The Urine Calcium/Creatinine Ratio and Uricemia during Hyponatremia of Different Origins: Clinical Implications
Abstract
:1. Introduction
2. Materials and Methods
3. Results
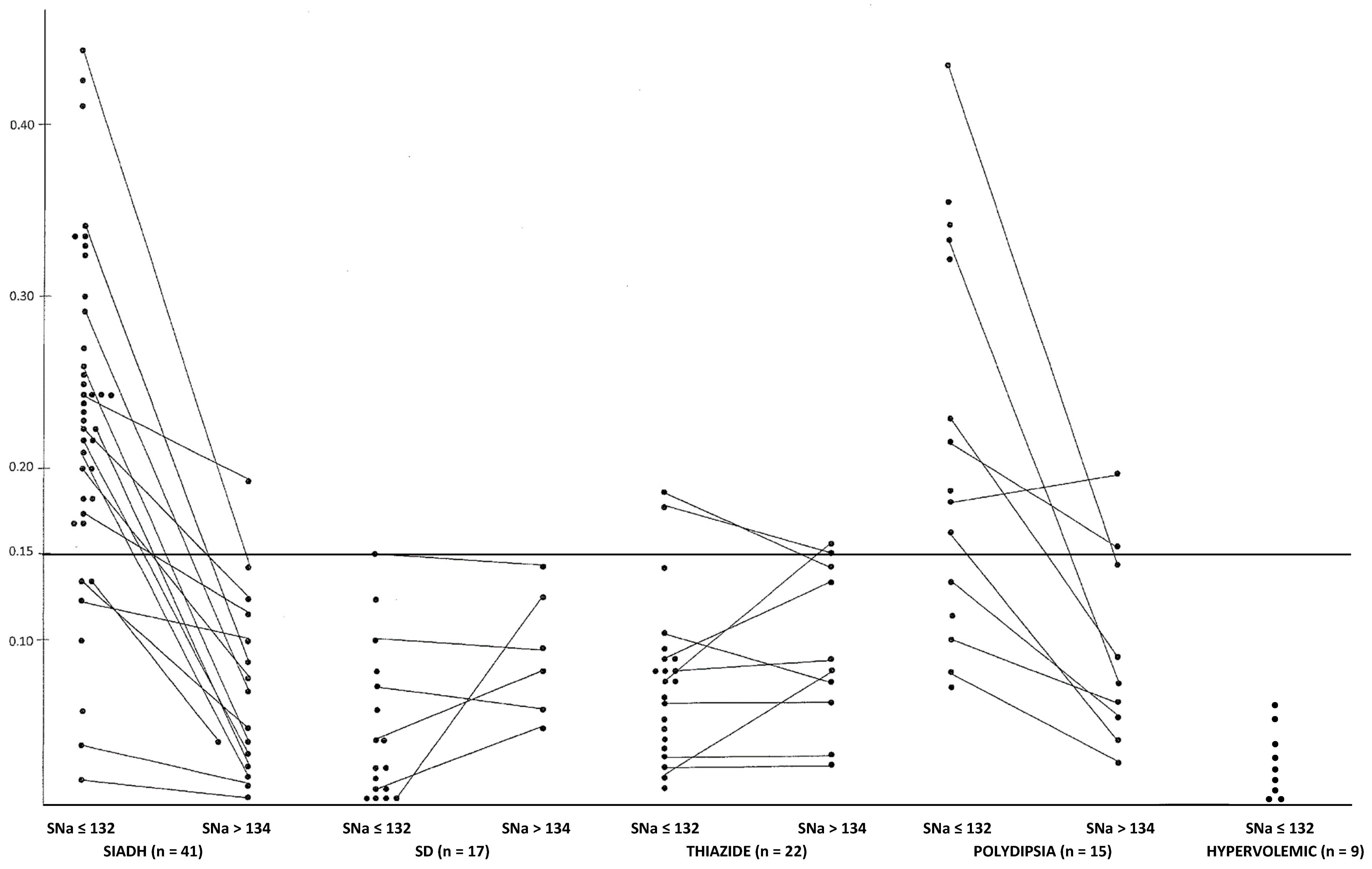
3.1. SIADH Patients
3.2. Salt Depletion
3.3. Diuretics
3.4. Polydipsia
3.5. Hypervolemic Hyponatremia due to Cirrhosis (n = 7) or Cardiac Failure (n = 2) (see Table 1 and Figure 1)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usala, R.L.; Fernandez, S.J.; Mete, M.; Cowen, L.; Shara, N.M.; Barsony, J.; Verbalis, J.G. Hyponatremia is associated with increased osteoporosis and bone fractures in a large US health system population. J. Clin. Endocrinol. Metab. 2015, 100, 3021–3031. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G.; Barsony, J.; Sugimura, Y.; Tian, Y.; Adams, D.J.; Carter, A.E.; Resnick, H.E. Hyponatremia-induced osteoporosis. JBMR 2010, 25, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsony, J.; Verbalis, J.G.; Sugimura, Y. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J. Biol. Chem. 2011, 286, 10864–11875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamma, R.; Sun, L.; Cuscito, C.; Lu, P.; Corcelli, M.; Li, J.; Colaianni, G.; Moonga, S.S.; Di Benedetto, A.; Grano, M.; et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc. Natl. Acad. Sci. USA 2013, 110, 18644–18649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsony, J.; Manigrasso, M.B.; Xu, Q.; Tam, H.; Verbalis, J.G. Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats. Age 2013, 35, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Decaux, G. Measurement of urinary-creatinine in chronic SIADH can be used to estimate solute and fluid intake. NDT 2021, 36, 1551–1553. [Google Scholar] [CrossRef]
- Fujisawa, C.; Umegaki, H.; Sugimoto, T.; Samizo, S.; Huang, C.H.; Fujisawa, H.; Sugimura, Y.; Kuzuya, M.; Toba, K.; Sakurai, T. Mild hyponatremia is associated with low skeletal muscle mass, physical function impairment, and depressive mood in the elderly. BMC Geriatr. 2021, 21, 15. [Google Scholar] [CrossRef]
- Wu, C.H.; Yang, K.C.; Chang, H.H.; Yen, J.F.; Tsai, K.S.; Huang, K.C. Sarcopenia is related to increased risk for low bone mineral density. J. Clin. Densitom. 2013, 16, 98–103. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Bone disease and idiopathic hypercalciuria. Semin. Nephrol. 2008, 28, 133–142. [Google Scholar] [CrossRef]
- Anastasio, P.; Trepiccione, F.; De Santo, N.G.; Capasso, G.; Viggiano, D.; Capolongo, G. Regulation of urinary calcium excretion by vasopressin. Clin. Kidney J. 2020, 13, 873–877. [Google Scholar] [CrossRef]
- Decaux, G.; Van Laethem, Y.; Van Kuyck, M.; Mockel, J. Hypercalciuria in the syndrome of inappropriate secretion of antidiuretic hormone. Miner. Electrolyte Metab. 1982, 7, 192–196. [Google Scholar]
- Grellier, J.; Jaafar, A.; Martin, A.; El Alaoui, M.; Lebely, C.; Tack, I.; Vallet, M. Syndrome of inappropriate anti-diuresis induces volume-dependent hypercalciuria. Osteoporos. Int. 2017, 28, 3161–3168. [Google Scholar] [CrossRef]
- Maniero, C.; Fassina, A.; Seccia, T.M.; Toniato, A.; Iacobone, M.; Plebani, M.; De Caro, R.; Calò, L.A.; Pessina, A.C.; Rossi, G.P. Mild hyperparathyroidism: A novel surgically correctable feature of primary aldosteronism. J. Hypertens. 2012, 30, 390–395. [Google Scholar] [CrossRef]
- Salcuni, A.S.; Palmieri, S.; Carnevale, V.; Morelli, V.; Battista, C.; Guarnieri, V.; Guglielmi, G.; Desina, G.; Eller-Vainicher, C.; Beck-Peccoz, P.; et al. Bone involvement in aldosteronism. J. Bone Miner. Res. 2012, 27, 2217–2222. [Google Scholar] [CrossRef]
- Ceccoli, L.; Ronconi, V.; Giovannini, L.; Marcheggiani, M.; Turchi, F.; Boscaro, M.; Giacchetti, G. Bone health and aldosterone excess. Osteoporos. Int. 2013, 24, 2801–2807. [Google Scholar] [CrossRef]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate and magnesium homeostasis. CJASN 2015, 10, 1257–1272. [Google Scholar] [CrossRef] [Green Version]
- Beck, L.H. Hypouricemia in the syndrome of inappropriate secretion of antidiuretic hormone. N. Engl. J. Med. 1979, 301, 528–530. [Google Scholar] [CrossRef]
- Decaux, G. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH). Semin. Nephrol. 2009, 29, 239–256. [Google Scholar] [CrossRef]
- Decaux, G.; VanderGheynst, F.; Bouko, Y.; Parma, J.; Vassart, G.; Vilain, C. Nephrogenic syndrome of inappropriate antidiuresis in adults: High phenotypic variability in men and women from a large pedigree. J. Am. Soc. Nephrol. 2007, 18, 606–612. [Google Scholar] [CrossRef]
- Musch, W.; Decaux, G. Utility and limitations of biochemical parameters in the evaluation of hyponatremia in the elderly. Int. Urol. Nephrol. 2001, 32, 475–493. [Google Scholar] [CrossRef]
- Culleton, B.F.; Larson, M.G.; Kannel, W.B.; Levy, D. Serum uric acid and risk for cardiovascular disease and death: The Framingham heart study. Ann. Intern. Med. 1999, 131, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Musch, W.; Decaux, G. Severe solute depletion in patients with hyponatremia due to diuretics despite biochemical pictures similar than those observed in the syndrome of inappropriate secretion of antidiuretic hormone. Nephron 2018, 140, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Decaux, G.; Unger, J.; Brimioulle, S.; Mockel, J. Hyponatremia in the syndrome of inappropriate secretion of antidiuretic hormone: Rapid correction with urea, sodium chloride, and water restriction therapy. JAMA 1982, 274, 471–474. [Google Scholar] [CrossRef]
- Better, O.S.; Gonick, H.C.; Chapman, L.C.; Varrady, P.D.; Kleeman, C.R. Effect of urea-saline diuresis on renal clearance of calcium, magnesium and inorganic phosphate in man. Proc. Soc. Exp. Biol. Med. 1966, 121, 592–596. [Google Scholar] [CrossRef]
- Delva, N.J.; Crammer, J.L.; Jarzylo, S.V.; Lawson, J.S.; Owen, J.A.; Sribney, M.; Weir, B.J.; Yendt, E.R. Osteopenia, pathological fracture, and increased urinary calcium excretion in schiziphrenic patients with polydipsia. Biol. Phychiatry 1989, 26, 781–793. [Google Scholar] [CrossRef]
- Decaux, G.; Prospert, F.; Namias, B.; Soupart, A. Hyperuriciemia as a clue for cnetral diabetes insipidus (lack of V1 effect) in the differential diagnoses of polydipsia. Am. J. Med. 1997, 103, 376–382. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Favus, M.J.; Langman, C.B.; Coe, F.L. Mechanism of chronic hypercalciuria with furosemide: Increased calcium absorption. Am. J. Physiol. 1986, 251, F17–F24. [Google Scholar] [CrossRef]
- Charoenphandhu, N.; Nakkrasae, L.-I.; Kraidith, K.; Teerapornpuntakit, J.; Thongchote, K.; Thongon, N.; Krishnamra, N. Two-step stimulation of intestinal Ca2+ absorption during lactation by long-term prolactin exposure and suckling-induced prolactin surge. Am. J. Physiol. Metab. 2009, 297, E609–E619. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, N.; Fernandez, S.J.; Mete, M.; Shara, N.M.; Verbalis, J.G. Hyponatremia and the risk of kidney stones: A matched case-control study in a large U.S. health system. PLoS ONE 2018, 13, e0203942. [Google Scholar] [CrossRef]
| Controls in Adults | SIADH (n = 41) | SD (n = 17) | Thiazides (n = 22) | Polydipsia (n = 15) | Cirrhosis (7) Cardiac Failure (2) (n = 9) |
|---|---|---|---|---|---|
| SNa (mEq/L) (135–145) | 123.3 ± 4.5 | 125.2 ± 3.3 | 123.4 ± 4.5 | 123.2 ± 4.4 | 127 ± 2.3 |
| Urea (mg/dL) (17–42) | 22.1 ± 8.4 | 35 ± 18 * | 42 ± 18 * | 20.4 ± 10 ** | 28 ± 19 |
| Uric acid (mg/dL) (2.5–7) | 2.7 ± 0.9 | 5.4 ± 1.9 * | 4.8 ± 1.85 * | 4.6 ± 2.25 | 4.7 ± 2.2 |
| Uosm (mOsm/kgH2O) (50–1100) | 462 ± 123 | 552 ± 155 | 432 ± 132 | 122 ± 40 | 450 ± 140 |
| UNa (mEq/L) (<170) | 89 ± 39 | 24 ± 14 * | 45 ± 24 * | 13 ± 14 * | 25 ± 20 * |
| SProt (g/dL) (6–8) | 6.6 ± 0.5 | 7.0 ± 0.8 | 7.4 ± 0.6 * | 7.0 ± 0.8 | 6.5 ± 1.0 |
| SCa (mg/dL) (8.1–10.6) | 9.0 ± 0.55 | 9.03 ± 0.50 | 9.40 ± 0.64 *** | 9.12 ± 0.66 | 8.3 ± 0.8 |
| UCa/UCr (before breakfast: ≤0.15) | 0.23 ± 0.096 | 0.056 ± 0.038 * | 0.075 ± 0.047 * | 0.205 ± 0.10 ** | 0.034 ± 0.01 * |
| SIADH (n = 15) | SD (n = 6) | Thiazides (n = 10) | Polydipsia (n = 9) | |||||
|---|---|---|---|---|---|---|---|---|
| SNa (mEq/L) | 124 ±3.6 | 137 ± 2.2 * | 126 ± 2.4 | 134 ± 1.3 * | 123 ± 4.9 | 134 ± 1.6 * | 121.5 ± 5 | 136 ± 3 * |
| SUrea (mg/dL) | 24 ± 11 | 39 ± 13 * | 36 ± 8 | 25 ± 6.7 *** | 39.3 ± 18 | 33 ± 10 | 19 ± 9 | 17 ± 7 |
| SUric acid (mg/dL) | 3 ± 0.45 | 4 ± 1.37 * | 5.7 ± 2 | 4.6 ± 1.5 *** | 5 ± 2.1 | 4.1 ± 2 * | 4.3 ± 2.4 | 4.5 ± 1.5 |
| Uosm (mOsm/kgH2O) | 391 ± 87 | 703 ± 240 * | 650 ± 135 | 604 ± 180 | 392 ± 120 | 430 ± 170 | 125 ± 40 | 306 ± 150 ** |
| SCa (mg/dL) | 9.15 ± 0.33 | 9.44 ± 0.40 | 9.16 ± 0.52 | 8.7 ± 0.17 | 9.45 ± 0.6 | 9.0 ± 0.25 *** | 9.0 ± 0.5 | 8.8 ± 0.5 |
| SPO4 (mg/dL) | 3.5 ± 0.5 | 3.8 ± 0.8 | 3.2 ± 0.9 | 3.1 ± 0.5 | 3.2 ± 0.6 | 2.9 ± 0.6 | 3.3 ± 1 | 3.2 ± 0.6 |
| UCa/UCr | 0.25 ± 0.1 | 0.09 ± 0.05 * | 0.06 ± 0.03 | 0.064 ± 0.036 | 0.09 ± 0.05 | 0.07 ± 0.05 | 0.21 ± 0.10 | 0.10 ± 0.07 *** |
| Sex—Age (Year)—Body Weight (kg) | SNa (mEq/L) | Urine Ca/24 h (mg) | Treatment | SNa (mEq/L) | Urine Ca/24 h (mg) | Etiology of SIADH |
|---|---|---|---|---|---|---|
| ♂—65—59 | 128 | 203 | Urea (15 g/day) | 133 | 160 | Brain damage |
| ♂—76—60 | 120 | 160 | Satavaptan (50 mg/day) | 139 | 86 | Carbamazepine |
| ♂—74—70 | 122 | 360 | Urea (30 g/day) | 141 | 34 | NSIAD |
| ♂—40—69 | 125 | 275 | WR | 139 | 192 | Carbamazepine |
| ♂—35—76 | 128 | 470 | WR | 139 | 298 | Carbamazepine |
| ♀—83—55 | 130 | 155 | Urea (15 g/day) | 133 | 77 | Idiopathic |
| ♀—27—63 | 124 | 434 | Urea (30 g/day) | 138 | 102 | Brain tumor |
| ♂—18—55 | 129 | 240 | Urea (30 g/day) | 137 | 80 | Brain damage |
| ♀—50—60 | 120 | 175 | Urea (30 g/day) | 136 | 70 | Carbamazepine |
| 52 ± 20 63 ± 6.7 | 125.1 ± 3.6 | 275 ± 112 | 137 ± 2.6 * | 122 ± 77 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decaux, G.; Musch, W. The Urine Calcium/Creatinine Ratio and Uricemia during Hyponatremia of Different Origins: Clinical Implications. J. Clin. Med. 2023, 12, 723. https://doi.org/10.3390/jcm12020723
Decaux G, Musch W. The Urine Calcium/Creatinine Ratio and Uricemia during Hyponatremia of Different Origins: Clinical Implications. Journal of Clinical Medicine. 2023; 12(2):723. https://doi.org/10.3390/jcm12020723
Chicago/Turabian StyleDecaux, Guy, and Wim Musch. 2023. "The Urine Calcium/Creatinine Ratio and Uricemia during Hyponatremia of Different Origins: Clinical Implications" Journal of Clinical Medicine 12, no. 2: 723. https://doi.org/10.3390/jcm12020723





