rivkin, M.J.; Raffini, L. Thrombotic complications in children with Coronavirus disease 2019 and Multisystem Inflammatory Syndrome of Childhood. J. ThrombHaemost. 2023, 21, 2313–2326. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.M. Multisystem Inflammatory Syndrome in Children (MIS-C). Curr. Allergy Asthma Rep. 2022, 22, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dambrauskas, S.; Vasquez-Hoyos, P.; Camporesi, A.; Cantillano, E.M.; Dallefeld, S.; Dominguez-Rojas, J.; Francoeur, C.; Gurbanov, A.; Mazzillo-Vega, L.; Shein, S.L.; et al. Paediatric critical COVID-19 and mortality in a multinational prospective cohort. Lancet Reg. Health Am. 2022, 12, 100272. [Google Scholar] [CrossRef] [PubMed]
- Beslow, L.A.; Linds, A.B.; Fox, C.K.; Kossorotoff, M.; ZuñigaZambrano, Y.C.; Hernández-Chávez, M.; Hassanein, S.M.A.; Byrne, S.; Lim, M.; Maduaka, N.; et al. Pediatric Ischemic Stroke: An Infrequent Complication of SARS-CoV-2. Ann. Neurol. 2021, 89, 657–665. [Google Scholar] [CrossRef]
- Carney, P.R.; Stevenson, D.W.; Riggs, E.; Dervisevic, M.; Carney, C.X.; Gomez, C.R. Thrombectomy of an Acute Ischemic Stroke in a Child with COVID-19 and MIS-C: Case Analysis and Literature Context. Children 2023, 10, 851. [Google Scholar] [CrossRef]
- Khosravi, B.; Moradveisi, B.; Abedini, M.; Behzadi, S.; Karimi, A. Stroke in a child with SARS-CoV-2 infection: A case report. eNeurologicalSci 2021, 23, 100345. [Google Scholar] [CrossRef]
- Jillella, D.V.; Philbrook, B.; Ortolani, E.; Grossberg, J.A.; Stani, T.; Samuels, O.; Pimentel, C.; Harrison, A.; Polu, A.R.; Siegel, B.I.; et al. Successful Endovascular Therapy in COVID-19 Associated Pediatric Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 106152. [Google Scholar] [CrossRef]
- Shanmugam, C.; Mohammed, A.R.; Ravuri, S.; Luthra, V.; Rajagopal, N.; Karre, S. COVID-2019—A comprehensive pathology insight. Pathol. Res. Pract. 2020, 216, 153222. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Zareef, R.; Diab, M.; Al Saleh, T.; Makarem, A.; Younis, N.K.; Bitar, F.; Arabi, M. Aspirin in COVID-19: Pros and Cons. Front. Pharmacol. 2022, 13, 849628. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.K.; Mayman, N.A.; Dhamoon, M.S.; Fifi, J.T. The emerging association between COVID-19 and acute stroke. Trends Neurosci. 2021, 44, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ribes, A.; Vardon-Bounes, F.; Mémier, V.; Poette, M.; Au-Duong, J.; Garcia, C.; Minville, V.; Sié, P.; Bura-Rivière, A.; Voisin, S.; et al. Thromboembolic events and COVID-19. Adv. Biol. Regul. 2020, 77, 100735. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; Dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.M.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article—Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682–689. [Google Scholar] [CrossRef]
- Chiotos, K.; Bassiri, H.; Behrens, E.M.; Blatz, A.M.; Chang, J.; Diorio, C.; Fitzgerald, J.C.; Topjian, A.; John, A.R.O. Multisystem Inflammatory Syndrome in Children During the Coronavirus 2019 Pandemic: A Case Series. J. Pediatr. Infect. Dis. Soc. 2020, 9, 393–398. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, S.-Y.; Choi, J.-W.; Korean Society of Kawasaki Disease. Clinical features, diagnosis, and outcomes of multisystem inflammatory syndrome in children associated with coronavirus disease 2019. Clin. Exp. Pediatr. 2021, 64, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Holm, M.; Hartling, U.B.; Glenthøj, J.; Schmidt, L.S.; Nordly, S.B.; Matthesen, A.T.; von Linstow, M.-L.; Espenhain, L. Incidence and clinical phenotype of multisystem inflammatory syndrome in children after infection with the SARS-CoV-2 delta variant by vaccination status: A Danish nationwide prospective cohort study. Lancet Child. Adolesc. Health 2022, 6, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Kamidani, S.; Tarquinio, K.M.; Maddux, A.B.; et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12–18 Years—United States, July–December 2021. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.D.; Zambrano, L.D.; Yousaf, A.R.; Abrams, J.Y.; Meng, L.; Wu, M.J.; Melgar, M.; Oster, M.E.; Godfred Cato, S.E.; Belay, E.D.; et al. Multisystem Inflammatory Syndrome in Children-United States, February 2020–July 2021. Clin. Infect. Dis. 2022, 75, e1165–e1175. [Google Scholar] [CrossRef]
- Miller, A.D.; Yousaf, A.R.; Bornstein, E.; Wu, M.J.; Lindsey, K.; Melgar, M.; Oster, M.E.; Zambrano, L.D.; Campbell, A.P. Multisystem Inflammatory Syndrome in Children During Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Delta and Omicron Variant Circulation-United States, July 2021–January 2022. Clin. Infect. Dis. 2022, 75 (Suppl. 2), S303–S307. [Google Scholar] [CrossRef]
- Levy, N.; Koppel, J.H.; Kaplan, O.; Yechiam, H.; Shahar-Nissan, K.; Cohen, N.K.; Shavit, I. Severity and Incidence of Multisystem Inflammatory Syndrome in Children During 3 SARS-CoV-2 Pandemic Waves in Israel. JAMA 2022, 327, 2452–2454. [Google Scholar] [CrossRef]
- Cloete, J.; Kruger, A.; Masha, M.; du Plessis, N.M.; Mawela, D.; Tshukudu, M.; Manyane, T.; Komane, L.; Venter, M.; Jassat, W.; et al. Paediatrichospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: A multicentre observational study. Lancet Child. Adolesc. Health 2022, 6, 294–302. [Google Scholar] [CrossRef]
- Gottlieb, M.; Bridwell, R.; Ravera, J.; Long, B. Multisystem inflammatory syndrome in children with COVID-19. Am. J. Emerg. Med. 2021, 49, 148–152. [Google Scholar] [CrossRef]
| WHO Criteria for Multisystem Inflammatory Syndrome in Children |
|---|
| Children and adolescents 0–19 years of age with fever ≥ 3 days |
| AND two of the following: |
|
| AND |
| elevated markers of inflammation such as Erythrocyte Sedimentation Rate (ESR), C-reactive protein, or procalcitonin. |
| AND |
| no other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal or streptococcal shock syndromes. |
| AND |
| evidence of COVID-19 (RT-PCR, antigen test or serology positive), or likely contact with patients with COVID-19. |
| On Admission | After 8 h | After 24 h | After 48 h | After 72 h | Reference Range | Unit | |
|---|---|---|---|---|---|---|---|
| RBC | 4.18 | N/A | 4.9 | N/A | 5.11 | 4.32–5.75 | mln/µL |
| Hb | 12.7 | N/A | 14.6 | N/A | 15.5 | 13.5–17.5 | g/dL |
| MCV | 85.9 | N/A | 84.9 | N/A | 86.5 | 81.2–95.1 | fl |
| Platelets | 134 | N/A | 203 | N/A | 286 | 140–420 | K/µL |
| CRP | 15 | 17.85 | 11.05 | 8.88 | 5.26 | 0–0.5 | mg/dL |
| Procalcitonin | 3.590 | 5.560 | 1.920 | 1.080 | 0.692 | <0.5 | mg/mL |
| LDH | 318 | N/A | N/A | N/A | N/A | 0–266 | U/L |
| D-dimer | 6046 | 9157 | 4191 | N/A | N/A | <500 | ng/mL |
| CK | 490 | 624 | N/A | N/A | N/A | 0–270 | U/L |
| CK-MB | 41.5 | 45.2 | N/A | N/A | N/A | 0–25 | U/L |
| Creatine | 1.16 | 1.67 | 0.72 | N/A | 0.73 | 0.7–1.2 | mg/dL |
| Urea | 81.9 | 92.4 | 42.8 | N/A | 36.7 | 18–45 | mg/dL |
| NT-proBNP | 14823 | 29415 | N/A | N/A | 4647 | 0–125 | pg/mL |
| cTnI | 14.65 | 6.16 | 1.83 | 1.32 | 0.62 | <0.04 | ng/mL |
| APTT | 40.6 | 45.7 | N/A | N/A | N/A | 25.4–36.9 | s |
| Fibrinogen | 5.94 | 6.75 | N/A | N/A | N/A | 1.9–4.0 | g/L |
| Antithrombin III | 46 | 89 | 91 | N/A | N/A | 83–128 | % |
| Calcium | 1.96 | 2.00 | 2.04 | 2.02 | 1.95 | 2.1–2.55 | mmoL/L |
| SARS-CoV-2, IgM | 4.01 | N/A | N/A | N/A | N/A | <1.1 | BAU/mL |
| SARS-CoV-2, IgG | 529 | N/A | N/A | N/A | N/A | <33.8 | BAU/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syzdoł, B.; Rzewuska, A.M.; Sielwanowska, W.; Żybowska, M.; Wilczek, N.A.; Woźniak, M.M. Ischemic Stroke in the Course of COVID-19 in a 16-Year-Old Boy. J. Clin. Med. 2023, 12, 6963. https://doi.org/10.3390/jcm12226963
Syzdoł B, Rzewuska AM, Sielwanowska W, Żybowska M, Wilczek NA, Woźniak MM. Ischemic Stroke in the Course of COVID-19 in a 16-Year-Old Boy. Journal of Clinical Medicine. 2023; 12(22):6963. https://doi.org/10.3390/jcm12226963
Chicago/Turabian StyleSyzdoł, Bartłomiej, Anna Maria Rzewuska, Wiktoria Sielwanowska, Monika Żybowska, Natalia Anna Wilczek, and Magdalena Maria Woźniak. 2023. "Ischemic Stroke in the Course of COVID-19 in a 16-Year-Old Boy" Journal of Clinical Medicine 12, no. 22: 6963. https://doi.org/10.3390/jcm12226963
APA StyleSyzdoł, B., Rzewuska, A. M., Sielwanowska, W., Żybowska, M., Wilczek, N. A., & Woźniak, M. M. (2023). Ischemic Stroke in the Course of COVID-19 in a 16-Year-Old Boy. Journal of Clinical Medicine, 12(22), 6963. https://doi.org/10.3390/jcm12226963





