Mobility Assessment Using Multi-Positional MRI in Children with Cranio-Vertebral Junction Anomalies
Abstract
:1. Introduction
2. Materials and Methods
2.1. mMRI Protocol
2.2. Occipito-Cervical Parameter Measurements
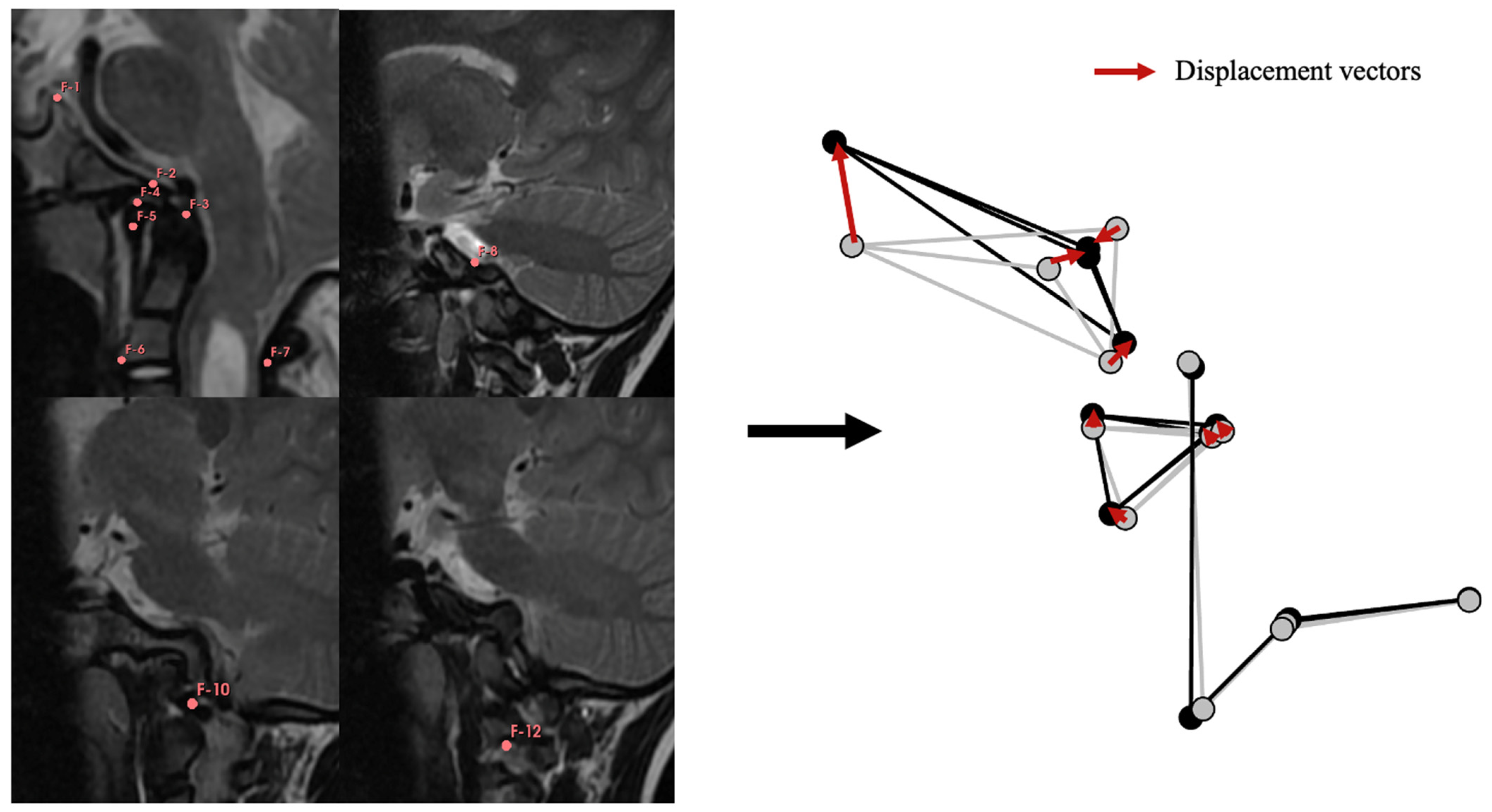
2.3. Geometric Modeling of the CVJ
2.4. Anatomical Study of the CVJ
2.5. Statistical Analysis
3. Results
3.1. Demographic and Morphometric Characteristics of the Cohort
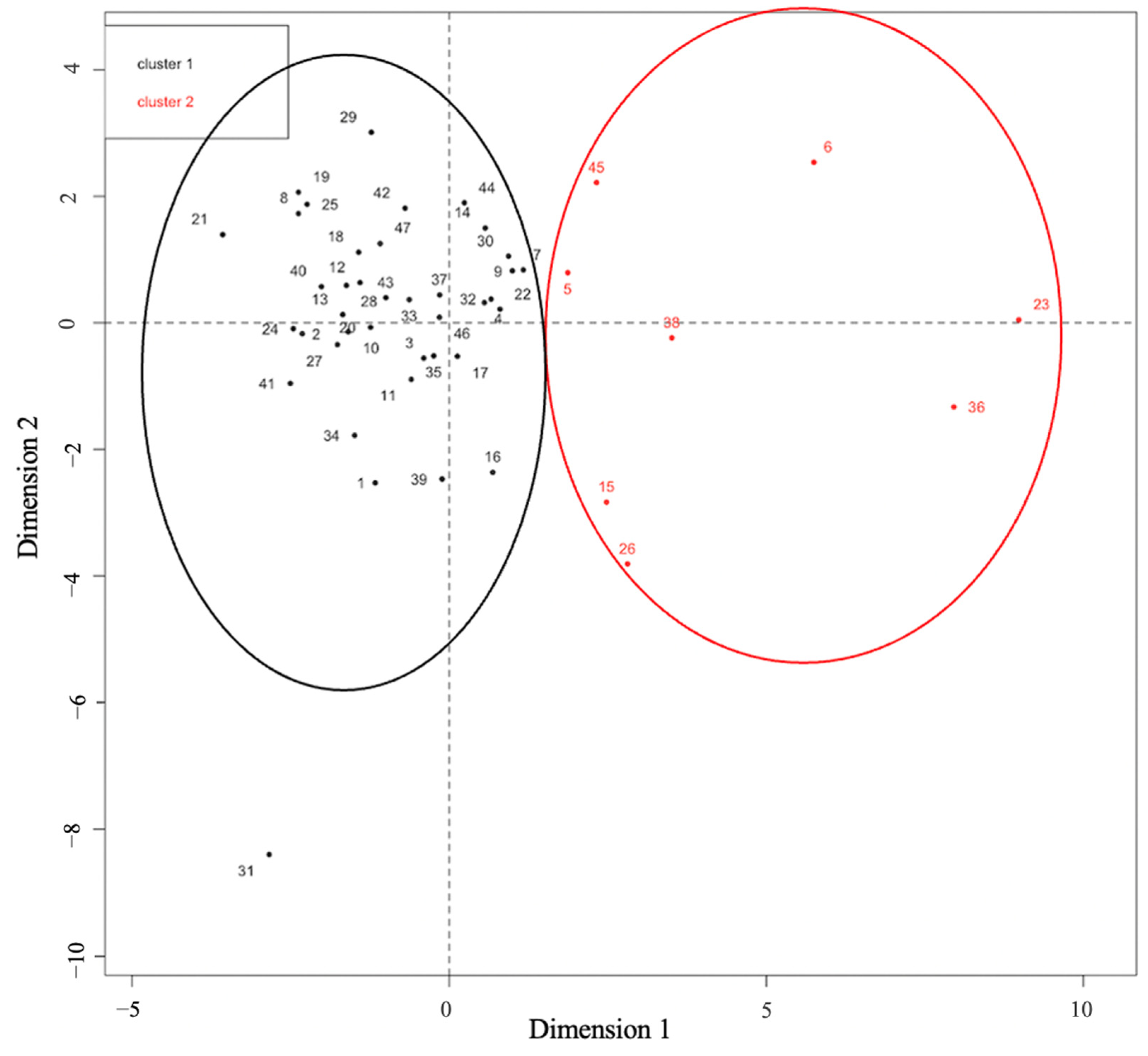
3.2. Mobility Assessment and Identification of CVJ Instability
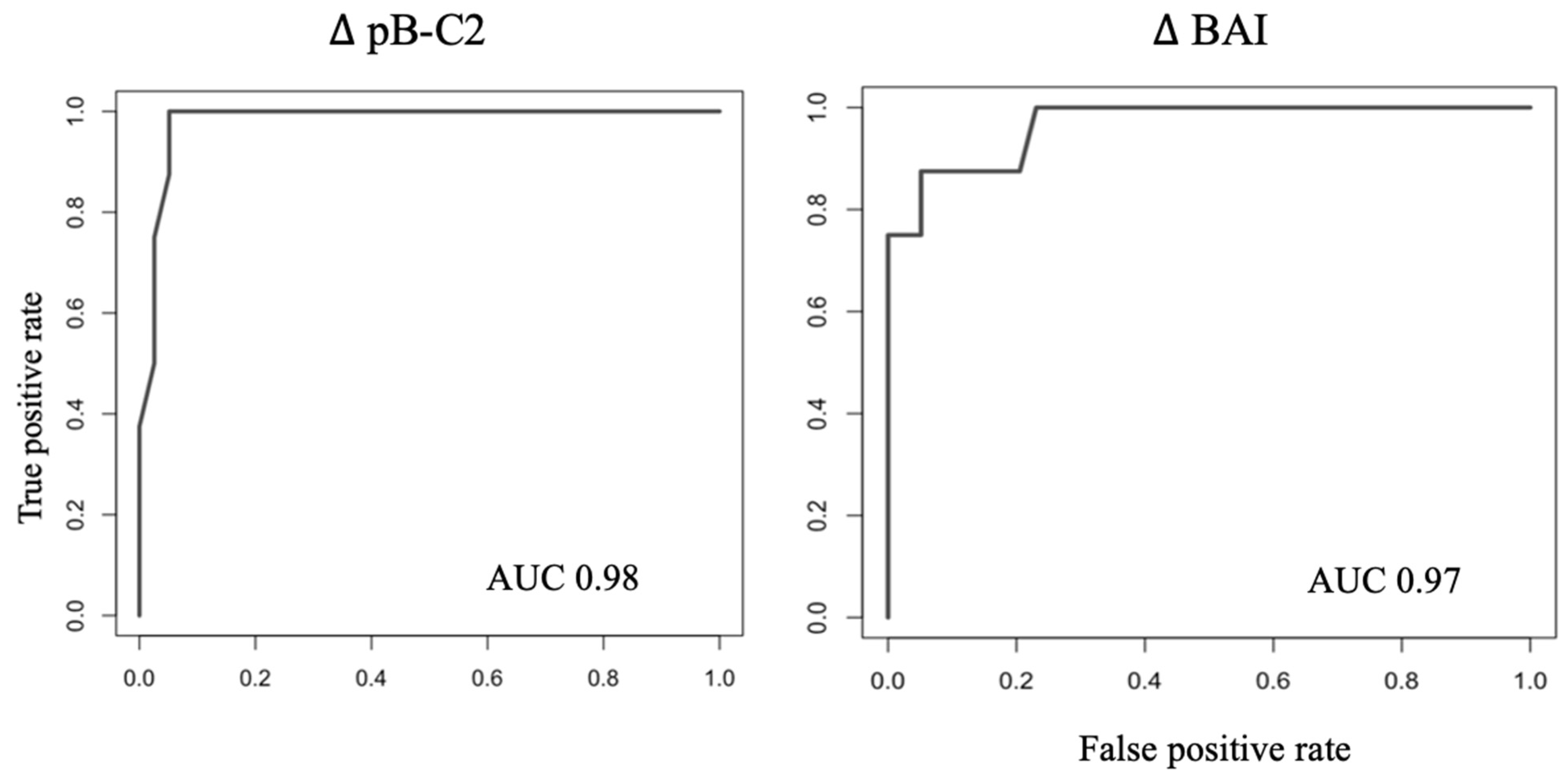
3.3. Identification of Instability Cut-Offs
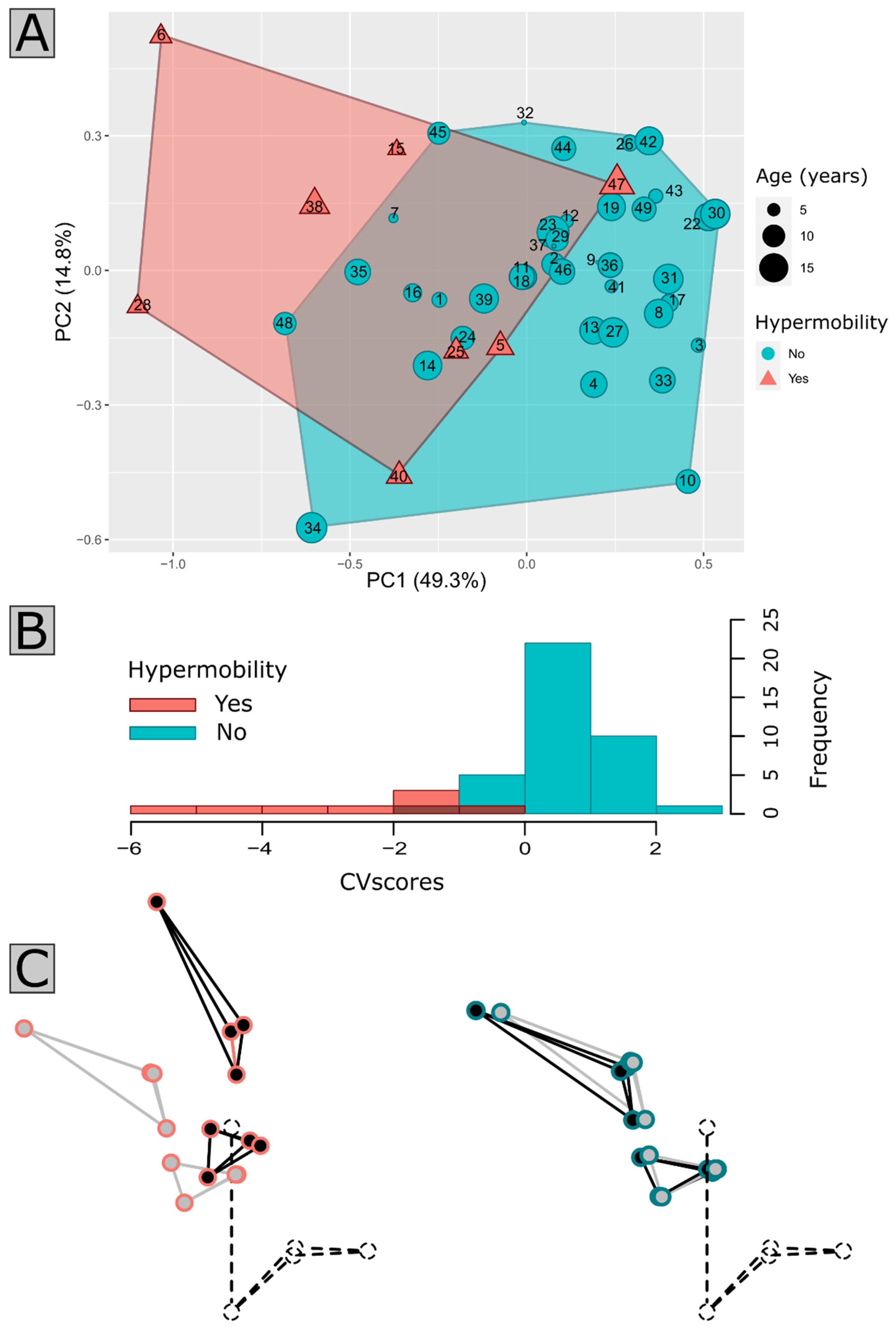
3.4. Anatomical Qualitative Analysis of the CVJ
3.5. Clinical Characteristics of Patients with Suspected Instability
4. Discussion
4.1. Current Practice and Limitations
4.2. Insights of Patient Clustering and Clinical Correlation
4.3. Limitations and Future Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goel, A. Central or axial atlantoaxial instability: Expanding understanding of craniovertebral junction. J. Craniovertebral Junction Spine 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.H. Craniovertebral junction database analysis: Incidence, classification, presentation, and treatment algorithms. Childs Nerv. Syst. 2008, 24, 1101–1108. [Google Scholar] [CrossRef]
- Massimi, L.; Peretta, P.; Erbetta, A.; Solari, A.; Farinotti, M.; Ciaramitaro, P.; Saletti, V.; Caldarelli, M.; Canheu, A.C.; Celada, C.; et al. Diagnosis and treatment of Chiari malformation type 1 in children: The International Consensus Document. Neurol. Sci. 2022, 43, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: International consensus document. Neurol. Sci. 2022, 43, 1327–1342. [Google Scholar] [CrossRef]
- Kim, H.J. Cervical spine anomalies in children and adolescents. Curr. Opin. Pediatr. 2013, 25, 72–77. [Google Scholar] [CrossRef]
- Miglioretti, D.L.; Johnson, E.; Williams, A.; Greenlee, R.T.; Weinmann, S.; Solberg, L.I.; Feigelson, H.S.; Roblin, D.; Flynn, M.J.; Vanneman, N.; et al. The Use of Computed Tomography in Pediatrics and the Associated Radiation Exposure and Estimated Cancer Risk. JAMA Pediatr. 2013, 167, 700. [Google Scholar] [CrossRef]
- Epstein, N.E.; Hyman, R.A.; Epstein, J.A.; Rosenthal, A.D. “Dynamic” MRI scanning of the cervical spine. Spine 1988, 13, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Kuwazawa, Y.; Bashir, W.; Pope, M.H.; Takahashi, K.; Smith, F.W. Biomechanical Aspects of the Cervical Cord: Effects of Postural Changes in Healthy Volunteers Using Positional Magnetic Resonance Imaging. Clin. Spine Surg. 2006, 19, 348–352. [Google Scholar] [CrossRef]
- Zhong, G.; Buser, Z.; Lao, L.; Yin, R.; Wang, J.C. Kinematic relationship between missed ligamentum flavum bulge and degenerative factors in the cervical spine. Spine J. 2015, 15, 2216–2221. [Google Scholar] [CrossRef]
- Reijnierse, M.; Breedveld, F.C.; Kroon, H.M.; Hansen, B.; Pope, T.L.; Bloem, J.L. Are magnetic resonance flexion views useful in evaluating the cervical spine of patients with rheumatoid arthritis? Skelet. Radiol. 2000, 29, 85–89. [Google Scholar] [CrossRef]
- Mackenzie, W.G.; Dhawale, A.A.; Demczko, M.M.; Ditro, C.; Rogers, K.J.; Bober, M.B.; Campbell, J.W. Flexion-Extension Cervical Spine MRI in Children With Skeletal Dysplasia: Is It Safe and Effective? J. Pediatr. Orthop. 2013, 33, 91–98. [Google Scholar] [CrossRef]
- Yecies, D.; Fogel, N.; Edwards, M.; Grant, G.; Yeom, K.W.; Cheshier, S. Safety of Dynamic Magnetic Resonance Imaging of the Cervical Spine in Children Performed without Neurosurgical Supervision. World Neurosurg. 2018, 116, e1188–e1193. [Google Scholar] [CrossRef] [PubMed]
- Koenigsberg, R.A.; Vakil, N.; Hong, T.A.; Htaik, T.; Faerber, E.; Maiorano, T.; Dua, M.; Faro, S.; Gonzales, C. Evaluation of Platybasia with MR Imaging. Am. J. Neuroradiol. 2005, 26, 89–92. [Google Scholar] [PubMed]
- Nascimento, J.J.C.; Neto, E.J.S.; Mello-Junior, C.F.; Valença, M.M.; Araújo-Neto, S.A.; Diniz, P.R.B. Diagnostic accuracy of classical radiological measurements for basilar invagination of type B at MRI. Eur. Spine J. 2019, 28, 345–352. [Google Scholar] [CrossRef]
- Kao, S.; Waziri, M.; Smith, W.; Sato, Y.; Yuh, W.; Franken, E. MR imaging of the craniovertebral junction, cranium, and brain in children with achondroplasia. Am. J. Roentgenol. 1989, 153, 565–569. [Google Scholar] [CrossRef]
- Thakar, S.; Sivaraju, L.; Jacob, K.S.; Arun, A.A.; Aryan, S.; Mohan, D.; Sai Kiran, N.A.; Hegde, A.S. A points-based algorithm for prognosticating clinical outcome of Chiari malformation Type I with syringomyelia: Results from a predictive model analysis of 82 surgically managed adult patients. J. Neurosurg. Spine 2018, 28, 23–32. [Google Scholar] [CrossRef]
- Cronin, C.G.; Lohan, D.G.; Mhuircheartigh, J.N.; Meehan, C.P.; Murphy, J.; Roche, C. CT evaluation of Chamberlain’s, McGregor’s, and McRae’s skull-base lines. Clin. Radiol. 2009, 64, 64–69. [Google Scholar] [CrossRef]
- Grabb, P.A.; Oakes, W.J. Ventral Brain Stem Compression in Pediatric and Young Adult Patients with Chiari I Malformations. Neurosurgery 1999, 44, 255–277, discussion 527–528. [Google Scholar] [CrossRef]
- Smoker, W.R. Craniovertebral junction: Normal anatomy, craniometry, and congenital anomalies. Radiographics 1994, 14, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.A.; Bertozzi, J.C.; Martinez, C.R.; Whitlow, J. Reassessment of the Craniocervical Junction: Normal Values on CT. Am. J. Neuroradiol. 2007, 28, 1819–1823. [Google Scholar] [CrossRef]
- Pan, Z.; Xi, Y.; Huang, W.; Kim, K.N.; Yi, S.; Shin, D.A.; Huang, K.; Chen, Y.; Huang, Z.; He, D.; et al. Independent Correlation of the C1–2 Cobb Angle with Patient-Reported Outcomes After Correcting Chronic Atlantoaxial Instability. Neurospine 2019, 16, 267–276. [Google Scholar] [CrossRef]
- Zimmerman, R.D.; Russell, E.J.; Leeds, N.E. Axial CT Recognition of Anteroposterior Displacement of Fourth Ventricle. Am. J. Neuroradiol. 1980, 1, 65–70. [Google Scholar] [PubMed]
- Cabet, S.; Szathmari, A.; Mottolese, C.; Franco, P.; Guibaud, L.; Rossi, M.; Di Rocco, F. New insights in craniovertebral junction MR changes leading to stenosis in children with achondroplasia. Childs Nerv. Syst. 2022, 38, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Mazerand, E.; Benichi, S.; Taverne, M.; Paternoster, G.; Rolland, A.; Antherieu, P.; Todeschi, J.; Kamdem Noumoye, L.; Gilard, V.; Bretonnier, M.; et al. Chiari malformation type I surgery in children: French multicenter 10-year cohort. J. Neurosurg. Pediatr. 2022, 30, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, H.E.; Anderson, R.C.E. Craniovertebral Junction Instability in the Setting of Chiari I Malformation. Neurosurg. Clin. 2015, 26, 561–569. [Google Scholar] [CrossRef]
- Leet, A.I.; Sampath, J.S.; Scott, C.I.; MacKenzie, W.G. Cervical Spinal Stenosis in Metatropic Dysplasia. J. Pediatr. Orthop. 2006, 26, 347–352. [Google Scholar] [CrossRef]
- Nakamura, K.; Miyoshi, K.; Haga, N.; Kurokawa, T. Risk factors of myelopathy at the atlantoaxial level in spondyloepiphyseal dysplasia congenita. Arch. Orthop. Trauma Surg. 1998, 117, 468–470. [Google Scholar] [CrossRef]
- Bollo, R.J.; Riva-Cambrin, J.; Brockmeyer, M.M.; Brockmeyer, D.L. Complex Chiari malformations in children: An analysis of preoperative risk factors for occipitocervical fusion: Clinical article. J. Neurosurg. Pediatr. 2012, 10, 134–141. [Google Scholar] [CrossRef]
- Gire, J.D.; Roberto, R.F.; Bobinski, M.; Klineberg, E.O.; Durbin-Johnson, B. The utility and accuracy of computed tomography in the diagnosis of occipitocervical dissociation. Spine J. 2013, 13, 510–519. [Google Scholar] [CrossRef]
- Chamnan, R.; Chantarasirirat, K.; Paholpak, P.; Wiley, K.; Buser, Z.; Wang, J.C. Occipitocervical measurements: Correlation and consistency between multi-positional magnetic resonance imaging and dynamic radiographs. Eur. Spine J. 2020, 29, 2795–2803. [Google Scholar] [CrossRef]
- Romano, A.; Albertini, G.; Guida, D.; Cornia, R.; Settecasi, C.; Condoluci, C.; Moraschi, M.; Fantozzi, L.M.; Bozzao, A.; Pierallini, A. A Cervical Flexion-Extension MRI Study in Down Syndrome. Indian J. Pediatr. 2015, 82, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Kirkpatrick, C.M.; Rizk, E.; Chern, J.J.; Oskouian, R.J.; Oakes, W.J. Do the cerebellar tonsils move during flexion and extension of the neck in patients with Chiari I malformation? A radiological study with clinical implications. Childs Nerv. Syst. 2016, 32, 527–530. [Google Scholar] [CrossRef]
- Pakkasjärvi, N.; Mattila, M.; Remes, V.; Helenius, I. Upper Cervical Spine Fusion in Children With Skeletal Dysplasia. Scand. J. Surg. 2013, 102, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Helman, S.N.; Badhey, A.; Kadakia, S.; Myers, E. Revisiting Crouzon syndrome: Reviewing the background and management of a multifaceted disease. Oral Maxillofac. Surg. 2014, 18, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Saenz, A.; Silva, A.H.D.; Jeelani, N.U.O.; James, G.; Tahir, M.Z. A rare case of atlantoaxial rotatory fixation after posterior calvarial vault expansion surgery in a Crouzon patient. Childs Nerv. Syst. 2022, 38, 2235–2238. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Hawaldar, A.; Lunawat, A.; Dandpat, S.; Goel, A. Report of two cases with omovertebral bone and Klippel–Feil syndrome with craniovertebral junction instability. J. Craniovertebral Junction Spine 2021, 12, 95. [Google Scholar] [CrossRef]
- Jae-Min Park, A.; Nelson, S.E.; Mesfin, A. Klippel-Feil Syndrome: Clinical Presentation and Management. JBJS Rev. 2022, 10, e21. [Google Scholar] [CrossRef]
- Virk, S.S.; Niedermeier, S.; Yu, E.; Khan, S.N. Adjacent Segment Disease. Orthopedics 2014, 37, 547–555. [Google Scholar] [CrossRef]
- Menezes, A.H. Craniovertebral junction anomalies: Diagnosis and management. Semin. Pediatr. Neurol. 1997, 4, 209–223. [Google Scholar] [CrossRef]
- Deora, H.; Behari, S.; Sardhara, J.; Singh, S.; Srivastava, A.K. Is Cervical Stabilization for All Cases of Chiari-I Malformation an Overkill? Evidence Speaks Louder Than Words! Neurospine 2019, 16, 195–206. [Google Scholar] [CrossRef]
- Goel, A. Craniovertebral Junction Instability: A Review of Facts about Facets. Asian Spine J. 2015, 9, 636. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, S.; Wang, F. Os odontoideum: Diagnosis and role of imaging. Surg. Radiol. Anat. 2020, 42, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Arvin, B.; Fournier-Gosselin, M.-P.; Fehlings, M.G. Os Odontoideum: Etiology and Surgical Management. Neurosurgery 2010, 66, A22–A31. [Google Scholar] [CrossRef] [PubMed]
- Goel, A. Basilar Invagination: Instability Is the Cause and Stabilization Is the Treatment. Neurospine 2020, 17, 585–587. [Google Scholar] [CrossRef] [PubMed]
| Variables | n (47) |
|---|---|
| Age (years; mean [range]) | 10.2 [3–18] |
| Diagnosis | |
| CM-1 | 17 |
| Syndromic craniosynostosis | 10 |
| Skeletal dysplasia | 4 |
| Achondroplasia | 2 |
| Mucopolysaccharidosis | 3 |
| Klippel-Feil syndrome | 3 |
| Filaminopathy type B with C0–C1 fusion | 1 |
| NF1 with C0–C1 fusion | 1 |
| FM stenosis with BI | 3 |
| Os odontoideum | 1 |
| C1–C2 panus | 1 |
| Surgery (occipito-cervical/cervical fixation) | 5 |
| MRI variables mean [range]: | |
| Static morphometric parameters | |
| Platybasia | 130.7 [109–157] |
| Boogaard angle | 137.1 [107–178] |
| Tentorial angle | 45.2 [17–121] |
| C2 retroversion | 74.7 [56–95] |
| McRae’s line | 31.2 [13–41] |
| Dynamic morphometric parameters (neutral; flexion; extension) | |
| pB-C2 | 10.4 [6.3–19]; 10.8 [6.4–20.6]; 9.9 [4–17] |
| CXA | 124 [82–180]; 120 [89–180]; 131 [101–180] |
| BAI | 12.6 [5–26]; 13 [0–28]; 11 [0–22] |
| BDI | 6.3 [0–16]; 6.2 [0–17]; 6 [0–16] |
| C1–C2 cobb angle | 17.6 [5–50]; 14.9 [4.8–42]; 19.2 [4–40] |
| Klaus index | 30.7 [9.3–41]; 28.7 [6.1–40]; 31,5 [8–41.7] |
| C2-opisthion interval | 25.4 [13–37.2]; 24.7 [12.4–37]; 24.9 [10.9–34] |
| Qualitative MRI variables n (%) | |
| Myelopathy | 9 (19%) |
| Syringomyelia | 18 (38%) |
| Herniation of cerebellar tonsils | 24 (51%) |
| Cluster №1 | Cluster №2 | |||||||
|---|---|---|---|---|---|---|---|---|
| K | p-Value | Mean | V | p-Value | Mean | V | p-Value | |
| n | 39 | 8 | ||||||
| pB-C2 | 0.6279325 | 3.2 × 10−11 | 0.89 [0–3.5] | /−5.374467 | 7.7 × 10−8 | 3.9 [2.4–6.4] | 5.374467 | 7.7 × 10−8 |
| BAI | 0.5627157 | 1.3 × 10−9 | 1.4 [0–4.5] | /−5.087723 | 3.6 × 10−7 | 6 [2.5–12] | 5.087723 | 3.6 × 10−7 |
| CXA | 0.2412186 | 4.6 × 10−4 | 9.3 [0.24] | /−3.331074 | 8.6 × 10−4 | 20.4 [4–38] | 3.331074 | 8.6 × 10−4 |
| BDI | 0.1803084 | 2.9 × 10−3 | 1.3 [0–4.5] | /−2.879963 | 4.0 × 10−3 | 3.1 [0.2–9.7] | 2.879963 | 4.0 × 10−3 |
| KI | 0.1726853 | 3.7 × 10−3 | 1 [0–3.7] | /−2.818426 | 4.8 × 10−3 | 2.8 [0.3–4.9] | 2.818426 | 4.8 × 10−3 |
| Case | Sex | Age (Years) | Diagnosis | Neurological Status/Symptoms | MRI Features | CT-Scan Features + Qualitative Analysis | Fixation Surgery |
|---|---|---|---|---|---|---|---|
| 1 | F | 9 | SD | Asymptomatic | FM stenosis, increased in flexion; AMS CSF absent in flexion; odontoid hypoplasia | N/A | yes |
| 2 | M | 8 | SD | Pyramidal syndrome | FM stenosis, increased in flexion; AMS CSF absent in flexion; odontoid hypoplasia; myelopathy | N/A | yes |
| 3 | F | 5 | Crouzon | Asymptomatic; mild alteration of SEP; normal polysomnography | BI, increased in flexion; Tonsillar herniation | C2–C3 fusion (post. arch, inf. articular facet); C2 dysplasia (post. arch, inf. articular facets: lateral mass; C2 vertical inf. articular facets | no |
| 4 | M | 7 | Crouzon | Mild SAS and SEP alteration | BI, increased in flexion; C2–C3 and C4–C5 fusion; Tonsillar herniation | C2–C3 fusion (post. arch, inf. articular facets, lateral masses C2 dysplasia (lateral mass) | yes |
| 5 | M | 6 | Klippel Feil | Asymptomatic | FM stenosis and BI, increased in flexion; myelopathy | Global C2–C3 fusion C2 dysplasia (lateral mass) | Indication but lost to follow-up |
| 6 | M | 10 | Klippel Feil with Sprengel malformation | Tetrapyramidal syndrome; SEP alteration | BI; odontoid deformation; FM stenosis increased in flexion with anterior spinal cord compression; syringomyelia | Global C2–C3 fusion C2 dysplasia (lateral mass) | yes |
| 7 | F | 8 | CM-1 | Asymptomatic (incidental finding); normal SEP and polysomnography | BI increased in flexion; Syringomyelia | N/A | no |
| 8 | M | 12 | Os odontoideum | Distal paresthesia; pyramidal syndrome | Myelopathy, C0–C1 luxation, C1–C2 cervical spinal stenosis | Odontoideum; C2 vertical inf. articular facets | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grenier-Chartrand, F.; Taverne, M.; James, S.; Guida, L.; Paternoster, G.; Loiselet, K.; Beccaria, K.; Dangouloff-Ros, V.; Levy, R.; de Saint Denis, T.; et al. Mobility Assessment Using Multi-Positional MRI in Children with Cranio-Vertebral Junction Anomalies. J. Clin. Med. 2023, 12, 6714. https://doi.org/10.3390/jcm12216714
Grenier-Chartrand F, Taverne M, James S, Guida L, Paternoster G, Loiselet K, Beccaria K, Dangouloff-Ros V, Levy R, de Saint Denis T, et al. Mobility Assessment Using Multi-Positional MRI in Children with Cranio-Vertebral Junction Anomalies. Journal of Clinical Medicine. 2023; 12(21):6714. https://doi.org/10.3390/jcm12216714
Chicago/Turabian StyleGrenier-Chartrand, Flavie, Maxime Taverne, Syril James, Lelio Guida, Giovanna Paternoster, Klervie Loiselet, Kevin Beccaria, Volodia Dangouloff-Ros, Raphaël Levy, Timothée de Saint Denis, and et al. 2023. "Mobility Assessment Using Multi-Positional MRI in Children with Cranio-Vertebral Junction Anomalies" Journal of Clinical Medicine 12, no. 21: 6714. https://doi.org/10.3390/jcm12216714





