Active Neurodynamics at Home in Patients with Knee Osteoarthritis: A Feasibility Study
Abstract
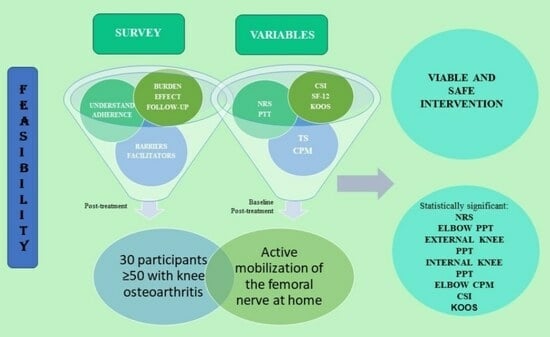
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Intervention
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Recruitment
3.3. Feasibility
3.4. Outcome Measurements
4. Discussion
4.1. Feasibility
4.2. Outcome Measurements
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bricca, A.; Juhl, C.B.; Steultjens, M.; Wirth, W.; Roos, E.M. Impact of exercise on articular cartilage in people at risk of, or with established, knee osteoarthritis: A systematic review of randomised controlled trials. Br. J. Sports Med. 2019, 53, 940–947. [Google Scholar] [CrossRef]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases and Injuries, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602, Erratum in Lancet 2017, 389, e1.
- Carlson, A.K.; Rawle, R.A.; Wallace, C.W.; Brooks, E.G.; Adams, E.; Greenwood, M.C.; Olmer, M.; Lotz, M.K.; Bothner, B.; June, R.K. Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.; Sullivan, M.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef]
- Md, R.; van Der Leeden, M.; Heymans, M.W.; Holla, J.F.M.; Häkkinen, A.; Lems, W.F.; Roorda, L.D.; Veenhof, C.; Sanchez-Ramirez, D.C.; de Vet, H.C.W.; et al. Course and predictors of pain and physical functioning in patients with hip osteoarthritis: Systematic review and meta-analysis. J. Rehabil. Med. 2016, 48, 245–252. [Google Scholar]
- Kus, G.; Yeldan, I. Strengthening the quadriceps femoris muscle versus other knee training programs for the treatment of knee osteoarthritis. Rheumatol. Int. 2019, 39, 203–218. [Google Scholar] [CrossRef]
- Kittelson, A.J.; George, S.Z.; Maluf, K.S.; Stevens-Lapsley, J.E. Future directions in painful knee osteoarthritis: Harnessing complexity in a heterogeneous population. Phys. Ther. 2014, 94, 422–432. [Google Scholar] [CrossRef]
- Fingleton, C.; Smart, K.; Moloney, N.; Fullen, B.; Doody, C. Pain sensitization in people with knee osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 1043–1056. [Google Scholar] [CrossRef]
- Tanaka, R.; Ozawa, J.; Kito, N.; Moriyama, H. Effect of the frequency and duration of land-based therapeutic exercise on pain relief for people with knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. J. Phys. Ther. Sci. 2014, 26, 969–975. [Google Scholar] [CrossRef]
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2015, 49, 1554–1557. [Google Scholar] [CrossRef]
- Tsokanos, A.; Livieratou, E.; Billis, E.; Tsekoura, M.; Tatsios, P.; Tsepis, E.; Fousekis, K. The Efficacy of Manual Therapy in Patients with Knee Osteoarthritis: A Systematic Review. Medicina 2021, 57, 696. [Google Scholar] [CrossRef]
- Azma, K.; RezaSoltani, Z.; Rezaeimoghaddam, F.; Dadarkhah, A.; Mohsenolhosseini, S. Efficacy of tele-rehabilitation compared with office-based physical therapy in patients with knee osteoarthritis: A randomized clinical trial. J. Telemed. Telecare 2018, 24, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, M.W.; Hough, A.D.; Dilley, A. Different nerve-gliding exercises induce different magnitudes of median nerve longitudinal excursion: An in vivo study using dynamic ultrasound imaging. J. Orthop. Sports Phys. Ther. 2009, 39, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.K.; Smith, M.P.; Sobczak, S.; James, C.R.; Sizer, P.S.; Brismée, J. Effects of lower limb neurodynamic mobilization on intraneural fluid dispersion of the fourth lumbar nerve root: An unembalmed cadaveric investigation. J. Man. Manip. Ther. 2015, 23, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Shacklock, M. Neurodynamics. Physiotherapy 1995, 81, 9–16. [Google Scholar] [CrossRef]
- Herrington, L. Effect of different neurodynamic mobilization techniques on knee extension range of motion in the slump position. J. Man. Manip. Ther. 2006, 14, 101–107. [Google Scholar] [CrossRef]
- Lau, Y.N.; Ng, J.; Lee, S.Y.; Chin, L.; Man, C.; Ming, S.; Lam, B.P.; Ngai, C. A brief report on the clinical trial on neural mobilization exercise for joint pain in patients with rheumatoid arthritis. Z. Rheumatol. 2019, 78, 474–478. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Wiggers, J.; Williams, A.; Campbell, E.; Wolfenden, L.; Yoong, S.; Robson, E.K.; McAuley, J.; Haskins, R.; Kamper, S.J.; et al. Randomised controlled trial of referral to a telephone-based weight management and healthy lifestyle programme for patients with knee osteoarthritis who are overweight or obese: A study protocol. BMJ Open 2016, 6, e010203. [Google Scholar] [CrossRef]
- Xiao, C.M.; Li, J.J.; Kang, Y.; Zhuang, Y.C. Follow-up of a wuqinxi exercise at home programme to reduce pain and improve function for knee osteoarthritis in older people: A randomised controlled trial. Age Ageing 2021, 50, 570–575. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, X.; Huang, H.; Liu, C.; Wan, Q.; Shang, S. The effects of a home-based exercise intervention on elderly patients with knee osteoarthritis: A quasi-experimental study. BMC Musculoskelet. Disord. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Brand, R.A.; Walls, R.J.; Ong, J.C.; Emerson, B.S.; O’Byrne, J.M.; Moyna, N.M. Effects of home-based resistance training and neuromuscular electrical stimulation in knee osteoarthritis: A randomized controlled trial. BMC Musculoskelet. Disord. 2012, 13, 118. [Google Scholar] [CrossRef]
- Aily, J.B.; de Almeida, A.C.; de Noronha, M.; Mattiello, S.M. Effects of a periodized circuit training protocol delivered by telerehabilitation compared to face-to-face method for knee osteoarthritis: A protocol for a non-inferiority randomized controlled trial. Trials 2021, 22, 887. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Iijima, H.; Tashiro, Y.; Kajiwara, Y.; Zeidan, H.; Shimoura, K.; Nishida, Y.; Bito, T.; Nakai, K.; Tatsumi, M.; et al. Home exercise therapy to improve muscle strength and joint flexibility effectively treats pre-radiographic knee OA in community-dwelling elderly: A randomized controlled trial. Clin. Rheumatol. 2019, 38, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.; Nelligan, R.K.; Schwartz, S.; Kasza, J.; Kimp, A.; Crofts, S.J.; Hinman, R.S. Behavior change text messages for home exercise adherence in knee osteoarthritis: Randomized trial. J. Med. Internet Res. 2020, 22, e21749. [Google Scholar] [CrossRef]
- Aily, J.B.; Barton, C.J.; Mattiello, S.M.; De Oliveira Silva, D.; De Noronha, M. Telerehabilitation for knee osteoarthritis in brazil: A feasibility study. Int. J. Telerehabil. 2020, 12, 137–148. [Google Scholar] [CrossRef]
- Yilmaz, M.; Sahin, M.; Algun, Z.C. Comparison of effectiveness of the home exercise program and the home exercise program taught by physiotherapist in knee osteoarthritis. J. Back Musculoskelet. Rehabil. 2019, 32, 161–169. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Butler, D.S. The Neurodynamic Techniques: A Definitive Guide from the Noigroup Team; Noigroup Publications: Adelaide, Australia, 2005. [Google Scholar]
- Taylor, L.J.; Harris, J.; Epps, C.D.; Herr, K. Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and cognitively intact older adults. Rehabil. Nurs. 2005, 30, 55–61. [Google Scholar] [CrossRef]
- Lluch, E.; Dueñas, L.; Falla, D.; Baert, I.; Meeus, M.; Sánchez-Frutos, J.; Nijs, J. Preoperative pain neuroscience education combined with knee joint mobilization for knee osteoarthritis: A randomized controlled trial. Clin. J. Pain 2018, 34, 44. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Vilagut, G.; Garin, O.; Cunillera, O.; Tresserras, R.; Brugulat, P.; Mompart, A.; Medina, A.; Ferrer, M.; Alonso, J. Normas de referencia para el Cuestionario de Salud SF-12 versión 2 basadas en población general de Cataluña—Reference guidelines for the 12-Item Short-Form Health Survey version 2 based on the Catalan general population. Med. Clin. 2012, 139, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Lizaur-Utilla, A.; Miralles-Munoz, F.A.; Gonzalez-Parreno, S.; Lopez-Prats, F.A. Validation of the spanish version of the knee injury and osteoarthritis outcome score (KOOS) for elderly patients with total knee replacement. J. Orthop. Res. 2019, 37, 2157. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, revised ed.; Lawrence Earlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014, 1, 10–25. [Google Scholar]
- Bowen, D.J.; Kreuter, M.; Spring, B.; Cofta-Woerpel, L.; Linnan, L.; Weiner, D.; Bakken, S.; Kaplan, C.P.; Squiers, L.; Fabrizio, C.; et al. How we design feasibility studies. Am. J. Prev. Med. 2009, 36, 452–457. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Beani, E.; Menici, V.; Ferrari, A.; Cioni, G.; Sgandurra, G. Feasibility of a home-based action observation training for children with unilateral cerebral palsy: An explorative study. Front. Neurol. 2020, 11, 16. [Google Scholar] [CrossRef]
- Carraro, N.; Gaudreau, P. Spontaneous and experimentally induced action planning and coping planning for physical activity: A meta-analysis. Psychol. Sport Exerc. 2013, 14, 228–248. [Google Scholar] [CrossRef]
- Sandlund, M.; Lindgren, H.; Pohl, P.; Melander-Wikman, A.; Bergvall-Kåreborn, B.; Lundin-Olsson, L. Towards a mobile exercise application to prevent falls: A participatory design process. Int. J. Child. Health Hum. Dev. 2016, 9, 389–398. [Google Scholar]
- Da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, 21. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.B.; Gibson, S.J. Treatment of chronic non-malignant pain in the elderly: Safety considerations. Drug Saf. 2009, 32, 457. [Google Scholar] [CrossRef] [PubMed]
- Kanavaki, A.M.; Rushton, A.; Efstathiou, N.; Alrushud, A.; Klocke, R.; Abhishek, A.; Duda, J.L. Barriers and facilitators of physical activity in knee and hip osteoarthritis: A systematic review of qualitative evidence. BMJ Open 2017, 7, e017042. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.A.; Steffen, A.D.; Fernández-De-Las-Peñas, C.; Kim, J.; Chmell, S.J. Joint mobilization enhances mechanisms of conditioned pain modulation in individuals with osteoarthritis of the knee. J. Orthop. Sports Phys. Ther. 2016, 46, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.H.; Anwer, S.; Sarkar, B.; Paul, A.K.; Anwar, D. Effect of 6-week retro or forward walking program on pain, functional disability, quadriceps muscle strength, and performance in individuals with knee osteoarthritis: A randomized controlled trial (retro-walking trial). BMC Musculoskelet. Disord. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Bhagat, M.; Neelapala, Y.R.; Gangavelli, R. Immediate effects of mulligan’s techniques on pain and functional mobility in individuals with knee osteoarthritis: A randomized control trial. Physiother. Res. Int. 2020, 25, e1812. [Google Scholar] [CrossRef]
- Kabiri, S.; Halabchi, F.; Angoorani, H.; Yekaninejad, S. Comparison of three modes of aerobic exercise combined with resistance training on the pain and function of patients with knee osteoarthritis: A randomized controlled trial. Phys. Ther. Sport 2018, 32, 22–28. [Google Scholar] [CrossRef]
- Rewald, S.; Lenssen, A.T.; Emans, P.J.; de Bie, R.A.; van Breukelen, G.; Mesters, I. Aquatic cycling improves knee pain and physical functioning in patients with knee osteoarthritis: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2020, 101, 1288–1295. [Google Scholar] [CrossRef]
- Van Oosterwijck, J.; Meeus, M.; Paul, L.; De Schryver, M.; Pascal, A.; Lambrecht, L.; Nijs, J. Pain physiology education improves health status and endogenous pain inhibition in fibromyalgia: A double-blind randomized controlled trial. Clin. J. Pain 2013, 29, 873–882. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C. Body mass index and risk of knee osteoarthritis: Systematic review and meta-analysis of prospective studies. BMJ Open 2015, 5, e007568. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef]
- Salaha, Z.F.M.; Ammarullah, M.I.; Abdullah, N.N.A.A.; Aziz, A.U.A.; Gan, H.-S.; Abdullah, A.H.; Kadir, M.R.A.; Ramlee, M.H. Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model. Materials 2023, 16, 3298. [Google Scholar] [CrossRef] [PubMed]
- Lamura, M.D.P.; Hidayat, T.; Ammarullah, M.I.; Bayuseno, A.P.; Jamari, J. Study of contact mechanics between two brass solids in various diameter ratios and friction coefficient. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2023, 237, 1613–1619. [Google Scholar] [CrossRef]
- Thompson, J.A.; Hast, M.W.; Granger, J.F.; Piazza, S.J.; Siston, R.A. Biomechanical effects of total knee arthroplasty component malrotation: A computational simulation. J. Orthop. Res. 2011, 29, 969–975. [Google Scholar] [CrossRef]
- Twiggs, J.; Miles, B.; Roe, J.; Fritsch, B.; Liu, D.; Parker, D.; Dickison, D.; Shimmin, A.; BarBo, J.; McMahon, S.; et al. Can TKA outcomes be predicted with computational simulation? Generation of a patient specific planning tool. Knee 2021, 33, 38–48. [Google Scholar] [CrossRef]
- Twiggs, J.; Miles, B.; Parker, D.; Liu, D.; Shimmin, A.; Fritsch, B.; Roe, J.; Baré, J.; Solomon, M.; Dickison, D.; et al. Patient-reported impairment following TKA is reduced when a computationally simulated predicted ideal alignment is achieved. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Twiggs, J.G.; Wakelin, E.A.; Fritsch, B.A.; Liu, D.W.; Solomon, M.I.; Parker, D.A.; Klasan, A.; Miles, B.P. Clinical and Statistical Validation of a Probabilistic Prediction Tool of Total Knee Arthroplasty Outcome. J. Arthroplast. 2019, 34, 2624–2631. [Google Scholar] [CrossRef] [PubMed]


| Frequency (%) | ||
|---|---|---|
| Gender | Male Female | 9 (30) 21 (70) |
| Kellgren–Lawrence scale | Grade I Grade II | 6 (20) 24 (80) |
| Lower limb deformities | Yes No | 2 (6.66) 28 (93.33) |
| Analgesic intake | Yes No | 5 (16.66) 25 (83.3) |
| Mean (SD) | Median (IQRs) | |
| Age (years) | 66.27 (±8.93) | 67.5 (72–67.5) |
| Height (m) | 1.64 (±0.08) | 1.65 (1.58–1.69) |
| Weight (kg) | 70.05 (±14.78) | 67.5 (60.25–79) |
| BMI (kg/m2) | 25.88 (±4.1) | 25.5 (23.11–27.29) |
| Comprehension | Participants (30) | |
|---|---|---|
| Understanding of the activity during the training session (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 29 (96.66) 1 (3.33) 0 (0) 0 (0) 0 (0) |
| Doubts during the intervention period (n (%)) | Yes No | 1 (3.33) 29 (96.66) |
| Watched video during the intervention period (n (%)) | Yes No | 14 (46.66) 16 (53.33) |
| Video views | Mean (SD) | 0.83 (1.02) |
| Calls to the researcher (n (%)) | 0 (0) | |
| Additional sessions (n (%)) | Yes No | 1 (3.33) 29 (96.66) |
| Adherence | ||
| Participation (n (%)) | Yes No | 30 (100) 0 (0) |
| Participants who completed the programme (n (%)) | Yes No | 30 (100) 0 (0) |
| Number of days the programme was carried out (n (%)) | 56 days 52 days 51 days 50 days 49 days 46 days | 14 (46.66) 8 (26.66) 1 (3.33) 2 (6.66) 4 (13.33) 1 (3.33) |
| Time of day when the programme was performed (n (%)) | Morning and afternoon Morning or afternoon only | 22 (73.33) 8 (26.66) |
| Daily time (minutes) to carry out the programme | Mean (SD) | 3.5 (±1.22) |
| Fixed schedule to carry out the programme (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 3 (10) 14 (46.66) 8 (26.66) 3 (10) 2 (6.66) |
| Load | ||
| It was appropriate (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 21 (70) 9 (30) 0 (0) 0 (0) 0 (0) |
| It was difficult (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 0 (0) 0 (0) 0 (0) 10 (33.33) 20 (66.66) |
| The difficulty increased over the days (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 0 (0) 0 (0) 0 (0) 11 (36.66) 19 (63.33) |
| The difficulty decreased over the days (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 3 (10) 7 (23.33) 11 (36.66) 7 (23.33) 2 (6.66) |
| Easy to perform at home (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 21 (70) 9 (30) 0 (0) 0 (0) 0 (0) |
| Difficult to perform without physiotherapist personal supervision | Strongly agree Agree Neutral Disagree Strongly disagree | 0 (0) 0 (0) 1 (3,33) 11 (36.66) 18 (60) |
| Why (n (%)) | Easy Video Both: video and easy | 18 (60) 8 (26.66) 4 (13.33) |
| The programme was burdensome (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 0 (0) 0 (0) 5 (16.66) 6 (20) 19 (63.33) |
| It was difficult to combine it with the ADL (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 0 (0) 0 (0) 1 (3.33) 8 (26.66) 21 (70) |
| It was necessary to modify any ADL to perform the programme (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 0 (0) 0 (0) 0 (0) 7 (23.33) 23 (76.66) |
| It was necessary to stop doing any ADL to carry out the programme (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 0 (0) 0 (0) 0 (0) 6(20) 24 (80) |
| I had the required equipment at home (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 28 (93.33) 2 (6.66) 0 (0) 0 (0) 0 (0) |
| I had to make some home modifications to perform the programme (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 0 (0) 0 (0) 0 (0) 4 (13.33) 26 (86.66) |
| Someone helped me to carry out the programme (n (%)) | Strongly agree Agree Neutral Disagree Strongly disagree | 0 (0) 0 (0) 0 (0) 2 (6.66) 28 (93.33) |
| Self-perceived effect | ||
| The programme was good (n (%)) | Strongly agreeAgreeNeutralDisagreeStrongly disagree | 15 (50)13 (43.33)2 (6.66)0 (0)0 (0) |
| I felt improvements in (n (%)) | Pain Mobility Pain and mobility Pain, mobility, and swelling Flexibility Flexibility and pain Did not improve | 15 (50) 3 (10) 8 (26.66) 1 (3.33) 1 (3.33) 1 (3.33) 1 (3.33) |
| There were no changes in (n (%)) | Anything Pain Mobility Swelling | 23 (76.66) 1 (3.33) 2 (6.66) 4 (13.33) |
| Barriers | ||
| Barriers (n (%)) | Anything Lack of consistency Remembering exercise Family obligations Better to do once a day | 24 (80) 3 (10) 1 (3.33) 1 (3.33) 1 (3.3) |
| Facilitators | ||
| Facilitators (n (%)) | Simplicity Duration Single exercise Video Motivation to improve Home treatment | 23 (76.66) 2 (6.66) 2 (6.66) 1 (3.33) 1 (3.33) 1 (3.33) |
| T0 | T1 | |||
|---|---|---|---|---|
| Means (SD) | Medians (IQR) | Means (SD) | Medians (IQR) | |
| NRS | 5 (1.90) | 5 (3.35–6.18) | 1.93 (1.25) | 1.75 (.94-.3.2) |
| Elbow PPT | 2.54 (0.77) | 2.5 (1.83–3.1) | 2.90 (1.03) | 2.75 (1.98–3.58) |
| External knee PPT | 2.65 (1.15) | 2.58 (1.71–3.38) | 3.15 (1.49) | 2.58 (2.21–3.98) |
| Internal knee PPT | 2.5 (1.11) | 2.38 (1.5–3.21) | 3.12 (1.72) | 2.5 (1.83–3.94) |
| Elbow TS | 1.48 (0.69) | 1.33 (1–1.67) | 1.49 (0.46) | 1.33 (1.25–1.58) |
| External knee TS | 1.53 (0.54) | 1.33 (1.2–1.69) | 1.45 (0.35) | 1.37 (1.22–1.58) |
| Internal knee TS | 1.55 (0.62) | 1.33 (1.23–1.67) | 1.64 (0.66) | 1.53 (1.26–1.71) |
| Elbow CPM | 1.32 (0.41) | 1.24 (1.08–1.35) | 1.68 (0.62) | 1.5 (1.33–2.08) |
| External knee CPM | 1.38 (0.66) | 1.12 (1–1.47) | 1.59 (0.56) | 1.5 (1.31–2) |
| Internal knee CPM | 1.48 (0.69) | 1.27 (1.07–1.67) | 1.94 (1.24) | 1.67 (1.33–2) |
| CSI | 27.97 (9.76) | 28 (20–32) | 24.20 (9.26) | 23 (16.75–29) |
| SF12 | 32.17 (2.21) | 32.5 (30–34) | 31.90 (2.25) | 32 (30.75–33) |
| KOOSS | 59.52 (21.27) | 60.71 (42.86–75) | 76.43 (14.67) | 67.86 (66.96–92-86) |
| KOOSR | 52.13 (15.96) | 51.39 (41.69.44) | 66.48 (13.51) | 68.06 (54.86–75) |
| KOOSADL | 58.48 (14.14) | 60.29 (45.59.66.54) | 72.35 (15.92) | 74.27 (54.41–83.82) |
| KOOSSP | 25.60 (21.71) | 22.5 (5–50) | 38.63 (23.76) | 35 (15–60) |
| KOOSQL | 40.21 (19.26) | 30.5 (25–57.81) | 50.83 (18.7) | 50 (37.5–62.5) |
| Means (SD) | CI | Cohen’s d (CI) | |
|---|---|---|---|
| NRS | −3.06 (1.91) | −3.78; −2.35 | −1.61 (−1.05; −2.14) |
| external knee PPT | 0.51 (0.96) | 0.15; 0.87 | 0.53 (0.91; 0.15) |
| CSI | −3.77 (4.1) | −5.3; −2.24 | -0.92 (−1.34; −0.48) |
| KOOSP | 14.35 (11.11) | 10.20; 18.50 | 1.29 (0.8; 1.77) |
| - | z | N | r |
| elbow PPT | −3.097 | 60 | −0.4 |
| internal knee PPT | −3.172 | 60 | −0.41 |
| elbow CPM | −3.028 | 60 | −0.391 |
| internal knee CPM | −2.099 | 60 | −0.271 |
| KOOSS | −4.431 | 60 | −0.572 |
| KOOSADL | −4.705 | 60 | −0.607 |
| KOOSSR | −3.856 | 60 | −0.498 |
| KOOSQL | −3.398 | 60 | −439 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-García, B.; Forriol-Campos, F.; Zuil-Escobar, J.C. Active Neurodynamics at Home in Patients with Knee Osteoarthritis: A Feasibility Study. J. Clin. Med. 2023, 12, 6635. https://doi.org/10.3390/jcm12206635
Serrano-García B, Forriol-Campos F, Zuil-Escobar JC. Active Neurodynamics at Home in Patients with Knee Osteoarthritis: A Feasibility Study. Journal of Clinical Medicine. 2023; 12(20):6635. https://doi.org/10.3390/jcm12206635
Chicago/Turabian StyleSerrano-García, Beatriz, Francisco Forriol-Campos, and Juan Carlos Zuil-Escobar. 2023. "Active Neurodynamics at Home in Patients with Knee Osteoarthritis: A Feasibility Study" Journal of Clinical Medicine 12, no. 20: 6635. https://doi.org/10.3390/jcm12206635