Ultrasound-Guided Injections of HYADD4 for Knee Osteoarthritis Improves Pain and Functional Outcomes at 3, 6, and 12 Months without Changes in Measured Synovial Fluid, Serum Collagen Biomarkers, or Most Synovial Fluid Biomarker Proteins at 3 Months
Abstract
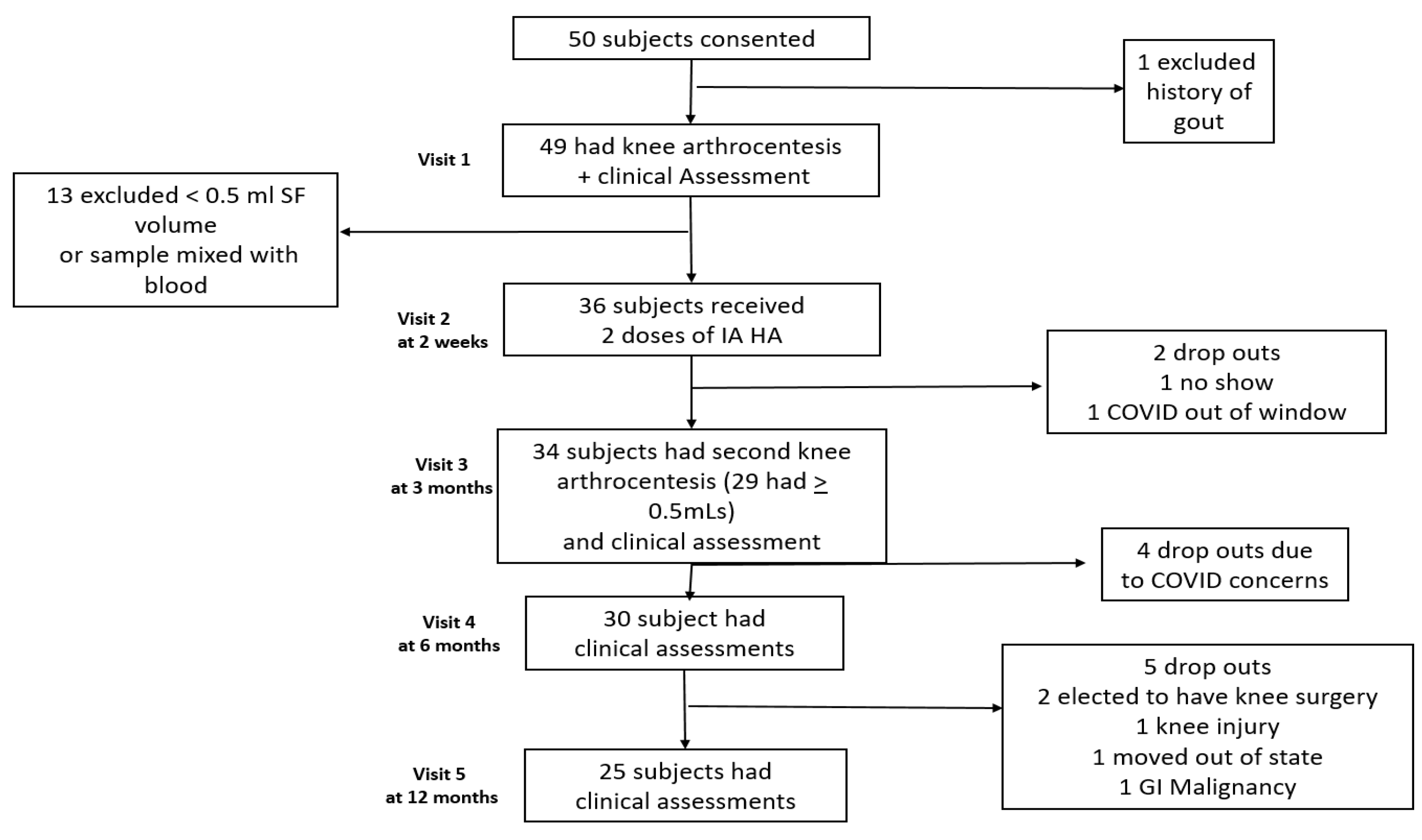
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Aspiration and Injection Technique
2.3. Sample Preparation and Analysis of SF and Serum Proteins
2.4. Clinical Efficacy Variables
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Injection and Aspiration Technique
4.2. SF Volume Measurements
4.3. Serum and SF Biomarkers
4.4. Strengths
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of hip and knee osteoarthritis A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- US Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers (CPI-U): U.S. City Average by Expenditure Cate-gory. Available online: https://www.bls.gov/news.release/cpi.t01.htm (accessed on 5 July 2023).
- Schieker, M.; Conaghan, P.G.; Mindeholm, L.; Praestgaard, J.; Solomon, D.H.; Scotti, C.; Gram, H.; Thuren, T.; Roubenoff, R.; Ridker, P.M. Effects of interleukin-1β inhibition on incident hip and knee replacement: Exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2020, 173, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Heijman, M.W.J.; Fiolet, A.T.J.; Mosterd, A.; Tijssen, J.G.P.; van den Bemt, B.J.F.; Schut, A.; Eikelboom, J.W.; Thompson, P.L.; van den Ende, C.H.M.; Nidorf, S.M.; et al. Association of low-dose colchicine with incidence of knee and hip replacements. Ann. Intern. Med. 2023, 176, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Acuna, A.J.; Samuel, L.T.; Grits, D.; Kamath, A.F. Hyaluronic acid injections for knee osteoarthritis, has utilization among Medicare beneficiaries changed between 2021 and 2018? J. Bone Jt. Surg. Am. 2022, 104, e43. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [PubMed]
- Ene, R.; Sinescu, R.D.; Ene, P.; Cirstoiu, M.M.; Cirstoiu, F.C. Synovial inflammation in patients with different stages of knee osteoarthritis. Rom. J. Morphol. Embryol. 2015, 56, 169–173. [Google Scholar]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Felson, D.T.; Niu, J.; Nevitt, M.C.; Crema, M.D.; Lynch, J.A.; Lewis, C.E.; Torner, J.; Zhang, Y. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30 months follow up: The MOST study. Ann. Rheum. Dis. 2011, 70, 1804–1809. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The Role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzi, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cell Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Mobasheri, A.; Bay-Jensen, A.C.; van Spil, W.E.; Larkin, J.; Levesque, M.C. Osteoarthritis year in review 2016: Biomarkers (biochemical markers). Osteoarthr. Cartil. 2017, 25, 199–208. [Google Scholar]
- Bay-Jenson, A.C.; Thudium, C.S.; Mobasheri, A. Development and use of biochemical markers in osteoarthritis: Current update. Cur. Opin. Rheumatol. 2018, 30, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.L.; Bruyere, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Republished: Value of biomarkers in osteoarthritis: Current status and perspectives. Postgrad. Med. J. 2014, 90, 171–178. [Google Scholar]
- Meehan, R.T.; Regan, E.A.; Hoffman, E.D.; Wolf, M.L.; Gill, M.T.; Crooks, J.L.; Parmar, P.J.; Scheuring, R.A.; Hill, J.C.; Pacheco, K.A.; et al. Synovial fluid cytokines, chemokines and MMP levels in osteoarthritis patients with knee pain display a profile similar to many Rheumatoid arthritis patients. J. Clin. Med. 2021, 10, 5027. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Mango, A.; Forefinger, A.; Nazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systemic review. BMC Musculoskelet. Discord. 2015, 16, 321–331. [Google Scholar] [CrossRef]
- Altman, R.; Beda, A.; Mango, A.; Nazi, F.; Shaw, P.; Meese, P. Anti-Inflammatory effects of intra-articular hyaluronic acid: A systemic review. Cartilage 2019, 10, 43–52. [Google Scholar] [CrossRef]
- Moreland, L.W. Intra-articular hyaluronic (hyaluronic acid) in Humans? For treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Bonassar, L.J. Frictional characterization of injectable hyaluronic acid is more predictive of clinical outcomes than traditional rheological or viscoelastic characterization. PLoS ONE 2019, 14, e0216702. [Google Scholar]
- Priano, F. Early efficacy of intra-articular HYADDR4 (HymovisR) injections for symptomatic knee osteoarthritis. Joints 2017, 5, 79–84. [Google Scholar]
- Berkoff, D.J.; Miller, L.E.; Block, J.E. Clinical utility of ultrasound guidance for intra-articular knee injections: A review. Clin. Interv. Aging 2012, 7, 89–95. [Google Scholar] [PubMed]
- Wu, T.; Dong, Y.; Song, H.x.; Fu, Y.; Li, J.H. Ultrasound-guided versus landmark in knee arthrocentesis: A systemic review. Semin. Arthritis Rheum. 2016, 45, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Meehan, R.; Wilson, C.; Hoffman, E.; Altimier, L.; Kaessner, M.; Regan, E.A. Ultrasound measurement of knee synovial fluid during external pneumatic compression. J. Orthop. Res. 2019, 37, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; Collins, J.E.; Hargrove, D.; Losina, E.; Nevitt, M.; Katz, J.N.; Wang, S.X.; Sandell, L.J.; Hoffman, S.C.; Hunter, D.J. Predictive validity of biochemical biomarkers in knee osteoarthritis: Data from the FNIH OA biomarkers consortium. Ann. Rheum. Dis. 2017, 76, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Ware, J.E.; Snow, K.; Klosinski, M.; Gandek, B. SF 36 Health Survey; Manual and Interpretations Guide; The Health Institute New England Medical Centre: Boston, MA, USA, 1993. [Google Scholar]
- Patel, A.A.; Donegan, D.; Albert, T. The 36-Item Short. J. Am. Acad. Orthop. Surg. 2007, 15, 126–134. [Google Scholar] [CrossRef]
- Pham, T.; van der Heijde, D.; Altman, R.D.; Anderson, J.J.; Bellamy, N.; Hochberg, M.; Simon, L.; Strand, V.; Woodworth, T.; Dougados, M. OMERACT-OARSI Initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. OsteoArthritis Cartil. 2004, 12, 389–399. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 5 July 2023).
- Maricar, N.; Parkes, M.J.; Callaghan, M.J.; Felson, D.T.; O’Neill, T.W. Where and how to inject the knee—A systemic review. Semin. Arthritis Rheum. 2013, 43, 195–203. [Google Scholar] [CrossRef]
- Iqbal, A.; Brahmabhatt, S.; Muruganandam, M.; Trost, J.R.; Farshami, F.J.; Cisneros, D.R.; Kiani, A.N.; McElwee, M.K.; Hayward, W.A.; Haseler, L.J.; et al. Extraction of Synovial fluid from the non-effusive pathologic knee with pneumatic compression. Authorea 2022. [Google Scholar] [CrossRef]
- Bhavsar, T.B.; Sibbitt, W.L.; Band, P.A.; Cabacungan, R.J.; Moore, T.S.; Salayandia, L.C.; Fields, R.A.; Kettwich, S.K.; Roldan, L.P.; Emil, N.S.; et al. Improvement in diagnostic and therapeutic arthrocentesis via constant compression. Clin. Rheumatol. 2018, 37, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Bisicchia, S.; Bernardi, G.; Tudisco, C. HYADD4 versus methylprednisolone acetate in symptomatic knee osteoarthritis: A single-centre single blind prospective randomized controlled clinical study with a 1 year follow up. Clin. Exp. Rheumatol. 2016, 34, 857–863. [Google Scholar] [PubMed]
- Benazzo, F.; Perticarnini, L.; Padolino, A.; Castelli, A.; Gifuni, P.; Lovato, M.; Manzini, C.; Giordan, N. A multi-centre, open label, long-term follow-up study to evaluate the benefits of a new viscoelastic hydrogel (HymovisR) in the treatment of knee osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 959–968. [Google Scholar]
- Hummer, C.D.; Angst, F.; Ngai, W.; Whittington, C.; Yoon, S.S.; Duarte, L.; Manitt, C.; Schemitsch, E. High molecular weight intraarticular hyaluronic acid for the treatment of knee osteoarthritis: A network meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 702. [Google Scholar] [CrossRef] [PubMed]
- Ferkel, E.; Manjoo, A.; Martins, D.; Bhandari, M.; Sethi, P.; Nicholls, M. Intra-articular Hyaluronic acid treatments review of product properties. Cartilage 2023. [Google Scholar] [CrossRef]
- Henrotin, Y.; Bannuru, R.; Malaise, M.; Ea, H.K.; Confavreux, C.; Bentin, J.; Urbin-Choffray, D.; Conrozier, T.; Brasseur, J.P.; Thomas, P.; et al. Hyaluronan derivative HYMOVISR increases cartilage volume and Type II collagen turnover in osteoarthritic knee: Data from MOKHA study. BMC Musculoskelet. Disord. 2019, 20, 293. [Google Scholar] [CrossRef]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis, a randomized clinical trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef]
- Latourte, A.; Rat, A.C.; Omorou, A.; Ngueyon-Sime, W.; Eymard, F.; Sellam, J.; Roux, C.; Ea, H.K.; Cohen-Solal, M.; Bardin, T.; et al. Do glucocorticoid injections increase the risk of knee osteoarthritis progression over 5 Years? Arthritis Rheumatol. 2022, 74, 1343–1351. [Google Scholar] [CrossRef]
- Bucci, J.; Chen, X.; LaValley, M.; Nevitt, M.; Torner, J.; Lewis, C.E.; Felson, D.T. Progression of knee osteoarthritis with use of intraarticular glucocorticoids versus hyaluronic acid. Arthritis Rheumatol. 2022, 74, 223–226. [Google Scholar] [CrossRef]
- Klocke, R.; Levasseur, K.; Kitas, G.D.; Smith, J.P.; Hirsch, G. Cartilage turnover and intra-articular corticosteroid injections in knee osteoarthritis. Rheumatol. Int. 2018, 38, 455–459. [Google Scholar] [CrossRef]
- Raynauld, J.P.; Buckland-Wright, C.; Ward, R.; Choquette, D.; Haraoui, B.; Martel-Pelletier, J.; Uthman, I.; Khy, V.; Tremblay, J.L.; Bertrand, C.; et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003, 48, 370–377. [Google Scholar] [CrossRef]
- Posey, K.L.; Hecht, J.T. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr. Drug Targets 2008, 9, 869–877. [Google Scholar] [CrossRef]
- Sasaki, E.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Inoue, R.; Chiba, D.; Fujita, H.; Takahashi, I.; Umeda, T.; Nakaji, S.; et al. Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis—A five year prospective cohort study. Arthritis Res. Ther. 2015, 17, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Barksby, H.E.; Milner, J.M.; Patterson, A.M.; Peak, N.J.; Hui, W.; Robson, T.; Lakey, R.; Middleton, J.; Cawston, T.E.; Richards, C.D.; et al. Matrix Metalloproteinase 10 promotion of collagenolysis via procollagenase activation. Arthritis Rhem. 2006, 54, 3244–3253. [Google Scholar] [CrossRef] [PubMed]
- Mehana, E.S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Falcinelli, E.; Giordan, N.; Luccioli, F.; Piselle, E.; La Paglia, G.M.C.; Momi, S.; Mirabelli, G.; Petito, E.; Alunno, A.; Gresele, P.; et al. Randomized Trial of HymovisR versus SynviscR on Matrix Metalloproteinase in Knee osteoarthritis. Muscles Ligaments Tendons J. 2020, 10, 553–561. [Google Scholar] [CrossRef]
- Vincent, H.K.; Percival, S.S.; Conrad, B.P.; Seay, A.N.; Montero, C.; Vincent, K.K. Hyaluronic acid (HA) Viscosupplementation on synovial fluid inflammation in knee osteoarthritis: A Pilot study. Open Orthop. J. 2013, 7, 378–384. [Google Scholar] [CrossRef]
- Sezgin, M.; Demirel, A.C.; Karaca, C.; Ortancil, O.; Ulkar, G.B.; Kanik, A.; Cakci, A. Does Hyaluronan affect inflammatory cytokines in knee osteoarthritis? Rheumatol. Int. 2005, 25, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; McAlindon, T.E.; Sullivan, M.C.; Wong, J.B.; Kent, D.M.; Schmid, C.H. Effectiveness and implications of alternative placebo treatments, a systemic review and network meta-analysis of osteoarthritis trials. Ann. Intern. Med. 2015, 163, 365–372. [Google Scholar] [CrossRef]
- Weitoft, T.; Uddenfeld, P. Importance of synovial fluid aspiration when injecting intra-articular corticosteroids. Ann. Rheum. Dis. 2000, 59, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Devji, T.; Bhandari, M.; Fierlinger, A.; Niazi, F.; Christensen, R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: A systemic review and meta-analysis of randomized trials. Semin. Arthritis Rheum. 2016, 46, 151–159. [Google Scholar] [CrossRef]
- Knight, V.; Long, T.; Meng, Q.H.; Linden, M.A.; Roads, D.D. Variability in the Laboratory Measurement of Cytokines: A Longitudinal Summary of a College of American Pathologists Proficiency Testing Survey. Arch. Pathol. Lab. Med. 2020, 144, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Jevsevar, D.S.; Brown, G.A.; Jones, D.L.; Matzkin, E.G.; Manner, P.A.; Mooar, P.; Schousboe, J.T.; Stovitz, S.; Sanders, J.O.; Bozic, K.J.; et al. American Academy of Orthopaedic Surgeons evidence-based guideline on treatment of osteoarthritis of the knee, 2nd edition. J. Bone Jt. Surg. Am. 2013, 95, 1885–1886. [Google Scholar] [CrossRef]
- Johansen, M.; Bahrt, H.; Altman, R.D.; Bartels, E.M.; Juhl, C.B.; Bliddal, H.; Lund, H.; Christensen, R. Exploring reasons for the observed inconsistent trial reports on intra-articular injections with hyaluronic acid in the treatment of osteoarthritis: Meta-regression analyses of randomized trials. Semin. Arthritis Rheum. 2016, 46, 34–48. [Google Scholar] [CrossRef]
- Pendleton, A.; Arden, N.; Dougados, M.; Doherty, M.; Bannwarth, B.; Bijlsma, J.; Cluzeau, F.; Cooper, C.; Dieppe, P.; Gunther, K.; et al. EULAR recommendations for the management of knee osteoarthrtitis: A report of a task force of the standing committee for the international clinical studies including therapeutic trials (ESCISIT). Ann. Rheum. Dis. 2000, 59, 936–944. [Google Scholar] [CrossRef]

| Age | 60.8 years (35–78) |
| Gender | 17 Male (50%)/17 Female (50%) |
| BMI kg/m2 | 28 (20–39) |
| Prior knee surgery | 10 (29%) |
| K-L grade | |
| II | 15 (44%) |
| III | 19 (56%) |
| Knee injected | |
| Right | 18 (53%) |
| Left | 16 (47%) |
| Lateral | 30 (88%) |
| Medial | 4 (12%) |
| Baseline Mean ± SD n = 36 | 3-Month Mean ± SD n = 34 | 6-Month Mean ± SD n = 30 | 12-Month Mean + SD n = 25 | |
|---|---|---|---|---|
| WOMAC score | 771 ± 394 | 463 ± 358 | 464 ± 352 | 402 + 333 |
| 40% decrease p < 0.0001 | 40% decrease p < 0.0001 | 50% decrease p < 0.0001 | ||
| VAS score | 4.9 ± 2.0 | 2.7 ± 1.7 | 2.4 ± 1.9 | 2.2 + 1.73 |
| 45% decrease p < 0.0001 | 51% decrease p < 0.0001 | 54% decrease p < 0.0001 | ||
| PCS score | 64.7 ± 18.1 | 74.6 ± 18.7 | 76.5 ± 18.1 | 81.2 + 11.9 |
| 15% increase p < 0.0001 | 18% increase p < 0.0001 | 24% increase p < 0.0001 | ||
| 6 MWD- | 404 ± 67 | 432 ± 83 | 422 ± 75 | 424 + 69 |
| Meters | 7% increase p < 0.007 | 5% increase NS | 5% increase NS | |
| SF before | 3.2 ± 2.2 | 3.1 ± 2.2 | 4.0 ± 2.9 | 4.2 +2.8 |
| inflation (mm) | 3% decrease NS | 25% increase NS | 31% increase NS | |
| SF after | 6.4 ± 3.7 | 5.2 ± 2.8 | 7.5 ± 4.0 | 7.5 + 3.4 |
| inflation (mm) | 18% decrease NS | 17% increase NS | 17% increase NS |
| Demographics | Responders n = 30 | Non Responders n = 6 | p-Values |
|---|---|---|---|
| Age mean and range | 59 (19–78) years | 62 (54–78) years | 0.48 |
| Gender and % | 13 F (43%)/17 M (57%) | 4 F (66%)/2 M (33%) | 0.39 |
| BMI mean and range | 28.1 (19–39) | 26.6 (21–32) | 0.61 |
| Prior Surgery and % | 9 (30%) | 3 (50%) | 0.38 |
| KL II or III and % | 18 KL II (60%)/12 KL III (40%) | 5 KL II (83%)/1 KL III (17%) | 0.39 |
| Functional Status, pain and US depth of SF | |||
| WOMAC score | 814 | 675 | 0.44 |
| VAS (1–10 scale) | 5.2 | 3.5 | 0.051 |
| PCS score | 64.1 | 64.6 | 0.96 |
| 6 MWD (meters) | 406 | 428 | 0.49 |
| US depth | |||
| Before inflation | 3.7 mm | 4.3 mm | 0.75 |
| After inflation | 7.7mm | 5.9 mm | 0.37 |
| Baseline Mean ± SD | 3-Month Mean ± SD | % Increase or Decrease from Baseline | p-Values | |
|---|---|---|---|---|
| n = 34 | n = 34 | |||
| C2C ng/mL | 278 ± 48 | 263 ± 52 | 5% decrease | 0.08 |
| COMP ng/mL | 828 ± 400 | 798 ± 435 | 4% decrease | 0.36 |
| HA ng/mL | 41 ± 29 | 52 ± 58 | 27% increase | 0.27 |
| CPII ng/mL | 1269 ± 508 | 1204 ± 549 | 5% decrease | 0.32 |
| Protein | Baseline Mean ± SD pg/mL | 3-Month Mean ± SD pg/mL | % Increase or Decrease from Baseline | p-Values |
|---|---|---|---|---|
| IL-1ra n = 16 | 345 ± 332 | 518 ± 564 | 50% increase | 0.127 |
| IL-4 n = 10 | 1971 ± 5243 | 251 ± 107 | 87% decrease | 0.395 |
| IL-6 n = 16 | 60 ± 98 | 40 ± 44 | 33% decrease | 0.905 |
| IL-7 n = 16 | 7 ± 1 | 8 ± 2 | 14% increase | 0.167 |
| IL-8 n = 16 | 36 ± 41 | 58 ± 77 | 61% increase | 0.273 |
| IL-15 n = 16 | 6 ± 3 | 7 ± 4 | 17% increase | 0.825 |
| IL-18 n = 16 | 109 ± 63 | 103 ± 63 | 6% decrease | 0.402 |
| IGFBP-1 n = 16 | 6376 ± 9346 | 6707 ± 12,560 | 5% increase | 0.406 |
| IGFBP-3 n = 16 | 36,517 ± 49,159 | 40,790 ± 58,870 | 12% increase | 0.808 |
| MMP-1 n = 10 | 7971 ± 6827 | 8323 ± 9046 | 4% increase | 0.541 |
| MMP-2 n = 10 | 283,599 ± 218,875 | 249,307 ± 194,342 | 12% decrease | 0.325 |
| MMP-3 n = 10 | 245,119 ± 153,269 | 235,275 ± 175,421 | 4% decrease | 0.293 |
| MMP-8 n = 10 | 1354 ± 503 | 1729 ± 1899 | 28% increase | 0.686 |
| MMP-9 n = 10 | 5014 ± 4464 | 6375 ± 9225 | 27% increase | 0.956 |
| MMP-10 n = 16 | 238 ± 206 | 200 ± 208 | 16% decrease | 0.0427 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meehan, R.T.; Gill, M.T.; Hoffman, E.D.; Coeshott, C.M.; Galvan, M.D.; Wolf, M.L.; Amigues, I.A.; Kastsianok, L.M.; Regan, E.A.; Crooks, J.L.; et al. Ultrasound-Guided Injections of HYADD4 for Knee Osteoarthritis Improves Pain and Functional Outcomes at 3, 6, and 12 Months without Changes in Measured Synovial Fluid, Serum Collagen Biomarkers, or Most Synovial Fluid Biomarker Proteins at 3 Months. J. Clin. Med. 2023, 12, 5541. https://doi.org/10.3390/jcm12175541
Meehan RT, Gill MT, Hoffman ED, Coeshott CM, Galvan MD, Wolf ML, Amigues IA, Kastsianok LM, Regan EA, Crooks JL, et al. Ultrasound-Guided Injections of HYADD4 for Knee Osteoarthritis Improves Pain and Functional Outcomes at 3, 6, and 12 Months without Changes in Measured Synovial Fluid, Serum Collagen Biomarkers, or Most Synovial Fluid Biomarker Proteins at 3 Months. Journal of Clinical Medicine. 2023; 12(17):5541. https://doi.org/10.3390/jcm12175541
Chicago/Turabian StyleMeehan, Richard T., Mary T. Gill, Eric D. Hoffman, Claire M. Coeshott, Manuel D. Galvan, Molly L. Wolf, Isabelle A. Amigues, Liudmila M. Kastsianok, Elizabeth A. Regan, James L. Crooks, and et al. 2023. "Ultrasound-Guided Injections of HYADD4 for Knee Osteoarthritis Improves Pain and Functional Outcomes at 3, 6, and 12 Months without Changes in Measured Synovial Fluid, Serum Collagen Biomarkers, or Most Synovial Fluid Biomarker Proteins at 3 Months" Journal of Clinical Medicine 12, no. 17: 5541. https://doi.org/10.3390/jcm12175541





