Systemic Immune Inflammation Index as a Key Predictor of Dialysis in Pediatric Chronic Kidney Disease with the Use of Random Forest Classifier
Abstract
:1. Introduction
2. Aim of Study
3. Materials and Methods
3.1. Patients’ Data
3.2. Classical Statistical Analysis
3.3. Machine Learning (ML) Statistical Scoring
3.4. Heuristic Algorithm
4. Results
4.1. CBC Values and Correlations with Clinical Parameters
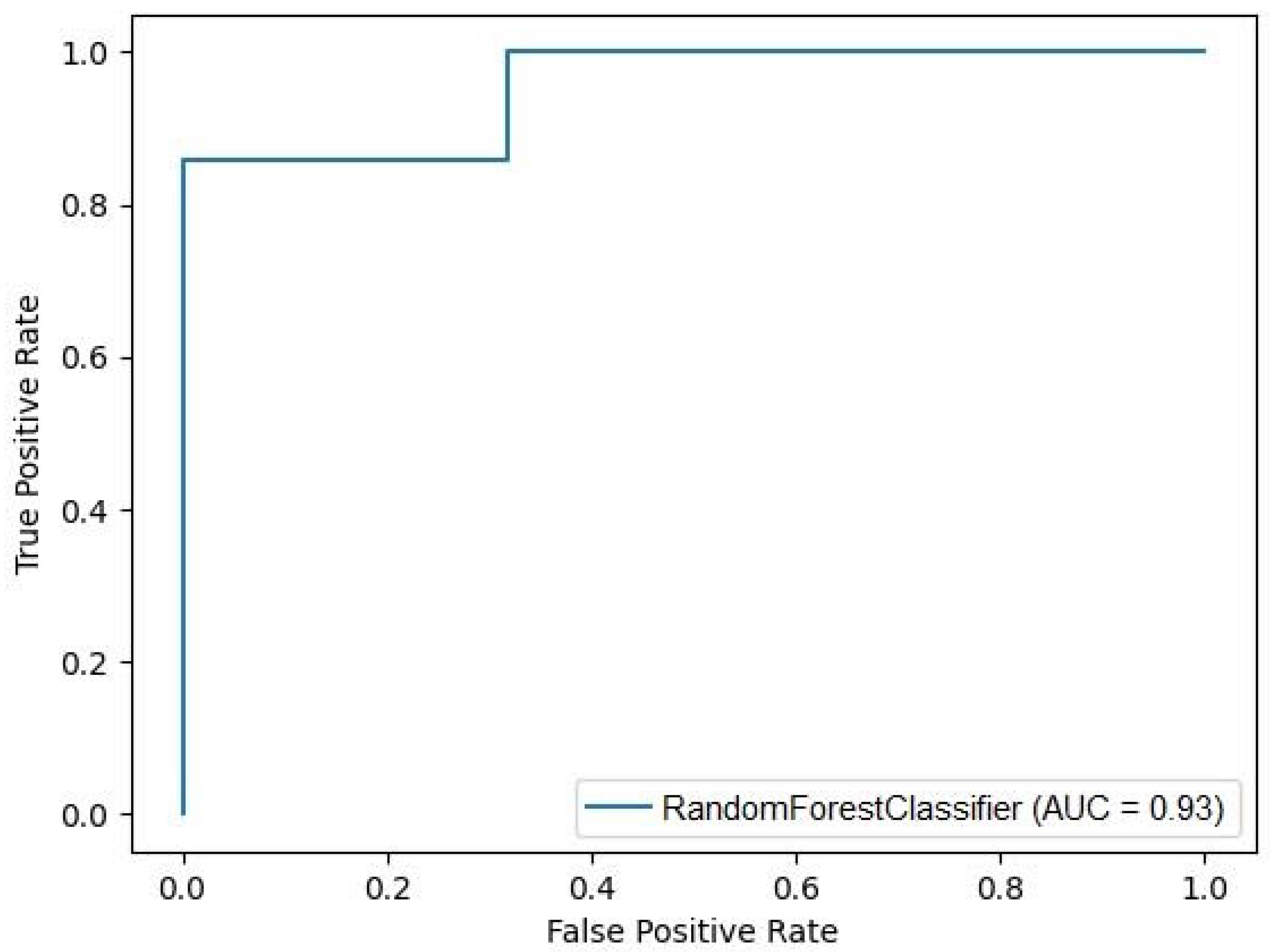
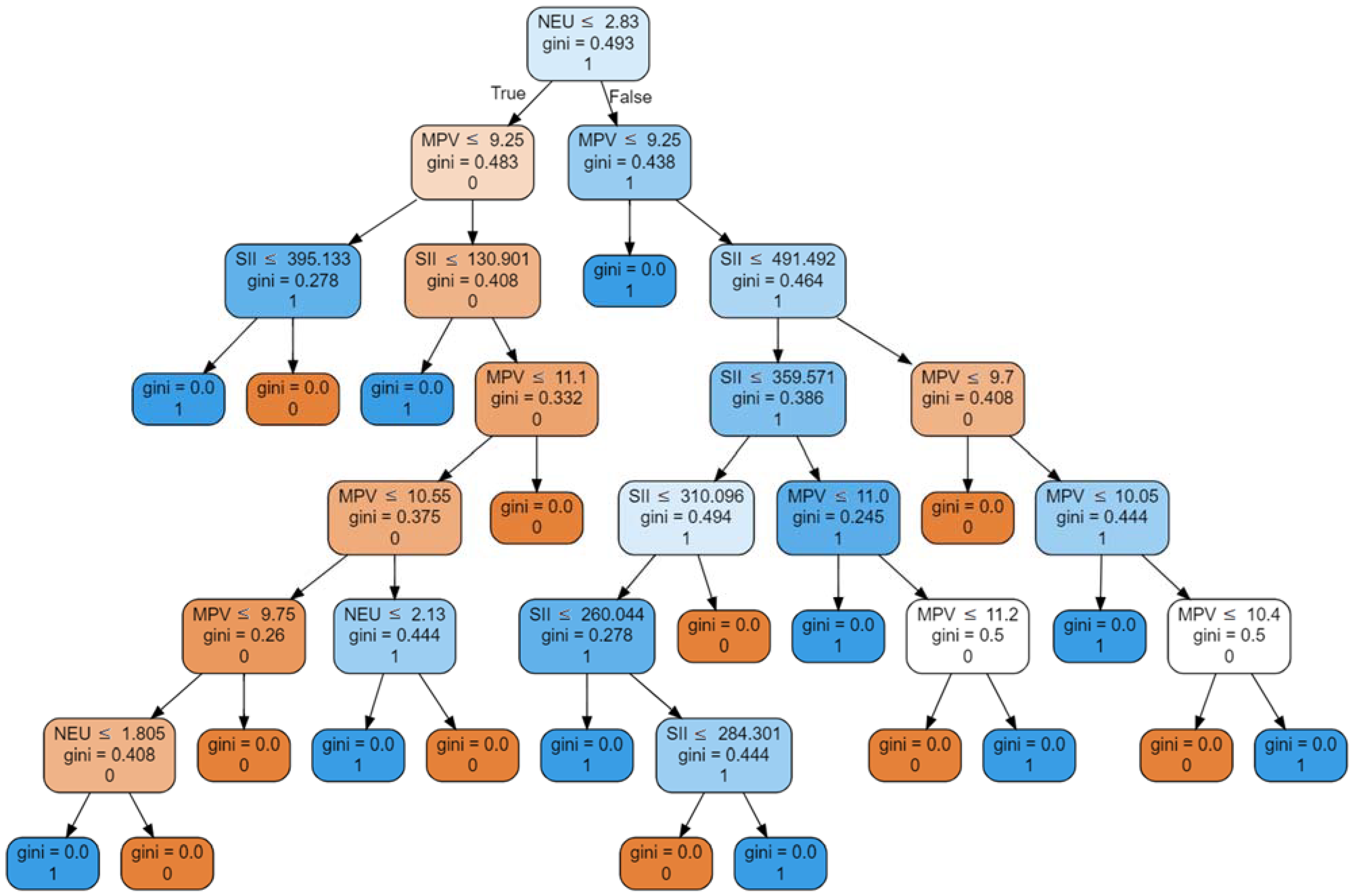
4.2. Machine Learning Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Syed-Ahmed, M.; Narayanan, M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. CRIC Study Investigators. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Shacham, Y. Inflammation in chronic kidney disease—Something old, something new. Int. J. Cardiol. 2023, 370, 407–408. [Google Scholar] [CrossRef]
- Tecklenborg, J.; Clayton, D.; Siebert, S.; Coley, S.M. The role of the immune system in kidney disease. Clin. Exp. Immunol. 2018, 192, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Golembiewska, E.; Lindholm, B.; Stenvinkel, P. End-Stage Renal Disease, Inflammation and Cardiovascular Outcomes. Contrib. Nephrol. 2017, 191, 32–43. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Kim, S.; Kim, S. An insight into recent advances on platelet function in health and disease. Int. J. Mol. Sci. 2022, 23, 6022. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yao, Y.; Zeng, R. Lymphocytes: Versatile participants in acute kidney injury and progression to chronic kidney disease. Front. Physiol. 2021, 12, 729084. [Google Scholar] [CrossRef]
- Espi, M.; Koppe, L.; Fouque, D.; Thaunat, O. Chronic kidney disease-associated immune dysfunctions: Impact of protein-bound uremic retention solutes on immune cells. Toxins 2020, 12, 300. [Google Scholar] [CrossRef]
- Agarwal, R.; Light, R.P. Patterns and prognostic value of total and differential leukocyte count in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Jia, J.; Li, J.; Huo, Y.; Zhang, Y. White blood cell count predicts the odds of kidney function decline in a Chinese community-based population. BMC Nephrol. 2017, 18, 190. [Google Scholar] [CrossRef]
- Uludag, K.; Arikan, T. Is White Blood Cell Count Associated With Mortality in Peritoneal Dialysis Patients?: A Retrospective Single-Center Analysis. Cureus 2021, 13, e19728. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Xian, H.; Li, T.; Al-Aly, Z. Association between Monocyte Count and Risk of Incident CKD and Progression to ESRD. Clin. J. Am. Soc. Nephrol. 2017, 12, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, R.; Nakayama, M.; Sakoh, T.; Fukui, A.; Katafuchi, E.; Seki, M.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren. Fail. 2019, 41, 238–243. [Google Scholar] [CrossRef]
- Liao, J.; Wei, D.; Sun, C.; Yang, Y.; Wei, Y.; Liu, X. Prognostic value of the combination of neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio and platelet-to-lymphocyte ratio on mortality in patients on maintenance hemodialysis. BMC Nephrol. 2022, 23, 393. [Google Scholar] [CrossRef]
- Jain, N.; Corken, A.L.; Kumar, A.; Davis, C.L.; Ware, J.; Arthur, J.M. Role of Platelets in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2021, 32, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Y.; Kim, J.K.; Hur, S.M.; Woo, S.A.; Park, K.A.; Park, M.Y.; Choi, S.J.; Hwang, S.D. Could mean platelet volume be a promising biomarker of progression of chronic kidney disease? Platelets 2015, 26, 143–147. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Y.; Chen, G.; Feng, J.; Gan, L. Association of mean platelet volume/lymphocyte ratio with inflammation in non-dialysis patients with chronic kidney disease stages 1–4: A retrospective study. Front. Immunol. 2022, 13, 1041356. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Russu, E.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Arbănași, E.M.; Voidăzan, S.T. The Predictive Value of NLR, MLR, and PLR in the Outcome of End-Stage Kidney Disease Patients. Biomedicines 2022, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Gok, M. Evaluation of the relationship between the mean platelet volume and the neutrophil/lymphocyte ratio with progression of chronic kidney disease in patients with autosomal dominant polycystic kidney disease. North Clin. Istanb. 2022, 9, 317–322. [Google Scholar] [CrossRef]
- Brito, G.M.C.; Fontenele, A.M.M.; Carneiro, E.C.R.L.; Nogueira, I.A.L.; Cavalcante, T.B.; Vale, A.A.M.; Monteiro, S.C.M.; Salgado Filho, N. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Nondialysis Chronic Kidney Patients. Int. J. Inflamm. 2021, 2021, 6678960. [Google Scholar] [CrossRef]
- Corken, A.; Ware, J.; Dai, J.; Arthur, J.M.; Smyth, S.; Davis, C.L.; Liu, J.; Harville, T.O.; Phadnis, M.A.; Mehta, J.L.; et al. Platelet-Dependent Inflammatory Dysregulation in Patients with Stages 4 or 5 Chronic Kidney Disease: A Mechanistic Clinical Study. Kidney360 2022, 3, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Li, H.; Wang, L.; Geng, J.; Yang, Q.; Su, B.; Liao, R. Systemic Immune-Inflammation Index Is Associated with Increased Urinary Albumin Excretion: A Population-Based Study. Front. Immunol. 2022, 13, 863640. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yuan, S.; Yuan, Y.; Yi, L. Prognostic and Clinicopathological Significance of the Systemic Immune-Inflammation Index in Patients with Renal Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2021, 11, 735803. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, S.R.; Huang, X.P.; Liu, X.Q.; Liu, J.B.; Tian, G. Prognostic value of systemic immune-inflammation index in patients with urinary system cancers: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1302–1310. [Google Scholar] [CrossRef]
- Karakaya, D.; Güngör, T.; Cakıcı, E.K.; Yazılıtaş, F.; Celikkaya, E.; Bulbul, M. Determining the effectiveness of the immature granulocyte percentage and systemic immune-inflammation index in predicting acute pyelonephritis. Postgrad. Med. 2023, 135, 155–160. [Google Scholar] [CrossRef]
- Kocaaslan, R.; Dilli, D.; Çitli, R. Diagnostic Value of the Systemic Immune-Inflammation Index in Newborns with Urinary Tract Infection. Am. J. Perinatol. 2022. ahead of print. [Google Scholar] [CrossRef]
- Ran, Y.; Wu, Q.N.; Long, Y.J.; Li, Q.; Wu, J.; Da, J.J.; He, P.H.; Yuan, J.; Zha, Y. Association of systemic immune-inflammation index with protein-energy wasting and prognosis in patients on maintenance hemodialysis. Zhonghua Yi Xue Za Zhi 2021, 101, 2223–2227. [Google Scholar] [CrossRef]
- Lai, W.; Xie, Y.; Zhao, X.; Xu, X.; Yu, S.; Lu, H.; Huang, H.; Li, Q.; Xu, J.Y.; Liu, J.; et al. Elevated systemic immune inflammation level increases the risk of total and cause-specific mortality among patients with chronic kidney disease: A large multi-center longitudinal study. Inflamm. Res. 2023, 72, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Song, Y.; Sun, Y.; Du, H.; Cai, Y.; You, Q.; Fu, H.; Shao, L. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011–2018. Front. Endocrinol. 2022, 13, 1071465. [Google Scholar] [CrossRef] [PubMed]
- Halpern, S.E.; Moris, D.; Shaw, B.I.; Krischak, M.K.; Olaso, D.G.; Kesseli, S.J.; Ravindra, K.; McElroy, L.M.; Barbas, A.S. The Systemic Immune-Inflammation Index Predicts Clinical Outcomes in Kidney Transplant Recipients. In Vivo 2020, 34, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, A.; Larkman, J.; Xu, Y.; Cardoso, V.R.; Gkoutos, G.V. A random forest based biomarker discovery and power analysis framework for diagnostics research. BMC Med. Genom. 2020, 13, 178. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Gholamy, A.; Kreinovich, V.; Kosheleva, O. Why 70/30 or 80/20 Relation Between Training and Testing Sets: A Pedagogical Explanation. Dep. Tech. Rep. 2018, 1209. Available online: https://scholarworks.utep.edu/cs_techrep/1209 (accessed on 24 October 2023).
- Yen, C.H.; Wu, I.W.; Lee, C.C.; Hsu, K.H.; Sun, C.Y.; Chen, C.Y.; Pan, H.C.; Hsu, H.J. The prognostic value of peripheral total and differential leukocyte count in renal progression: A community-based study. PLoS ONE 2021, 16, e0258210. [Google Scholar] [CrossRef]
- Cetın, N.; Kocaturk, E.; Tufan, A.K.; Kıraz, Z.K.; Alatas, O. Immature granulocytes as biomarkers of inflammation in children with predialysis chronic kidney disease. Pediatr. Nephrol. 2023, 38, 219–225. [Google Scholar] [CrossRef]
- Lee, T.W.; Bae, W.; Choi, J.; Bae, E.; Jang, H.N.; Chang, S.H.; Park, D.J. The neutrophil-to-lymphocyte ratio may indicate when to start hemodialysis. Ren. Fail. 2022, 44, 1401–1408. [Google Scholar] [CrossRef]
- Yilmaz, G.; Sevinc, C.; Ustundag, S.; Yavuz, Y.C.; Hacıbekiroglu, T.; Hatipoglu, E.; Baysal, M. The relationship between mean platelet volume and neutrophil/lymphocyte ratio with inflammation and proteinuria in chronic kidney disease. Saudi J. Kidney Dis. Transpl. 2017, 28, 90–94. [Google Scholar] [CrossRef]
- Woziwodzka, K.; Dziewierz, A.; Pawica, M.; Panek, A.; Krzanowski, M.; Gołasa, P.; Latacz, P.; Burkat, M.; Kuźniewski, M.; Krzanowska, K. Neutrophil-to-lymphocyte ratio predicts long-term all-cause mortality in patients with chronic kidney disease stage 5. Folia Med. Cracov. 2019, 59, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Stojanowski, J.; Konieczny, A.; Rydzyńska, K.; Kasenberg, I.; Mikołajczak, A.; Gołębiowski, T.; Krajewska, M.; Kusztal, M. Artificial neural network—An effective tool for predicting the lupus nephritis outcome. BMC Nephrol. 2022, 23, 381. [Google Scholar] [CrossRef] [PubMed]
- Rankin, S.; Han, L.; Scherzer, R.; Tenney, S.; Keating, M.; Genberg, K.; Rahn, M.; Wilkins, K.; Shlipak, M.; Estrella, M. A Machine Learning Model for Predicting Mortality within 90 Days of Dialysis Initiation. Kidney360 2022, 3, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Guinsburg, A.M.; Jiao, Y.; Bessone, M.I.D.; Monaghan, C.K.; Magalhães, B.; Kraus, M.A.; Kotanko, P.; Hymes, J.L.; Kossmann, R.J.; Berbessi, J.C.; et al. Predictors of shorter- and longer-term mortality after COVID-19 presentation among dialysis patients: Parallel use of machine learning models in Latin and North American countries. BMC Nephrol. 2022, 23, 340. [Google Scholar] [CrossRef]
- Konieczny, A.; Stojanowski, J.; Rydzyńska, K.; Kusztal, M.; Krajewska, M. Artificial Intelligence-A Tool for Risk Assessment of Delayed-Graft Function in Kidney Transplant. J. Clin. Med. 2021, 10, 5244. [Google Scholar] [CrossRef]

| CBC-Derived Ratios | Body Weight | CRP | Alkaline Phosphatase | Serum Albumin | Serum Creatinine | eGFR |
|---|---|---|---|---|---|---|
| SII | R = 0.19 | R = 0.31 | R = −0.38 | R = −0.27 | R = −0.02 | R = −0.012 |
| p = 0.11 | p = 0.014 | p = 0.004 | p = 0.039 | p = 0.84 | p = 0.92 | |
| NLR | R = 0.35 | R = 0.31 | R = −0.44 | R = −0.15 | R = −0.003 | R = 0.06 |
| p = 0.003 | p = 0.013 | p = 0.0007 | p = 0.23 | p = 0.98 | p = 0.63 | |
| LMR | R = −0.3 | R = −0.06 | R = 0.16 | R = 0.12 | R = −0.14 | R = 0.013 |
| p = 0.015 | p = 0.64 | p = 0.22 | p = 0.36 | p = 0.24 | p = 0.91 | |
| PLR | R = 0.26 | R = 0.14 | R = −0.41 | R = −0.32 | R = 0.15 | R = −0.12 |
| p = 0.035 | p = 0.29 | p = 0.002 | p = 0.012 | p = 0.22 | p = 0.32 |
| Feature | Training Set Mean ± SD (Min–Max) n = 52 | Testing Set Mean ± SD (Min–Max) n = 14 |
|---|---|---|
| Gender—male | 30 (57.7%) | 6 (42.9%) |
| Gender—female | 22 (42.3%) | 8 (57.1%) |
| sCr (mg/dL) * | 5.94 ± 2.8 (1.38–15.05) | 7.75 ± 3.74 (2.76–16.24) |
| WBC (×103/uL) | 6.9 ± 2.31 (2.6–14.05) | 6.32 ± 1.7 (4.1–11.16) |
| PLT (×103/uL) | 236.29 ± 81.64 (57–513) | 221.86 ± 48.10 (163–334) |
| MPV (fL) | 10.03 ± 0.85 (8.2–12.4) | 10.16 ± 1.12 (8.8–11.8) |
| NEU (×103/uL) | 3.62 ± 1.61 (1.09–8.8) | 3.16 ± 1.15 (1.17–4.97) |
| LYM (×103/uL) | 2.33 ± 0.96 (0.72–6.6) | 2.21 ± 1.01 (1–4.75) |
| MON (×103/uL) | 0.53 ± 0.18 (0.27–1.07) | 0.85 ± 1.19 (0.35–4.9) |
| CRP (mg/L) | 2.65 ± 6.77 (0.1–35.75) | 1.67 ± 2.98 (0.09–10.95) |
| SII | 389.21 ± 252.44 (77.34–1582.13) | 361.47 ± 202.14 (136.99–891.7) |
| PLR | 109.80 ± 40.51 (46.34–276.79) | 113.46 ± 38.73 (54.53–185) |
| NLR | 1.67 ± 0.9 (0.4–4.68) | 1.7 ± 1.11 (0.62–4.82) |
| LMR | 4.59 ± 1.66 (1–8.05) | 3.96 ± 1.74 (0.42–7.68) |
| CKD 4 * | 9 (17.3%) | 2 (14.3%) |
| CKD 5 * | 11 (21.2%) | 5 (35.7%) |
| Dialysis–target | 32 (61.5%) | 7 (50.0%) |
| HD * | 18 (34.6%) | 2 (14.3%) |
| APD * | 14 (26.9%) | 5 (35.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawalec, A.; Stojanowski, J.; Mazurkiewicz, P.; Choma, A.; Gaik, M.; Pluta, M.; Szymański, M.; Bruciak, A.; Gołębiowski, T.; Musiał, K. Systemic Immune Inflammation Index as a Key Predictor of Dialysis in Pediatric Chronic Kidney Disease with the Use of Random Forest Classifier. J. Clin. Med. 2023, 12, 6911. https://doi.org/10.3390/jcm12216911
Kawalec A, Stojanowski J, Mazurkiewicz P, Choma A, Gaik M, Pluta M, Szymański M, Bruciak A, Gołębiowski T, Musiał K. Systemic Immune Inflammation Index as a Key Predictor of Dialysis in Pediatric Chronic Kidney Disease with the Use of Random Forest Classifier. Journal of Clinical Medicine. 2023; 12(21):6911. https://doi.org/10.3390/jcm12216911
Chicago/Turabian StyleKawalec, Anna, Jakub Stojanowski, Paulina Mazurkiewicz, Anna Choma, Magdalena Gaik, Mateusz Pluta, Michał Szymański, Aleksandra Bruciak, Tomasz Gołębiowski, and Kinga Musiał. 2023. "Systemic Immune Inflammation Index as a Key Predictor of Dialysis in Pediatric Chronic Kidney Disease with the Use of Random Forest Classifier" Journal of Clinical Medicine 12, no. 21: 6911. https://doi.org/10.3390/jcm12216911





