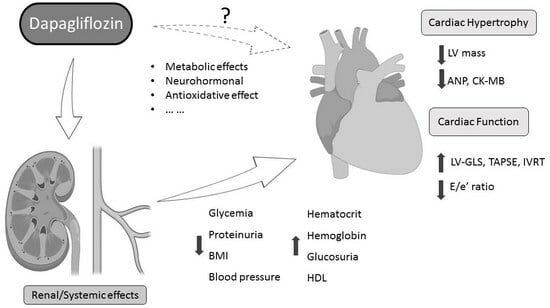
Dapagliflozin Improved Cardiac Function and Structure in Diabetic Patients with Preserved Ejection Fraction: Results of a Single Centre, Observational Prospective Study
Abstract
:1. Introduction
2. Material & Methods
2.1. Patients and Study Design
2.2. Echocardiography
2.3. Biochemical Analysis
2.4. Follow-Up, Adverse Events and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics and Follow-Up of the Study Population
3.2. Echocardiographic Data: Functional and Structural Changes
3.3. Biochemical Analysis: Changes in Hypertrophy Biomarkers
3.4. Association between Biomarkers and Echocardiographic Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Bennett, D.; Conrad, N.; Williams, T.M.; Basu, J.; Dwight, J.; Woodward, M.; Patel, A.; McMurray, J.; MacMahon, S. Risk prediction in patients with heart failure: A systematic review and analysis. JACC Heart Fail. 2014, 2, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Cavender, M.A.; Steg, P.G.; Smith, S.C., Jr.; Eagle, K.; Ohman, E.M.; Goto, S.; Kuder, J.; Im, K.; Wilson, P.W.F.; Bhatt, D.L.; et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: Outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation 2015, 132, 923–931. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Neal, B.; Perkovic, V.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Fabbrini, E.; Sun, T.; Li, Q.; et al. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018, 137, 323–334. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- McMurray, J.; Solomon, S.; Inzucchi, S.; Kober, L.; Kosiborod, M.; Martinez, F.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Rocca, H.-P.B.-L.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G.; et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Fegan, P.; Yeap, B.; Dwivedi, G. The effects of sodium-glucose cotransporter 2 inhibitors on left ventricular function: Current evidence and future directions. ESC Heart Fail. 2019, 6, 927–935. [Google Scholar] [CrossRef]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients with Type 2 Diabetes and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019, 140, 1693–1702. [Google Scholar] [CrossRef]
- Verma, S.; Garg, A.; Yan, A.T.; Gupta, A.K.; Al-Omran, M.; Sabongui, A.; Teoh, H.; Mazer, C.D.; Connelly, K.A. Effect of Empagliflozin on Left Ventricular Mass and Diastolic Function in Individuals With Diabetes: An Important Clue to the EMPA-REG OUTCOME Trial? Diabetes Care 2016, 39, e212–e213. [Google Scholar] [CrossRef]
- Soga, F.; Tanaka, H.; Tatsumi, K.; Mochizuki, Y.; Sano, H.; Toki, H.; Matsumoto, K.; Shite, J.; Takaoka, H.; Doi, T.; et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc. Diabetol. 2018, 17, 132. [Google Scholar] [CrossRef]
- Matsutani, D.; Sakamoto, M.; Kayama, Y.; Takeda, N.; Horiuchi, R.; Utsunomiya, K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 73. [Google Scholar] [CrossRef]
- Cohen, N.D.; Gutman, S.J.; Briganti, E.M.; Taylor, A.J. Effects of empagliflozin treatment on cardiac function and structure in patients with type 2 diabetes: A cardiac magnetic resonance study. Intern. Med. J. 2019, 49, 1006–1010. [Google Scholar] [CrossRef]
- Yu, Y.-W.; Zhao, X.-M.; Wang, Y.-H.; Zhou, Q.; Huang, Y.; Zhai, M.; Zhang, J. Effect of sodium–glucose cotransporter 2 inhibitors on cardiac structure and function in type 2 diabetes mellitus patients with or without chronic heart failure: A meta-analysis. Cardiovasc. Diabetol. 2021, 20, 25. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Vargas-Delgado, A.P.; Requena-Ibanez, J.A.; Garcia-Ropero, A.; Mancini, D.; Pinney, S.; Macaluso, F.; Sartori, S.; Roque, M.; Sabatel-Perez, F.; et al. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Grubić-Rotkvić, P.; Cigrovski-Berković, M.; Bulj, N.; Rotkvić, L.; Ćelap, I. Sodium-glucose cotransporter 2 inhibitors’ mechanisms of action in heart failure. World J. Diabetes 2020, 11, 269–279. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. 1), S13–S28. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Tuñón, J.; Blanco-Colio, L.M.; Cristóbal, C.; Tarín, N.; Higueras, J.; Huelmos, A.; Alonso, J.J.; Egido, J.; Asensio, D.; Lorenzo, Ó.; et al. Usefulness of a combination of monocyte chemoattractant protein-1, galectin-3, and N-terminal probrain natriuretic peptide to predict cardiovascular events in patients with coronary artery disease. Am. J. Cardiol. 2014, 113, 434–440. [Google Scholar] [CrossRef]
- Gamella-Pozuelo, L.; Fuentes-Calvo, I.; Gómez-Marcos, M.A.; Recio-Rodriguez, J.I.; Agudo-Conde, C.; Fernández-Martín, J.L.; Cannata-Andía, J.B.; López-Novoa, J.M.; García-Ortiz, L.; Martínez-Salgado, C.; et al. Plasma Cardiotrophin-1 as a Marker of Hypertension and Diabetes-Induced Target Organ Damage and Cardiovascular Risk. Med. Baltim. 2015, 94, e1218. [Google Scholar] [CrossRef]
- Hoffmann, U.; Espeter, F.; Weiss, C.; Ahmad-Nejad, P.; Lang, S.; Brueckmann, M.; Akin, I.; Neumaier, M.; Borggrefe, M.; Behnes, M.; et al. Ischemic biomarker heart-type fatty acid binding protein (hFABP) in acute heart failure—Diagnostic and prognostic insights compared to NT-proBNP and troponin I. BMC Cardiovasc. Disord. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Mustapic, I.; Bakovic, D.; Grabovac, Z.S.; Borovac, J.A. Impact of SGLT2 Inhibitor Therapy on Right Ventricular Function in Patients with Heart Failure and Reduced Ejection Fraction. J. Clin. Med. 2023, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wei, Y.; Rui, Y.; Fan, L. Echocardiographic evaluation of the effect of dapagliflozin on epicardial adipose tissue and left ventricular systolic function in type 2 diabetes mellitus. J. Diabetes Complicat. 2023, 37, 108509. [Google Scholar] [CrossRef] [PubMed]
- van Riet, E.E.S.; Hoes, A.W.; Limburg, A.; Landman, M.A.J.; van der Hoeven, H.; Rutten, F.H. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur. J. Heart Fail. 2014, 16, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzen, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef]
- Lorenzo-Almoros, A.; Tunon, J.; Orejas, M.; Cortes, M.; Egido, J.; Lorenzo, O. Diagnostic approaches for diabetic cardiomyopathy. Cardiovasc. Diabetol. 2017, 16, 28. [Google Scholar] [CrossRef]
- Poirier, P.; Bogaty, P.; Garneau, C.; Marois, L.; Dumesnil, J.G. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: Importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001, 24, 5–10. [Google Scholar] [CrossRef]
- Ha, J.-W.; Lee, H.-C.; Kang, E.-S.; Ahn, C.-M.; Kim, J.-M.; Ahn, J.-A.; Lee, S.-W.; Choi, E.-Y.; Rim, S.-J.; Oh, J.K.; et al. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: Implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart Br. Card. Soc. 2007, 93, 1571–1576. [Google Scholar] [CrossRef]
- Ferrannini, E.; Solini, A. SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat. Rev. Endocrinol. 2012, 7, 495–502. [Google Scholar] [CrossRef]
- Jhund, P.S.; Kondo, T.; Butt, J.H.; Docherty, K.F.; Claggett, B.L.; Desai, A.S.; Vaduganathan, M.; Gasparyan, S.B.; Bengtsson, O.; Lindholm, D.; et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: A patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat. Med. 2022, 28, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT-2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet Lond Engl. 2022, 400, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Bouthoorn, S.; Valstar, G.B.; Gohar, A.; Ruijter, H.M.D.; Reitsma, H.B.; Hoes, A.W.; Rutten, F.H. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diabetes Vasc. Dis. Res. 2018, 15, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, A.; Cantini, G.; Tani, A.; Coppini, R.; Zecchi-Orlandini, S.; Raimondi, L.; Luconi, M.; Mannucci, E. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: A new potential pharmacological target. Int. J. Cardiol. 2017, 243, 86–90. [Google Scholar] [CrossRef]
- Cortés, M.; Oliva, M.R.; Orejas, M.; Navas, M.A.; Rábago, R.M.; Martínez, M.E.; Taibo, M.; Palfy, J.; Rey, M.; Farré, J. Usefulness of speckle myocardial imaging modalities for differential diagnosis of left ventricular non-compaction of the myocardium. Int. J. Cardiol. 2016, 223, 813–818. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63 25 Pt A, 2751–2768. [Google Scholar] [CrossRef]
- Donal, E.; Thebault, C.; O’Connor, K.; Veillard, D.; Rosca, M.; Pierard, L.; Lancellotti, P. Impact of aortic stenosis on longitudinal myocardial deformation during exercise. Eur. J. Echocardiogr. 2011, 12, 235–241. [Google Scholar] [CrossRef]
- Koyama, J.; Falk, R.H. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc. Imaging 2010, 3, 333–342. [Google Scholar] [CrossRef]
- Tayyareci, Y.; Yildirimturk, O.; Aytekin, V.; Demiroglu, I.C.; Aytekin, S. Subclinical left ventricular dysfunction in asymptomatic severe aortic regurgitation patients with normal ejection fraction: A combined tissue Doppler and velocity vector imaging study. Echocardiography 2010, 27, 260–268. [Google Scholar] [CrossRef]
- Yajima, R.; Kataoka, A.; Takahashi, A.; Uehara, M.; Saito, M.; Yamaguchi, C.; Lee, K.; Komuro, I.; Funabashi, N. Distinguishing focal fibrotic lesions and non-fibrotic lesions in hypertrophic cardiomyopathy by assessment of regional myocardial strain using two-dimensional speckle tracking echocardiography: Comparison with multislice CT. Int. J. Cardiol. 2012, 158, 423–432. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.; Wu, Q. Atrial Natriuretic Peptide in Cardiovascular Biology and Disease (NPPA). Gene 2015, 569, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Takei, M.; Shiraishi, Y.; Suzuki, Y. Increased HematocritDuring Sodium-Glucose Cotransporter 2 Inhibitor Therapy Indicates Recovery of Tubulointerstitial Function in Diabetic Kidneys. J. Clin. Med. Res. 2016, 8, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Shulman, G.I. Sodium-glucose cotransporter-2 inhibitors: Understanding the mechanisms for therapeutic promise and persisting risks. J. Biol. Chem. 2020, 295, 14379–14390. [Google Scholar] [CrossRef] [PubMed]



| Variables | n = 31 |
|---|---|
| Age (mean (±SD)) | 66.4 (±8.4) |
| Male (n (%)) | 28 (90.3) |
| Hypertension (n (%)) | 22 (71.0) |
| Dyslipidaemia (n (%)) | 24(77.4) |
| Tobacco (n (%)) | 27 (87.1) |
| Obesity (n (%)) | 8 (25.8) |
| Cerebrovascular disease (n (%)) | 1 (3.2) |
| Peripheral vascular disease (n (%)) | 3 (9.7) |
| Chronic pulmonary disease (n (%)) | 3 (9.7) |
| Glomerular filtration (mL/min/1.73 m2 (±SD)) | 83.2 (±13.2) |
| Ischemic heart disease (n (%)) | 24 (77.4) |
| QRS >120 (n (%)) | 5 (16.1) |
| Baseline (±SD or IR) | Dapagliflozin (±SD or IR) | p Value | |
|---|---|---|---|
| Biochemichal variables (*) | |||
| Hemoglobin (g/dL) | 14.4 ± 1.4 | 14.9± 1.8 | 0.023 |
| Hematocrit (%) | 42.9 ± 3.8 | 45.1 ± 4.5 | <0.001 |
| Creatinin (mg/dL) | 0.9 (0.2) | 1 (0.4) | NS |
| GF (mL/min/1.73 m2) | 86.2 (22.2) | 84.4 (26.0) | NS |
| Potassium (mmol/L) | 4.5 ± 0.4 | 4.5 ± 0.4 | NS |
| Cholesterol (mg/dL) | 136.5 (58) | 135 (37) | NS |
| LDL cholesterol (mg/dL) | 61 (31) | 64.5 (18) | NS |
| HDL cholesterol (mg/dL) | 47.5 (14) | 50 (19) | 0.031 |
| Glucose (mg/dL) | 129.5 (46.5) | 124 (32.25) | 0.02 |
| Glyc. hemoglobin (%) | 6.9 (0.8) | 6.8 (0.9) | NS |
| Ferritin (ng/mL) | 55.5 (183) | 41.5 (123) | 0.018 |
| Proteinuria (%) | 17.4% | 8.7% | 0.005 |
| Urine pH | 5.7 ± 0.7 | 5.6 ± 0.7 | NS |
| NT-ProBNP (pg/mL) | 52.4 (76.5) | 51.5 (116) | NS |
| CK-MB (ng/mL) | 1.34 (1.1) | 1.32 (1.5) | 0.006 |
| MCP-1 (pg/mL) | 212 (95) | 228 (88) | NS |
| IL-6 (pg/mL) | 3.26 (2.78) | 2.68 (2.47) | NS |
| MMP-9 (ng/mL) | 489.9 (470.5) | 554.9 (473.7) | NS |
| hs-CRP (mg/mL) | 1.05 (2.07) | 1.26 (1.86) | NS |
| GAL-3 (ng/mL) | 6.9 ± 1.7 | 7.5 ± 2.4 | NS |
| TIM-1(pg/mL) | 91.3 (67.7) | 103 (72.5) | NS |
| ANP (ng/mL) | 19.4 (17.99) | 14.3 (12.29) | 0.007 |
| MMP-2 (ng/mL) | 277.1 ± 100.2 | 297.2 ± 88.7 | NS |
| H-FABP (ng/mL) | 1.01 (0.60) | 0.85 (0.8) | NS |
| Clinical variables (*) | |||
| Body weight (kg) | 80 (20) | 74.0 (22.0) | <0.001 |
| BMI (kg/m2) | 27.68 (5.02) | 26.5 (5.2) | <0.001 |
| Ecocardiographic variables (*) | |||
| LVEF (%) | 59.8 ± 3.5 | 60.5 ± 3.0 | NS |
| 3D LVEDV (mL) | 116.5 (65.9) | 118 (51) | NS |
| 3D LVESV (mL) | 48 (25.8) | 48 (19) | NS |
| 3D LV mass (g) | 162 (48) | 144 (35) | 0.028 |
| LV-GLS (%) | −19 (2.9) | −19.3 (2.3) | 0.036 |
| LA 3D-volume (mL/m2) | 37.7 ± 10.1 | 37.8 ± 13.4 | NS |
| PALS(%) | 34.1 ± 12.8 | 32.5 ± 9.7 | NS |
| PACS(%) | −16.7 ± 8.2 | −16.1± 7.2 | NS |
| LACS(%) | −17.0 (11.3) | −13.9 (8.1) | NS |
| TAPSE (mm) | 20.9 ± 3.3 | 23 ± 3.4 | 0.01 |
| RVFWSL(%) | −23.1 ± 5.8 | −23.4 ± 3.5 | NS |
| RV4CSL (%) | −19.1 ± 4.1 | −18.4 ± 2.4 | NS |
| é wave (cm/s) | 8.3 ± 1.9 | 9.1 ± 2.3 | NS |
| E/é (ratio) | 7.4 (2.5) | 6.6 (3.2) | 0.009 |
| IRVT (ms) | 89.5 ± 18.9 | 100.0 ± 17.6 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, M.; Lorenzo, O.; Lumpuy-Castillo, J.; Martínez-Albaladejo, S.; Taibo-Urquía, M.; Pello, A.M.; Bollas, A.J.; Orejas, M.; Navas, M.Á.; Macia, E.; et al. Dapagliflozin Improved Cardiac Function and Structure in Diabetic Patients with Preserved Ejection Fraction: Results of a Single Centre, Observational Prospective Study. J. Clin. Med. 2023, 12, 6698. https://doi.org/10.3390/jcm12206698
Cortés M, Lorenzo O, Lumpuy-Castillo J, Martínez-Albaladejo S, Taibo-Urquía M, Pello AM, Bollas AJ, Orejas M, Navas MÁ, Macia E, et al. Dapagliflozin Improved Cardiac Function and Structure in Diabetic Patients with Preserved Ejection Fraction: Results of a Single Centre, Observational Prospective Study. Journal of Clinical Medicine. 2023; 12(20):6698. https://doi.org/10.3390/jcm12206698
Chicago/Turabian StyleCortés, Marcelino, Oscar Lorenzo, Jairo Lumpuy-Castillo, Sacramento Martínez-Albaladejo, Mikel Taibo-Urquía, Ana María Pello, Antonio José Bollas, Miguel Orejas, Miguel Ángel Navas, Ester Macia, and et al. 2023. "Dapagliflozin Improved Cardiac Function and Structure in Diabetic Patients with Preserved Ejection Fraction: Results of a Single Centre, Observational Prospective Study" Journal of Clinical Medicine 12, no. 20: 6698. https://doi.org/10.3390/jcm12206698