Haematopoietic Stem Cell Transplantation for Chronic Granulomatous Disease
Abstract
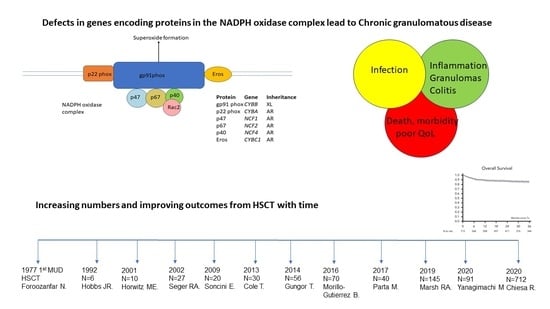
:1. Introduction
2. Pathophysiology and Clinical Outcome
3. Haematopoietic Stem Cell Transplantation for Chronic Granulomatous Disease—Historical Perspective
4. Haematopoietic Stem Cell Transplantation for Chronic Granulomatous Disease—The Modern Era
5. Most Appropriate Pre-Transplant Conditioning Regimen
6. Donor Selection
7. X-Linked Chronic Granulomatous Disease—Special Groups
7.1. Mcleod Phenotype
7.2. X-Linked-Carriers
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Janeway, C.A.; Craig, J.; Davidson, M.; Downey, W.; Gitlin, D.; Sullivan, J.C. Hypergammaglobulinemia associated with severe, recurrent and chronic non-specific infection. Am. J. Dis. Child. 1954, 88, 388–392. [Google Scholar]
- Bridges, R.A.; Berendes, H.; Good, R.A. A fatal granulomatous disease of childhood; The clinical, pathological, and laboratory features of a new syndrome. A.M.A. J. Dis. Child. 1959, 97, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 24, 1473–1507. [Google Scholar] [CrossRef] [PubMed]
- Salvator, H.; Mahlaoui, N.; Catherinot, E.; Rivaud, E.; Pilmis, B.; Borie, R.; Crestani, B.; Tcherakian, C.; Suarez, F.; Dunogue, B.; et al. Pulmonary manifestations in adult patients with chronic granulomatous disease. Eur. Respir. J. 2015, 45, 1613–1623. [Google Scholar] [CrossRef]
- Marciano, B.E.; Spalding, C.; Fitzgerald, A.; Mann, D.; Brown, T.; Osgood, S.; Yockey, L.; Darnell, D.N.; Barnhart, L.; Daub, J.; et al. Common severe infections in chronic granu-lomatous disease. Clin. Infect. Dis. 2015, 60, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.B.K.R.; McGrogan, P.; Flood, T.J.; Gennery, A.R.; Morton, L.; Thrasher, A.; Goldblatt, D.; Parker, L.; Cant, A.J. Special Article: Chronic granulomatous disease in the United Kingdom and Ireland: A comprehensive national patient-based registry. Clin. Exp. Immunol. 2008, 152, 211–218. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.M.; van Koppen, E.; Åhlin, A.; Belohradsky, B.H.; Bernatowska, E.; Corbeel, L.; Español, T.; Fischer, A.; Kurenko-Deptuch, M.; Mouy, R.; et al. Chronic Granulomatous Disease: The European Experience. PLoS ONE 2009, 4, e5234. [Google Scholar] [CrossRef]
- Mouy, R.; Fischer, A.; Vilmer, E.; Seger, R.; Griscelli, C. Incidence, severity, and prevention of infections in chronic granulom-atous disease. J. Pediatr. 1989, 114, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Martire, B.; Rondelli, R.; Soresina, A.; Pignata, C.; Broccoletti, T.; Finocchi, A.; Rossi, P.; Gattorno, M.; Rabusin, M.; Azzari, C.; et al. Clinical features, long-term follow-up and outcome of a large cohort of patients with Chronic Granulomatous Disease: An Italian multicenter study. Clin. Immunol. 2008, 126, 155–164. [Google Scholar] [CrossRef]
- Bach, F.; Albertini, R.; Joo, P.; Anderson, J.; Bortin, M. Bone-marrow transplantation in a patient with the wiskott-aldrich syndrome. Lancet 1968, 292, 1364–1366. [Google Scholar] [CrossRef]
- Gatti, R.A.; Meuwissen, H.J.; Allen, H.D.; Hong, R.; Good, R.A. Immunological reconstitution of sex-linked lymphopenic immu-nological deficiency. Lancet 1968, 292, 1366–1369. [Google Scholar] [CrossRef]
- De Koning, J.; Van Bekkum, D.; Dicke, K.; Dooren, L.; Van Rood, J.; Rádl, J. Transplantation of bone-marrow cells and fetal thymus in an infant with lymphopenic immunological deficiency. Lancet 1969, 293, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Foroozanfar, N.; Hobbs, J.R.; Hugh-Jones, K.; Humble, J.G.; James, D.C.O.; Selwyn, S.; Watson, J.G.; Yamamura, M. Bone marrow transplantation from an unrelated donor for chronic granulomatous disease. Lancet 1977, 309, 210–213. [Google Scholar]
- Hobbs, J.R.; Monteil, M.; McCluskey, D.R.; Jurges, E.; El Tumi, M. Chronic granulomatous disease 100% corrected by displacement bone marrow transplantation from a volunteer unrelated donor. Eur. J. Pediatr. 1992, 151, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.E.; Barrett, A.J.; Brown, M.R.; Carter, C.S.; Childs, R.; Gallin, J.I.; Gress, R.E.; Holland, S.M.; Linton, G.F.; Miller, J.A.; et al. Treatment of Chronic Granulomatous Disease with Nonmyeloablative Conditioning and a T-Cell–Depleted Hematopoietic Allograft. N. Engl. J. Med. 2001, 344, 881–888. [Google Scholar] [CrossRef]
- Seger, R.A.; Gungor, T.; Belohradsky, B.H.; Blanche, S.; Bordigoni, P.; Di Bartolomeo, P.; Flood, T.; Landais, P.; Müller, S.; Ozsahin, H.; et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: A survey of the European experience, 1985–2000. Blood 2002, 100, 4344–4350. [Google Scholar] [CrossRef] [PubMed]
- Soncini, E.; Slatter, M.A.; Jones, L.B.K.R.; Hughes, S.; Flood, T.J.; Barge, D.; Spickett, G.P.; Jackson, G.H.; Collin, M.P.; Abinun, M.; et al. Haematopoeitic Stem cell Transplantation for Chronic Granulomatous Disease: Long Term Outcome and Growth—A single Centre Experience. Br. J. Haematol. 2009, 145, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.; Pearce, M.S.; Cant, A.J.; Cale, C.M.; Goldblatt, D.; Gennery, A.R. Clinical outcome in children with Chronic Granulomatous Disease managed conservatively or with haematopoietic stem cell transplant. J. Allergy Clin. Immunol. 2013, 132, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.C.; Di Colo, G.; Dattani, V.; Braggins, H.; Kumararatne, D.; Williams, A.P.; Alachkar, H.; Jolles, S.; Battersby, A.; Cole, T.; et al. Long-term outcomes for adults with chronic granulomatous disease in the United Kingdom. J. Allergy Clin. Immunol. 2021, 147, 1104–1107. [Google Scholar] [CrossRef]
- Chiesa, R.; Wang, J.; Blok, H.-J.; Hazelaar, S.; Neven, B.; Moshous, D.; Schulz, A.; Hoenig, M.; Hauck, F.; Al Seraihy, A.; et al. Hematopoietic cell transplantation in chronic granulomatous disease: A study of 712 children and adults. Blood 2020, 136, 1201–1211. [Google Scholar] [CrossRef]
- Lankester, A.C.; Albert, M.H.; Booth, C.; Gennery, A.R.; Güngör, T.; Hönig, M.; Morris, E.C.; Moshous, D.; Neven, B.; Schulz, A.; et al. EBMT/ESID inborn errors working party guidelines for hematopoietic stem cell transplantation for inborn errors of immunity. Bone Marrow Transplant. 2021, 56, 2052–2062. [Google Scholar] [CrossRef]
- Yanagimachi, M.; Kato, K.; Iguchi, A.; Sasaki, K.; Kiyotani, C.; Koh, K.; Koike, T.; Sano, H.; Shigemura, T.; Muramatsu, H.; et al. Hematopoietic Cell Transplantation for Chronic Granulomatous Disease in Japan. Front. Immunol. 2020, 11, 1617. [Google Scholar] [CrossRef] [PubMed]
- Leiding, J.W.; Arnold, D.E.; Parikh, S.H.; Logan, B.R.; Marsh, R.A.; Griffith, L.M.; Wu, R.; Kidd, S.; Mallhi, K.K.; Chellapandian, D.; et al. Genotype, Oxidase Status, and Preceding Infection or Autoinflammation Do Not Affect Allogeneic HCT Outcomes for CGD. Blood 2023, in press. [Google Scholar] [CrossRef]
- Marsh, R.A.; Leiding, J.W.; Logan, B.R.; Griffith, L.M.; Arnold, D.E.; Haddad, E.; Falcone, E.L.; Yin, Z.; Patel, K.; Airbuckle, E.; et al. submitted on behalf of the Primary Immune Deficiency Treatment Consortium. Chronic Granulomatous Disease-Associated IBD Resolves and Does Not Adversely Impact Survival Following Allogeneic HCT. J. Clin. Immunol. 2019, 39, 653–667. [Google Scholar] [CrossRef]
- Güngör, T.; Teira, P.; Slatter, M.; Stussi, G.; Stepensky, P.; Moshous, D.; Vermont, C.; Ahmad, I.; Shaw, P.J.; da Cunha, J.M.T.; et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: A prospective multicentre study. Lancet 2014, 383, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Morillo-Gutierrez, B.; Beier, R.; Rao, K.; Burroughs, L.; Schulz, A.; Ewins, A.-M.; Gibson, B.; Sedlacek, P.; Krol, L.; Strahm, B.; et al. Treosulfan-based conditioning for allogeneic HSCT in children with chronic granulomatous disease: A multicenter experience. Blood 2016, 128, 440–448. [Google Scholar] [CrossRef]
- Parta, M.; Kelly, C.; Kwatemaa, N.; Theobald, N.; Hilligoss, D.; Qin, J.; Kuhns, D.B.; Zerbe, C.; Holland, S.M.; Malech, H.; et al. Allogeneic Reduced-Intensity Hematopoietic Stem Cell Transplantation for Chronic Granulomatous Disease: A Single-Center Prospective Trial. J. Clin. Immunol. 2017, 37, 548–558. [Google Scholar] [CrossRef]
- Slatter, M.A.; Maschan, M.A.; Gennery, A.R. T-lymphocyte depleted transplants for inborn errors of immunity. Exp. Rev. Clin. Immunol. 2023, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lum, S.H.; Flood, T.; Hambleton, S.; Abinun, M.; Owens, S.; Cigrovski, N.; Cant, A.; Gennery, A.R.; Slatter, M. Two decades of excellent transplant survival in children with chronic granulomatous disease: A report from a supraregional immunology transplant center in Europe. Blood 2019, 133, 2546–2549. [Google Scholar] [CrossRef]
- Parta, M.; Hilligoss, D.; Kelly, C.; Kwatemaa, N.; Theobald, N.; Zerbe, C.S.; Holland, S.M.; Malech, H.L.; Kang, E.M. Failure to Prevent Severe Graft-Versus-Host Disease in Haploidentical Hematopoietic Cell Transplantation with Post-Transplant Cyclophosphamide in Chronic Granulomatous Disease. J. Clin. Immunol. 2020, 40, 619–624. [Google Scholar] [CrossRef]
- Lhomme, F.; Peyrard, T.; Babinet, J.; Abou-Chahla, W.; Durieu, I.; Moshous, D.; Neven, B.; Rohrlich, P.-S.; Albinni, S.; Amiranoff, D.; et al. Chronic Granulomatous Disease with the McLeod Phenotype: A French National Retrospective Case Series. J. Clin. Immunol. 2020, 40, 752–762. [Google Scholar] [CrossRef]
- Battersby, A.C.; Braggins, H.; Pearce, M.S.; McKendrick, F.; Campbell, M.; Burns, S.; Cale, C.M.; Goldblatt, D.; Gennery, A.R. Health-Related Quality of Life and Emotional Health in X-Linked Carriers of Chronic Granulomatous Disease in the United Kingdom. J. Clin. Immunol. 2019, 39, 195–199. [Google Scholar] [CrossRef]
- Battersby, A.; Martin, A.J.; Tarn, J.; Ng, W.F.; Cale, C.; Goldblatt, D.; Gennery, A.R. Raised serum IL-8 levels are associated with excessive fatigue in female carriers of X-linked Chronic Granulomatous Disease in the United Kingdom. J. Clin. Immunol. 2017, 37, 279–281. [Google Scholar] [CrossRef]
- Marciano, B.E.; Zerbe, C.S.; Falcone, E.L.; Ding, L.; DeRavin, S.S.; Daub, J.; Kreuzburg, S.; Yockey, L.; Hunsberger, S.; Foruraghi, L.; et al. X-linked carriers of chronic granulomatous disease: Illness, lyonization, and stability. J. Allergy Clin. Immunol. 2018, 141, 365–371. [Google Scholar] [CrossRef]
- Tsilifis, C.; Torppa, T.; Williams, E.J.; Albert, M.H.; Hauck, F.; Soncini, E.; Kang, E.; Malech, H.; Schuetz, C.; von Bernuth, H.; et al. Allogeneic HSCT for Symptomatic Female X-linked Chronic Granulomatous Disease Carriers. J. Clin. Immunol. 2023, in press. [Google Scholar] [CrossRef]
- Perez-Heras, I.; Tsilifis, C.; Slatter, M.A.; Brynjólfsson, S.F.; Haraldsson, Á.; Gennery, A.R. HSCT in two brothers with CGD arising from mutations in CYBC1 corrects the defect in neutrophil function. Clin. Immunol. 2021, 229, 108799. [Google Scholar] [CrossRef]
- Cole, T.; McKendrick, F.; Titman, P.; Cant, A.J.; Pearce, M.S.; Cale, C.M.; Goldblatt, D.; Gennery, A.R. Health related quality of life and emotional health in children with chronic granulomatous disease: A comparison of those managed conservatively with those that have un-dergone haematopoietic stem cell transplant. J. Clin. Immunol. 2013, 33, 8–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slatter, M.A.; Gennery, A.R. Haematopoietic Stem Cell Transplantation for Chronic Granulomatous Disease. J. Clin. Med. 2023, 12, 6083. https://doi.org/10.3390/jcm12186083
Slatter MA, Gennery AR. Haematopoietic Stem Cell Transplantation for Chronic Granulomatous Disease. Journal of Clinical Medicine. 2023; 12(18):6083. https://doi.org/10.3390/jcm12186083
Chicago/Turabian StyleSlatter, M. A., and A. R. Gennery. 2023. "Haematopoietic Stem Cell Transplantation for Chronic Granulomatous Disease" Journal of Clinical Medicine 12, no. 18: 6083. https://doi.org/10.3390/jcm12186083