Clinical Characteristics of Autoimmune Hepatitis in a Middle Eastern Population: A Tertiary Care Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Chemistry Laboratory Profile
2.3. Assessment of the Serologic Profile and Histopathologic Evaluation of Liver Biopsies
2.4. The Criteria for the Diagnosis of AIH and the Evaluation of Response to Treatment
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
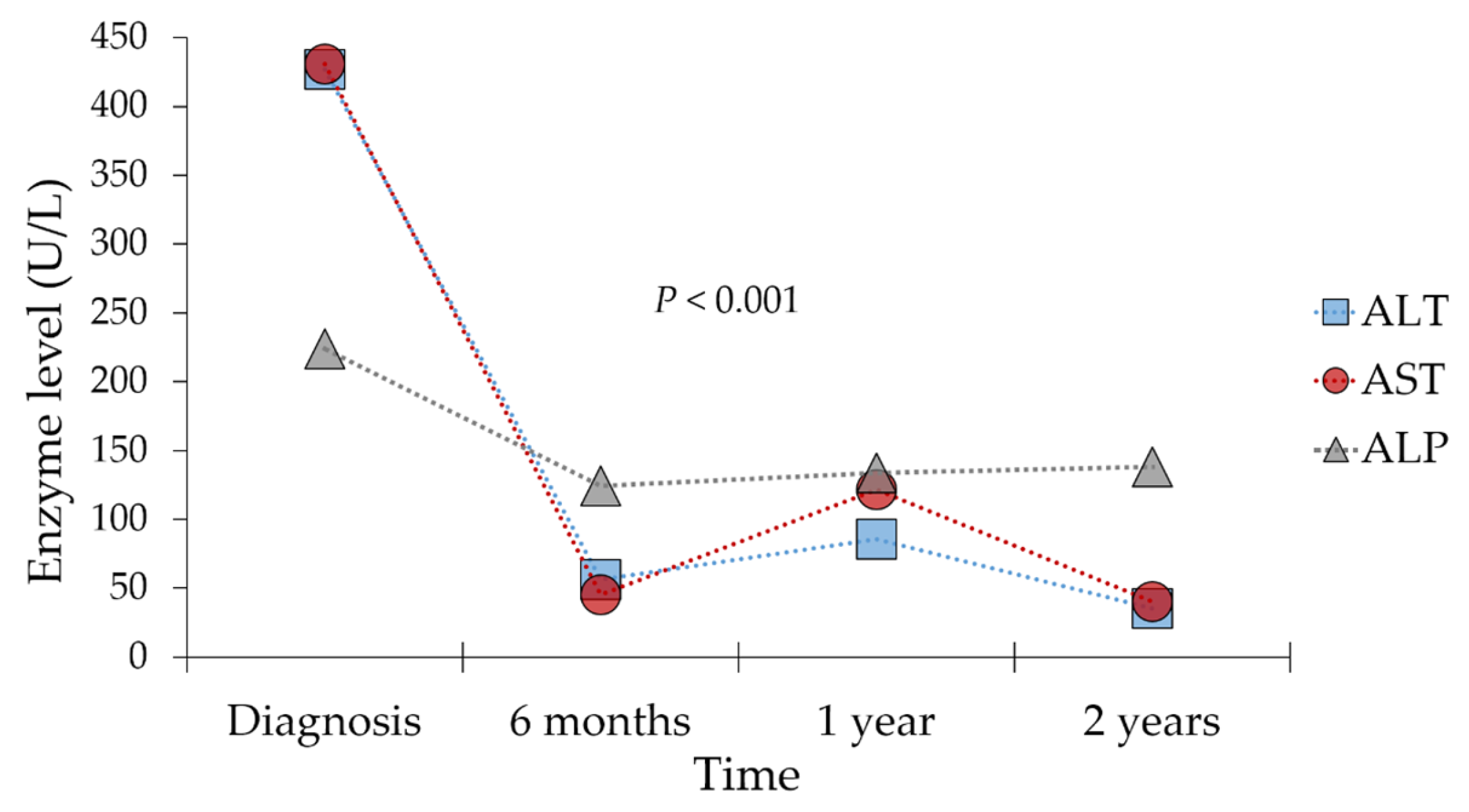
3.2. Baseline Laboratory Parameters at Time of Diagnosis
3.3. Histopathologic Evaluation of Liver Biopsies
3.4. Treatment Regimens, Response, and Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mieli-Vergani, G.; Vergani, D.; Czaja, A.J.; Manns, M.P.; Krawitt, E.L.; Vierling, J.M.; Lohse, A.W.; Montano-Loza, A.J. Autoimmune hepatitis. Nat. Rev. Dis. Prim. 2018, 4, 18017. [Google Scholar] [CrossRef]
- Makol, A.; Watt, K.D.; Chowdhary, V.R. Autoimmune hepatitis: A review of current diagnosis and treatment. Hepat. Res. Treat. 2011, 2011, 390916. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.L.; Adams, D.; Assis, D.N.; Kerkar, N.; Manns, M.P.; Mayo, M.J.; Vierling, J.M.; Alsawas, M.; Murad, M.H.; Czaja, A.J. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines from the American Association for the Study of Liver Diseases. Hepatology 2020, 72, 671–722. [Google Scholar] [CrossRef] [PubMed]
- Cardon, A.; Conchon, S.; Renand, A. Mechanisms of autoimmune hepatitis. Curr. Opin. Gastroenterol. 2021, 37, 79–85. [Google Scholar] [CrossRef]
- Gatselis, N.K.; Zachou, K.; Koukoulis, G.K.; Dalekos, G.N. Autoimmune hepatitis, one disease with many faces: Etiopathogenetic, clinico-laboratory and histological characteristics. World J. Gastroenterol. 2015, 21, 60–83. [Google Scholar] [CrossRef]
- Liberal, R.; Vergani, D.; Mieli-Vergani, G. Update on Autoimmune Hepatitis. J. Clin. Transl. Hepatol. 2015, 3, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, F.; Miao, Q.; Krawitt, E.L.; Gershwin, M.E.; Ma, X. The clinical phenotypes of autoimmune hepatitis: A comprehensive review. J. Autoimmun. 2016, 66, 98–107. [Google Scholar] [CrossRef]
- Manns, M.P.; Lohse, A.W.; Vergani, D. Autoimmune hepatitis—Update 2015. J. Hepatol. 2015, 62, S100–S111. [Google Scholar] [CrossRef]
- Grant, C.R.; Liberal, R. Liver immunology: How to reconcile tolerance with autoimmunity. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 6–16. [Google Scholar] [CrossRef]
- Teufel, A.; Weinmann, A.; Kahaly, G.J.; Centner, C.; Piendl, A.; Wörns, M.; Lohse, A.W.; Galle, P.R.; Kanzler, S. Concurrent autoimmune diseases in patients with autoimmune hepatitis. J. Clin. Gastroenterol. 2010, 44, 208–213. [Google Scholar] [CrossRef]
- Bittencourt, P.L.; Farias, A.Q.; Porta, G.; Cançado, E.L.; Miura, I.; Pugliese, R.; Kalil, J.; Goldberg, A.C.; Carrilho, F.J. Frequency of concurrent autoimmune disorders in patients with autoimmune hepatitis: Effect of age, gender, and genetic background. J. Clin. Gastroenterol. 2008, 42, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.; Grønbæk, L.; Vilstrup, H. Worldwide Incidence of Autoimmune Liver Disease. Dig. Dis. 2015, 33 (Suppl. 2), 2–12. [Google Scholar] [CrossRef] [PubMed]
- Terziroli Beretta-Piccoli, B.; Mieli-Vergani, G.; Vergani, D. Autoimmmune hepatitis. Cell. Mol. Immunol. 2022, 19, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.; Peters, M.G. Liver disease in women: The influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol. Hepatol. 2013, 9, 633–639. [Google Scholar]
- Liberal, R.; Mieli-Vergani, G.; Vergani, D. Clinical significance of autoantibodies in autoimmune hepatitis. J. Autoimmun. 2013, 46, 17–24. [Google Scholar] [CrossRef]
- Lemoinne, S.; Heurgue, A.; Bouzbib, C.; Hanslik, B.; Gournay, J.; Nguyen-Khac, E.; Bureau, C.; de Lédinghen, V.; Ganne-Carrié, N.; Bourlière, M. Non-invasive diagnosis and follow-up of autoimmune hepatitis. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101772. [Google Scholar] [CrossRef]
- Mieli-Vergani, G.; Vergani, D. Autoimmune hepatitis. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 320–329. [Google Scholar] [CrossRef]
- Francque, S.; Vonghia, L.; Ramon, A.; Michielsen, P. Epidemiology and treatment of autoimmune hepatitis. Hepat. Med. 2012, 4, 1–10. [Google Scholar] [CrossRef]
- Sebode, M.; Weiler-Normann, C.; Liwinski, T.; Schramm, C. Autoantibodies in Autoimmune Liver Disease-Clinical and Diagnostic Relevance. Front. Immunol. 2018, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Komori, A. Recent updates on the management of autoimmune hepatitis. Clin. Mol. Hepatol. 2021, 27, 58–69. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Aizawa, Y.; Hokari, A. Autoimmune hepatitis: Current challenges and future prospects. Clin. Exp. Gastroenterol. 2017, 10, 9–18. [Google Scholar] [CrossRef]
- Czaja, A.J. Diagnosis and management of the overlap syndromes of autoimmune hepatitis. Can. J. Gastroenterol. 2013, 27, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.; John, S. Autoimmune hepatitis: Appraisal of current treatment guidelines. World J. Hepatol. 2018, 10, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, D. Long-Term Outcomes of Autoimmune Hepatitis. Clin. Liver Dis. 2019, 14, 24–28. [Google Scholar] [CrossRef]
- Terziroli Beretta-Piccoli, B.; Mieli-Vergani, G.; Vergani, D. Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J. Gastroenterol. 2017, 23, 6030–6048. [Google Scholar] [CrossRef]
- Athamneh, R.Y.; Arıkan, A.; Sayan, M.; Mahafzah, A.; Sallam, M. Variable Proportions of Phylogenetic Clustering and Low Levels of Antiviral Drug Resistance among the Major HBV Sub-Genotypes in the Middle East and North Africa. Pathogens 2021, 10, 1333. [Google Scholar] [CrossRef]
- Sallam, M.; Batarseh, R.; Natsheh, A.; Abbadi, J.; Al-Fraihat, E.; Yaseen, A.; Kaddomi, D.; Khamees, N.; Mahafzah, A.; Şahin, G.Ö. An update on hepatitis C virus genotype distribution in Jordan: A 12-year retrospective study from a tertiary care teaching hospital in Amman. BMC Infect. Dis. 2019, 20, 3. [Google Scholar] [CrossRef]
- Fallatah, H.; Akbar, H.; Qari, Y. Autoimmune hepatitis: Single-center experience of clinical presentation, response to treatment and prognosis in Saudi Arabia. Saudi J. Gastroenterol. 2010, 16, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.B.; Mehta, K.J. Liver biopsy for assessment of chronic liver diseases: A synopsis. Clin. Exp. Med. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Hennes, E.M.; Zeniya, M.; Czaja, A.J.; Parés, A.; Dalekos, G.N.; Krawitt, E.L.; Bittencourt, P.L.; Porta, G.; Boberg, K.M.; Hofer, H.; et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008, 48, 169–176. [Google Scholar] [CrossRef]
- Chazouillères, O.; Wendum, D.; Serfaty, L.; Montembault, S.; Rosmorduc, O.; Poupon, R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: Clinical features and response to therapy. Hepatology 1998, 28, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Czaja, A.J.; Gorham, J.D.; Krawitt, E.L.; Mieli-Vergani, G.; Vergani, D.; Vierling, J.M. Diagnosis and management of autoimmune hepatitis. Hepatology 2010, 51, 2193–2213. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Q.; Bian, Z.; Ren, L.L.; Jia, J.; Ma, X. Autoimmune hepatitis: East meets west. J. Gastroenterol. Hepatol. 2015, 30, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.J.; Ryan, R.P.; Marshall, T.P.; Hirschfield, G.M. The Epidemiology of UK Autoimmune Liver Disease Varies with Geographic Latitude. Clin. Gastroenterol. Hepatol. 2021, 19, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Leung, P.S.C.; Adamopoulos, I.E.; Gershwin, M.E. The implication of vitamin D and autoimmunity: A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 217–226. [Google Scholar] [CrossRef]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef] [PubMed]
- Angum, F.; Khan, T.; Kaler, J.; Siddiqui, L.; Hussain, A. The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus 2020, 12, e8094. [Google Scholar] [CrossRef]
- Tunio, N.A.; Mansoor, E.; Sheriff, M.Z.; Cooper, G.S.; Sclair, S.N.; Cohen, S.M. Epidemiology of Autoimmune Hepatitis (AIH) in the United States Between 2014 and 2019: A Population-based National Study. J. Clin. Gastroenterol. 2021, 55, 903–910. [Google Scholar] [CrossRef]
- Muratori, P.; Czaja, A.-J.; Muratori, L.; Pappas, G.; Maccariello, S.; Cassani, F.; Granito, A.; Ferrari, R.; Mantovani, V.; Lenzi, M.; et al. Genetic distinctions between autoimmune hepatitis in Italy and North America. World J. Gastroenterol. 2005, 11, 1862–1866. [Google Scholar] [CrossRef]
- Behairy, O.G.A. Characteristics of autoimmune hepatitis in a sample of Egyptian children. Egypt. Pediatr. Assoc. Gaz. 2017, 65, 108–113. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Joshita, S.; Matsumoto, A.; Umemura, T.; Tanaka, E.; Morita, S.; Maejima, T.; Ota, M. Incidence and prevalence of autoimmune hepatitis in the Ueda area, Japan. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2016, 46, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Fedrizzi, R.S.; Coral, G.P.; Mattos, A.A.; Mattos, Â.Z.; Tovo, C.V. Evaluation of patients with autoimmune hepatitis in a specialized outpatient clinic in Southern Brazil. Arq. Gastroenterol. 2020, 57, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Baven-Pronk, M.A.M.C.; Biewenga, M.; van Silfhout, J.J.; van den Berg, A.P.; van Buuren, H.R.; Verwer, B.J.; van Nieuwkerk, C.M.J.; Bouma, G.; van Hoek, B. Role of age in presentation, response to therapy and outcome of autoimmune hepatitis. Clin. Transl.Gastroenterol. 2018, 9, 165. [Google Scholar] [CrossRef]
- Czaja, A.J.; Carpenter, H.A. Distinctive clinical phenotype and treatment outcome of type 1 autoimmune hepatitis in the elderly. Hepatology 2006, 43, 532–538. [Google Scholar] [CrossRef]
- Werner, M.; Prytz, H.; Ohlsson, B.; Almer, S.; Björnsson, E.; Bergquist, A.; Wallerstedt, S.; Sandberg-Gertzén, H.; Hultcrantz, R.; Sangfelt, P.; et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: A nationwide study. Scand. J. Gastroenterol. 2008, 43, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, G.; Somani, S.K.; Baba, C.S.; Alexander, G. Autoimmune hepatitis in India: Profile of an uncommon disease. BMC Gastroenterol. 2005, 5, 27. [Google Scholar] [CrossRef]
- Feld, J.J.; Dinh, H.; Arenovich, T.; Marcus, V.A.; Wanless, I.R.; Heathcote, E.J. Autoimmune hepatitis: Effect of symptoms and cirrhosis on natural history and outcome. Hepatology 2005, 42, 53–62. [Google Scholar] [CrossRef]
- Delgado, J.S.; Vodonos, A.; Malnick, S.; Kriger, O.; Wilkof-Segev, R.; Delgado, B.; Novack, V.; Rosenthal, A.; Menachem, Y.; Melzer, E.; et al. Autoimmune hepatitis in southern Israel: A 15-year multicenter study. J. Dig. Dis. 2013, 14, 611–618. [Google Scholar] [CrossRef]
- Czaja, A.J. Autoimmune hepatitis in diverse ethnic populations and geographical regions. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 365–385. [Google Scholar] [CrossRef]
- Lammert, C.; Chalasani, S.N.; Atkinson, E.J.; McCauley, B.M.; Lazaridis, K.N. Environmental risk factors are associated with autoimmune hepatitis. Liver Int. Off. J. Int. Assoc. Study Liver 2021, 41, 2396–2403. [Google Scholar] [CrossRef]
- Czaja, A.J.; Souto, E.O.; Bittencourt, P.L.; Cancado, E.L.; Porta, G.; Goldberg, A.C.; Donaldson, P.T. Clinical distinctions and pathogenic implications of type 1 autoimmune hepatitis in Brazil and the United States. J. Hepatol. 2002, 37, 302–308. [Google Scholar] [CrossRef]
- Malekzadeh, Z.; Haghazali, S.; Sepanlou, S.G.; Vahedi, H.; Merat, S.; Sotoudeh, M.; Nasseri-Moghaddam, S.; Malekzadeh, R. Clinical features and long term outcome of 102 treated autoimmune hepatitis patients. Hepat. Mon. 2012, 12, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J. Global Disparities and Their Implications in the Occurrence and Outcome of Autoimmune Hepatitis. Dig. Dis. Sci. 2017, 62, 2277–2292. [Google Scholar] [CrossRef] [PubMed]
- Homberg, J.C.; Abuaf, N.; Bernard, O.; Islam, S.; Alvarez, F.; Khalil, S.H.; Poupon, R.; Darnis, F.; Lévy, V.G.; Grippon, P.; et al. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: A second type of “autoimmune” hepatitis. Hepatology 1987, 7, 1333–1339. [Google Scholar] [CrossRef]
- Czaja, A.J. Performance parameters of the conventional serological markers for autoimmune hepatitis. Dig. Dis. Sci. 2011, 56, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Amarapurkar, D.; Dharod, M.; Amarapurkar, A. Autoimmune hepatitis in India: Single tertiary referral centre experience. Trop. Gastroenterol. Off. J. Dig. Dis. Found. 2015, 36, 36–45. [Google Scholar] [CrossRef]
- Karakoyun, M.; Ecevit, C.O.; Kilicoglu, E.; Aydogdu, S.; Yagci, R.V.; Ozgenc, F. Autoimmune hepatitis and long-term disease course in children in Turkey, a single-center experience. Eur. J. Gastroenterol. Hepatol. 2016, 28, 927–930. [Google Scholar] [CrossRef]
- Czaja, A.J.; Freese, D.K. Diagnosis and treatment of autoimmune hepatitis. Hepatology 2002, 36, 479–497. [Google Scholar] [CrossRef]
- Czaja, A.J.; Manns, M.P.; Homburger, H.A. Frequency and significance of antibodies to liver/kidney microsome type 1 in adults with chronic active hepatitis. Gastroenterology 1992, 103, 1290–1295. [Google Scholar] [CrossRef]
- Zahiruddin, A.; Farahmand, A.; Gaglio, P.; Massoumi, H. Clinical characteristics and response to therapy of autoimmune hepatitis in an urban Latino population. Gastroenterol. Hepatol. Bed Bench 2016, 9, 225–230. [Google Scholar]
- Sherigar, J.M.; Yavgeniy, A.; Guss, D.; Ngo, N.; Mohanty, S. Seronegative Autoimmune Hepatitis A Clinically Challenging Difficult Diagnosis. Case Rep. Med. 2017, 2017, 3516234. [Google Scholar] [CrossRef] [PubMed]
- Gadour, E. Autoimmune Hepatitis: Treatment Options and Management Review. Cureus 2021, 13, e15682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-X.; Yan, L.; Ma, X. Autoimmune Hepatitis in the Asia-Pacific Area. J. Clin. Transl. Hepatol. 2018, 6, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Talari, K.; Goyal, M. Retrospective studies-utility and caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef]

| Variable | Type 1 AIH | Type 2 AIH | AIH-PBC 12 Overlap Syndrome | Seronegative AIH | p Value 13 |
|---|---|---|---|---|---|
| Age | 45, 43 (32–61) | 32, 32 (21–43) | 45, 45 (34–55) | 43, 39 (35–53) | 0.816 |
| BMI 1 | 25.2, 25.8 (21.4–27.7) | 25.8, 25.8 (22.9–28.7) | 24.8, 24.8 (18.4–31.2) | 25.6, 23.3 (21–30.2) | 0.998 |
| ALT 2 | 390.9, 298 (61–667) | 1044.4, 1044.4 (893.6–1195.2) | 78.6, 78.6 (65–92.2) | 435.7, 195.9 (63–288) | 0.245 |
| AST 3 | 396.5, 337.2 (50–620) | 996.4, 996.4 (695.2–1297.5) | 52.5, 52.5 (30–74.9) | 454.1, 203.4 (46–357) | 0.209 |
| GGT 4 | 259, 197.3 (100–269) | 131, 131 (106–156) | 618, 618 (371–865) | 561.1, 128 (71–234) | 0.351 |
| ALP 5 | 233.2, 190 (124–294) | 205, 205 (139–271) | 247, 247 (117–377) | 204.8, 214 (168–232) | 0.979 |
| Total Bilirubin (mg/dL) | 5.761, 5.458 (1.09–8.2) | 8.626, 8.626 (2.975–14.276) | 0.53, 0.53 (0.5–0.56) | 11.274, 1.9 (1.45–20.98) | 0.213 |
| Direct Bilirubin (mg/dL) | 4.601, 3.92 (0.44–7.19) | 7.163, 7.163 (1.718–12.607) | 0.205, 0.205 (0.1–0.31) | 9.452, 1.6 (0.62–18.7) | 0.271 |
| PT 6 | 17.1, 16.3 (14.6–18.2) | 17.8, 17.8 (16.6–19) | 12.6, 12.6 (12.1–13) | 15.8, 14.3 (13.6–16.6) | 0.083 |
| PTT 7 | 34.1, 33.1 (30.1–36.4) | 35.2, 35.2 (33.1–37.3) | 29, 29 (28.4–29.5) | 34.3, 33.6 (30.8–38.5) | 0.446 |
| INR 8 | 1.35, 1.32 (1.12–1.41) | 1.42, 1.42 (1.25–1.59) | 0.93, 0.93 (0.89–0.97) | 1.2, 1.13 (1.03–1.3) | 0.080 |
| Albumin (g/dL) | 3.56, 3.8 (3.29–3.9) | 3.25, 3.25 (2.52–3.98) | 4.4, 4.4 (4.3–4.5) | 3.76, 3.8 (3.4–4.3) | 0.155 |
| Creatinine (mg/dL) | 0.42, 0.42 (0.3–0.49) | 0.62, 0.62 (0.5–0.74) | 0.63, 0.63 (0.58–0.68) | 0.5, 0.4 (0.14–0.46) | 0.135 |
| BUN 9 | 23.6, 21 (17–26) | 24, 24 (15.8–32.3) | 28.6, 28.6 (28.1–29) | 24.2, 20 (13–25) | 0.671 |
| GFR 10 | 135, 130 (119–155) | 118.5, 118.5 (99–138) | 109, 109 (104–114) | 142.9, 125.5 (114–160.5) | 0.426 |
| IgG 11 | 20.89, 19.56 (14.2–26.67) | 22.37, 22.37 (17.72–27.02) | 13.52, 13.52 (8.97–18.08) | 15.19, 15.84 (13.01–19.12) | 0.253 |
| Feature | Degree/Class | Number (%) |
|---|---|---|
| Presence of interface hepatitis | 18 (94.7%) | |
| Degree of interface hepatitis | Mild | 8 (42.1%) |
| Moderate | 9 (47.4%) | |
| Severe | 1 (5.3%) | |
| Presence of bridging necrosis | 9 (52.6%) | |
| Presence of lymphoplasmacytic infiltrate | 18 (94.7%) | |
| Degree of lymphoplasmacytic infiltrate | Mild | 4 (21.0%) |
| Moderate | 12 (63.2%) | |
| Severe | 2 (10.5%) | |
| Presence of rosette pattern | 3 (15.8%) | |
| Fibrosis score | F0 | 2 (10.5%) |
| F1 | 7 (36.8%) | |
| F2 | 6 (31.6%) | |
| F3 | 4 (21.0%) | |
| F4 | 0 | |
| Grade of inflammation | A0 | 1 (5.3%) |
| A1 | 3 (15.8%) | |
| A2 | 11 (57.9%) | |
| A3 | 4 (21.0%) |
| Outcome | Biochemical Remission at Six Months | Incomplete Response at Six Months | Failed Response at Six Months | Biochemical Remission at One Year | Incomplete Response at One Year | Failed Response at One Year |
|---|---|---|---|---|---|---|
| Total | 20 (100%) | 4 (100%) | 2 (100%) | 15 (100%) | - | 4 (100%) |
| Type 1 AIH 1 | 11 (55%) | 3 (75%) | 2 (100%) | 12 (80%) | - | 1 (25%) |
| Type 2 AIH | 1 (5%) | 1 (25%) | - | 0 (%) | - | - |
| Seronegative AIH | 8 (40%) | - | - | 3 (20%) | - | 3 (75%) |
| Acute presentation | 11 (55%) | 4 (100%) | 2 (100%) | 8 (53%) | - | 4 (100%) |
| Azathioprine at induction | 3 (15%) | 2 (50%) | 0 (0%) | 4 (27%) | - | 0 (0%) |
| Mean ALT at diagnosis 2 | 358.6 ± 389.6 | 594.5 ± 466.0 | 485.6 ± 401.1 | 291.3 ± 362.4 | - | 424.0 ± 451.9 |
| Mean AST at diagnosis 3 | 378.3 ± 440.5 | 470.9 ± 285.5 | 387.5 ± 266.6 | 265.7 ± 308.6 | - | 470.9 ± 580.7 |
| Total bilirubin at diagnosis 4 | 6.9 ± 7.7 | 4.5 ± 3.5 | 4.2 ± 3.5 | 4.1 ± 4.3 | - | 5.9 ± 7.1 |
| Direct bilirubin at diagnosis | 5.6 ± 6.7 | 3.5 ± 3.1 | 3.4 ± 3.1 | 3.13 ± 4.0 | - | 5.1 ± 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamimi, T.A.; Sallam, M.; Rayyan, D.; Farah, R.; Alkhulaifat, D.; Al-Ani, A.; Elmusa, R.; Sharawi, S.; Tanash, O.; Rayyan, Y. Clinical Characteristics of Autoimmune Hepatitis in a Middle Eastern Population: A Tertiary Care Center Experience. J. Clin. Med. 2023, 12, 629. https://doi.org/10.3390/jcm12020629
Tamimi TA, Sallam M, Rayyan D, Farah R, Alkhulaifat D, Al-Ani A, Elmusa R, Sharawi S, Tanash O, Rayyan Y. Clinical Characteristics of Autoimmune Hepatitis in a Middle Eastern Population: A Tertiary Care Center Experience. Journal of Clinical Medicine. 2023; 12(2):629. https://doi.org/10.3390/jcm12020629
Chicago/Turabian StyleTamimi, Tarek A., Malik Sallam, Deema Rayyan, Randa Farah, Dana Alkhulaifat, Abdallah Al-Ani, Reem Elmusa, Said Sharawi, Omar Tanash, and Yaser Rayyan. 2023. "Clinical Characteristics of Autoimmune Hepatitis in a Middle Eastern Population: A Tertiary Care Center Experience" Journal of Clinical Medicine 12, no. 2: 629. https://doi.org/10.3390/jcm12020629
APA StyleTamimi, T. A., Sallam, M., Rayyan, D., Farah, R., Alkhulaifat, D., Al-Ani, A., Elmusa, R., Sharawi, S., Tanash, O., & Rayyan, Y. (2023). Clinical Characteristics of Autoimmune Hepatitis in a Middle Eastern Population: A Tertiary Care Center Experience. Journal of Clinical Medicine, 12(2), 629. https://doi.org/10.3390/jcm12020629








