The Use of Capacitive and Resistive Energy Transfer in Postpartum Pain Management in Women after Perineal Trauma
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Examination, Data Collecting
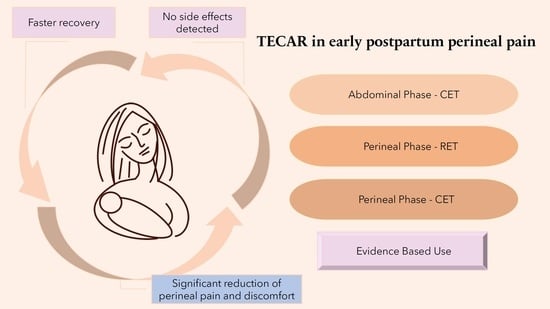
2.3. Intervention
2.4. Statistical Methods
3. Results
3.1. Characteristics of the Study Groups
3.2. Perineal Injuries
3.3. Medications Intake
3.4. Pain and Discomfort Level Outcomes
3.4.1. Between the Study Groups Analysis
3.4.2. Within the Study Groups Analysis
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leifer, G. Maternity Nursing an Introductory Text, Postpartum Assessment, and Nursing Care, 9th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2005; pp. 190–205. [Google Scholar]
- Karaçam, Z.; Eroǧlu, K. Effects of episiotomy on bonding and mothers’ health. J. Adv. Nurs. 2003, 43, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Kettle, C.; Tohill, S. Perineal care. BMJ Clin. Evid. 2008, 2008, 1401. [Google Scholar] [PubMed]
- Arnold, M.J.; Sadler, K.; Leli, K.A. Obstetric Lacerations: Prevention and Repair. Am. Fam. Physician 2021, 103, 745–752. [Google Scholar] [PubMed]
- Senol, D.K.; Aslan, E. The Effects of Cold Application to the Perineum on Pain Relief After Vaginal Birth. Asian Nurs. Res. 2017, 11, 276–282. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Basbaum, A.I. Spinal mechanisms of acute and persistent pain. Reg. Anesth. Pain Med. 1999, 24, 59–67. [Google Scholar] [CrossRef]
- Juárez, Á.; Ferretiz, G.; Rocha, A. From gate and neuromodulation to neuromatrix. Rev. Chil. Anest. 2019, 48, 288–297. [Google Scholar]
- Trout, K.K. The neuromatrix theory of pain: Implications for selected nonpharmacologic methods of pain relief for labor. J. Midwifery Women’s Health 2004, 49, 482–488. [Google Scholar] [CrossRef]
- Gulliver, B.G.; Fisher, J.; Roberts, L. A New Way to Assess Pain in Laboring Women: Replacing the Rating Scale With a “Coping” Algorithm. Nurs. Women’s Health 2008, 12, 404–408. [Google Scholar] [CrossRef]
- Simkin, P.; Bolding, A. Update on nonpharmacologic approaches to relieve labor pain and prevent suffering. J. Midwifery Women’s Health 2004, 49, 489–504. [Google Scholar] [CrossRef]
- Shepherd, E.; Grivell, R.M. Aspirin (single dose) for perineal pain in the early postpartum period. Cochrane Database Syst. Rev. 2022, 7, CD012129. Cochrane Database Syst. Rev. 2022, 7, CD012129. [Google Scholar]
- Gutton, C.; Bellefleur, J.P.; Puppo, S.; Brunet, J.; Antonini, F.; Leone, M.; Bretelle, F. Lidocaine versus ropivacaine for perineal infiltration post-episiotomy. Int. J. Gynecol. Obstet. 2013, 122, 33–36. [Google Scholar] [CrossRef]
- Eshkevari, L.; Trout, K.K.; Damore, J. Management of postpartum pain. J. Midwifery Women’s Health 2013, 58, 622–631. [Google Scholar] [CrossRef]
- Pitangui, A.C.R.; de Sousa, L.; Gomes, F.A.; Ferreira, C.H.J.; Nakano, A.M.S. High-frequency TENS in post-episiotomy pain relief in primiparous puerpere: A randomized, controlled trial. J. Obstet. Gynaecol. Res. 2012, 38, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Bretelle, F.; Fabre, C.; Golka, M.; Pauly, V.; Roth, B.; Bechadergue, V.; Blanc, J. Capacitive-resistive radiofrequency therapy to treat postpartum perineal pain: A randomized study. PLoS ONE 2020, 15, e0231869. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Maccagnano, G.; Gallone, M.F.; Covelli, I.; Tafuri, S.; Moretti, B. Short term efficacy of capacitive-resistive diathermy therapy in patients with low back pain: A prospective randomized controlled trial. J. Biol. Regul. Homeost. Agents 2017, 31, 509–515. [Google Scholar]
- De Sousa-De Sousa, L.; Tebar Sanchez, C.; Maté-Muñoz, J.L.; Hernández-Lougedo, J.; Barba, M.; Lozano-Estevan, M.D.C.; Garnacho-Castaño, M.V.; García-Fernández, P. Application of capacitive-resistive electric transfer in physiotherapeutic clinical practice and sports. Int. J. Environ. Res. Public Health 2021, 18, 12446. [Google Scholar] [CrossRef]
- Pavone, C.; Castrianni, D.; Romeo, S.; Napoli, E.; Usala, M.; Gambino, G.; Scaturro, D. TECAR therapy for Peyronie’s disease: A phase-one prospective study. Great evidence in patients with erectile dysfunction. Urologia 2013, 80, 148–153. [Google Scholar]
- Cooksey, F.S. Discussion on the role of physiotherapy in the prevention and treatment of post-natal disorders. Proc. R. Soc. Med. 1950, 43, 741–745. [Google Scholar]
- Krusen, F.H. The present of status of short wave diathermy. J. Am. Med. Assoc. 1938, 110, 1280–1287. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Medel, E.; Colastra, C.; Roldán, R.; Úbeda, A. Response of neuroblastoma cells to RF currents as a function of the signal frequency. BMC Cancer 2019, 19, 889. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Paíno, C.L.; Trillo, M.Á.; Úbeda, A. Electric Stimulation at 448 kHz Promotes Proliferation of Human Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2014, 34, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Martínez-Botas, J.; Trillo, M.Á.; Paíno, C.L.; Úbeda, A. Antiadipogenic effects of subthermal electric stimulation at 448 kHz on differentiating human mesenchymal stem cells. Mol. Med. Rep. 2016, 13, 3895–3903. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weber, T.; Kabelka, B. Noninvasive monopolar capacitive-coupled radiofrequency for the treatment of pain associated with lateral elbow tendinopathies: 1-year follow-up. PM&R 2012, 4, 176–181. [Google Scholar]
- Clijsen, R.; Leoni, D.; Schneebeli, A.; Cescon, C.; Soldini, E.; Li, L.; Barbero, M. Does the Application of Tecar Therapy Affect Temperature and Perfusion of Skin and Muscle Microcirculation? A pilot feasibility study on healthy subjects. J. Altern. Complement. Med. 2020, 26, 147–153. [Google Scholar]
- Kumaran, B.; Watson, T. Thermal build-up, decay and retention responses to local therapeutic application of 448 kHz capacitive resistive monopolar radiofrequency: A prospective randomised crossover study in healthy adults. Int. J. Hyperthermia 2015, 31, 883–895. [Google Scholar] [CrossRef]
- Klovning, A.; Avery, K.; Sandvik, H.; Hunskaar, S. Comparison of two questionnaires for assessing the severity of urinary incontinence: The ICIQ-UI SF versus the incontinence severity index. Neurourol. Urodyn. 2009, 28, 411–415. [Google Scholar] [CrossRef]
- Laycock, J.O.; Jerwood, D. Pelvic floor muscle assessment: The PERFECT scheme. Physiotherapy 2001, 87, 631–642. [Google Scholar] [CrossRef]
- González-Gutiérrez, M.D.; López-Garrido, Á.; Cortés-Pérez, I.; Obrero-Gaitán, E.; León-Morillas, F.; Ibáñez-Vera, A.J. Effects of non-invasive radiofrequency diathermy in pelvic floor disorders: A systematic review. Medicina 2022, 58, 437. [Google Scholar] [CrossRef]
- Martensson, L.; Bergh, I. Effect of treatment for labor pain: Verbal reports versus visual analogue scale scores-A prospective randomized study. Int. J. Nurs. Midwifery 2011, 3, 43–47. [Google Scholar]
- OECD. Average Length of Stay: Childbirth. Health: Key Tables from OECD, No. 51. 2014. Available online: https://www.oecd-ilibrary.org/social-issues-migration-health/average-length-of-stay-childbirth-2014-1_l-o-s-childbirth-table-2014-1-en (accessed on 30 June 2014).
- Beltrame, R.; Ronconi, G.; Ferrara, P.E.; Salgovic, L.; Vercelli, S.; Solaro, C.; Ferriero, G. Capacitive and resistive electric transfer therapy in rehabilitation: A systematic review. Int. J. Rehabil. Res. 2020, 43, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Duessen, A.; Ashwood, P.; Martis, R. Anesthesia for relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst. Rev. 2011, CD004908. [Google Scholar]
- Fahey, J.O. Best practices in management of postpartum pain. J. Perinat. Neonatal Nurs. 2017, 31, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Andrews, V.; Thakar, R.; Sultan, A.H.; Jones, P.W. Evaluation of postpartum perineal pain and dyspareunia—A prospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 152–156. [Google Scholar] [CrossRef]
- Batten, M.; Stevenson, E.; Zimmermann, D.; Isaacs, C. Implementation of a Hydrotherapy Protocol to Improve Postpartum Pain Management. J. Midwifery Women’s Health 2017, 62, 210–214. [Google Scholar] [CrossRef]
- Roskelly, M.; Wheeler, V. NSAIDs for Postpartum Perineal Pain. Am. Fam. Physician 2018, 97, 754C–754D. [Google Scholar]
- Vermelis, J.M.; Wassen, M.M.; Fiddelers, A.A.; Nijhuis, J.G.; Marcus, M.A. Prevalence and predictors of chronic pain after labor and delivery. Curr. Opin. Anesth. 2010, 23, 295–299. [Google Scholar] [CrossRef]






| Variables | Study Group (n = 62) | Sham Group (n = 59) | p-Value |
|---|---|---|---|
| Age (years) | 30.5 ± 3.8 | 31 ± 4.6 | 0.58 t |
| BMI (kg/m2) | 26 (24.3; 28.4) | 26.8 ± 3.8 | 0.84 w |
| Primaparas Multiparous | 32 (51.6%) 30 (48.4%) | 28 (47.5%) 31 (52.5%) | 0.78 c |
| I labor stage length (in hours) | 7 (4.1; 10) | 7 (4; 11.8) | 0.69 w |
| II labor stage length (in hours) | 1 (0.8; 1.5) | 1 (0.5; 1.5) | 0.20 w |
| Child weight (g) | 3400 (3055; 3582.5) | 3478.7 ± 461.9 | 0.16 w |
| Child length (cm) | 54 (52; 56) | 55 ± 2.4 | 0.08 w |
| Previous episiotomy | 21 (33.9%) | 24 (40.7%) | 0.56 c |
| Previous perineal tear | 4 (6.5%) | 6 (10.2%) | 0.85 c |
| Perineal Injury/Instrumental Delivery | Study Group (n = 62) | Sham Group (n = 59) | p-Value |
|---|---|---|---|
| Episiotomy | 54 (87.1%) | 51 (86.4%) | 1 c |
| 1st-degree perineal tear 2nd-degree perineal tear 3rd-degree perineal tear | 9 (14.5%) 6 (9.7%) 3 (4.8%) | 12 (20.3%) 2 (3.4%) 1 (1.7%) | 0.38 f |
| Vacuum delivery | 2 (3.2%) | 3 (5.1%) | 0.67 f |
| Forceps delivery | 0 (0%) | 0 (0%) | 1 f |
| Analgesics | Study Group (n = 62) | Sham Group (n = 59) | p-Value |
|---|---|---|---|
| Paracetamol (g) | |||
| 1st day | 0 (0; 1) | 0.2 (0; 1) | 0.52 w |
| 2nd day | 0 (0; 1) | 0 (0; 1) | 0.53 w |
| p-value | 0.33 w | 0.11 w | |
| Ibuprofen (g) | |||
| 1st day | 0.4 (0; 0.8) | 0.4 (0; 0.4) | 0.80 w |
| 2nd day | 0.4 (0; 0.4) | 0.4 (0; 0.4) | 0.72 w |
| p-value | 0.004 w | 0.08 w | |
| Variables | Study Group (n = 62) | Sham Group (n = 59) | p-Value w |
|---|---|---|---|
| Pain at rest (VAS) | |||
| Baseline | 5 (3; 6) | 3 (2; 5) | 0.01 |
| 1st intervention | 3 (2; 5) | 3 (2; 4) | 0.42 |
| 2nd intervention | 2.5 (2; 4) | 3 (2; 3.8) | 0.97 |
| Pain while sitting (VAS) | |||
| Baseline | 6 (4; 7) | 5 (3; 6) | 0.02 |
| 1st intervention | 4 (2; 6) | 4 (2; 5) | 0.62 |
| 2nd intervention | 3 (1.8; 5) | 3 (1.2; 4) | 0.55 |
| Pain while walking (VAS) | |||
| Baseline | 3.5 (2; 5) | 3 (0; 4.5) | 0.048 |
| 1st intervention | 2 (0; 4) | 2 (1; 4) | 0.61 |
| 2nd intervention | 2 (0; 3) | 2 (1; 3) | 0.90 |
| Discomfort while sitting (VAS) | |||
| Baseline | 6 (4.2; 7) | 5 (4; 6) | 0.01 |
| 1st intervention | 5 (3.2; 6) | 4 (3; 6) | 0.16 |
| 2nd intervention | 3 (2; 6) | 3 (2; 5) | 0.33 |
| Discomfort while walking (VAS) | |||
| Baseline | 4.5 (3; 6) | 3 (2; 5) | 0.02 |
| 1st intervention | 3 (2; 4.8) | 3 (2; 4.5) | 0.82 |
| 2nd intervention | 2 (1; 4) | 2 (1; 3) | 0.49 |
| Variables | ∆ Study Group (n = 62) Me (Min, Max) | ∆ Sham Group (n = 59) Me (Min, Max) | p-Value w |
|---|---|---|---|
| Pain at rest | |||
| 2 − baseline * | −2 (−3; −1) | −1 (−2; 0) | 0.045 |
| 1 − baseline ** | −1 (−2; 0) | 0 (−1.8; 1) | 0.049 |
| 2 − 1 *** | −1 (−1.2; 0) | −1 (−1; 0) | 0.86 |
| Pain while sitting | |||
| 2 − baseline | −2.5 (−4; −1) | −2 (−3; 0) | 0.15 |
| 1 − baseline | −1 (−3.2; 0) | −1 (−2; 0) | 0.13 |
| 2 − 1 | −1 (−2; 0) | −1 (−2; 0) | 0.6 |
| Pain while walking | |||
| 2 − baseline | −1 (−3; 0) | −1 (−2; 0) | 0.08 |
| 1 − baseline | −0.5 (−2;0) | 0 (−1; 1) | <0.01 |
| 2 − 1 | 0 (−1.2; 0) | −0.5 (−2; 0) | 0.26 |
| Discomfort while sitting | |||
| 2 − baseline | −2 (−4; 0) | −1 (−2.8; 0) | 0.26 |
| 1 − baseline | −1 (−3; 0) | −1 (−2; 0) | 0.52 |
| 2 − 1 | −1 (−2; 0) | −1 (−2; 0) | 0.49 |
| Discomfort while walking | |||
| 2 − baseline | −2 (−3; −1) | −1 (−2; 0) | 0.049 |
| 1 − baseline | −1 (−2; 0) | 0 (−1.8; 1) | 0.022 |
| 2 − 1 | −1 (−1; 0) | −1 (−2; 0) | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siereńska, J.; Sotomska, Z.; Madej-Łukasiak, D.; Wąż, P.; Grzybowska, M.E. The Use of Capacitive and Resistive Energy Transfer in Postpartum Pain Management in Women after Perineal Trauma. J. Clin. Med. 2023, 12, 6077. https://doi.org/10.3390/jcm12186077
Siereńska J, Sotomska Z, Madej-Łukasiak D, Wąż P, Grzybowska ME. The Use of Capacitive and Resistive Energy Transfer in Postpartum Pain Management in Women after Perineal Trauma. Journal of Clinical Medicine. 2023; 12(18):6077. https://doi.org/10.3390/jcm12186077
Chicago/Turabian StyleSiereńska, Joanna, Zofia Sotomska, Dorota Madej-Łukasiak, Piotr Wąż, and Magdalena Emilia Grzybowska. 2023. "The Use of Capacitive and Resistive Energy Transfer in Postpartum Pain Management in Women after Perineal Trauma" Journal of Clinical Medicine 12, no. 18: 6077. https://doi.org/10.3390/jcm12186077
APA StyleSiereńska, J., Sotomska, Z., Madej-Łukasiak, D., Wąż, P., & Grzybowska, M. E. (2023). The Use of Capacitive and Resistive Energy Transfer in Postpartum Pain Management in Women after Perineal Trauma. Journal of Clinical Medicine, 12(18), 6077. https://doi.org/10.3390/jcm12186077