Ultra-Early Treatment of Neurosurgical Emergencies with Endoscopic Endonasal Approach: Experience from Three Italian Referral Centers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Procedure
2.2. Statistical Analysis
3. Results
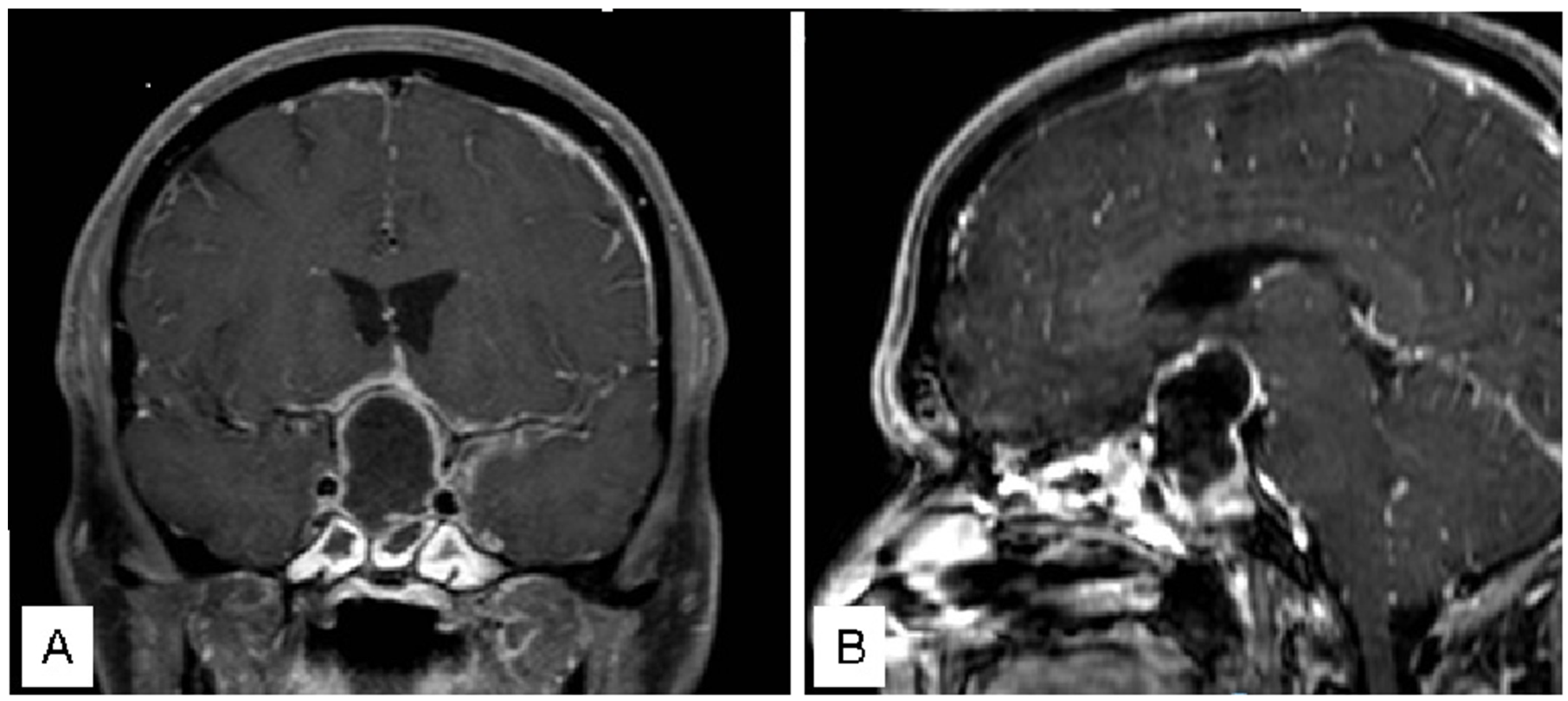
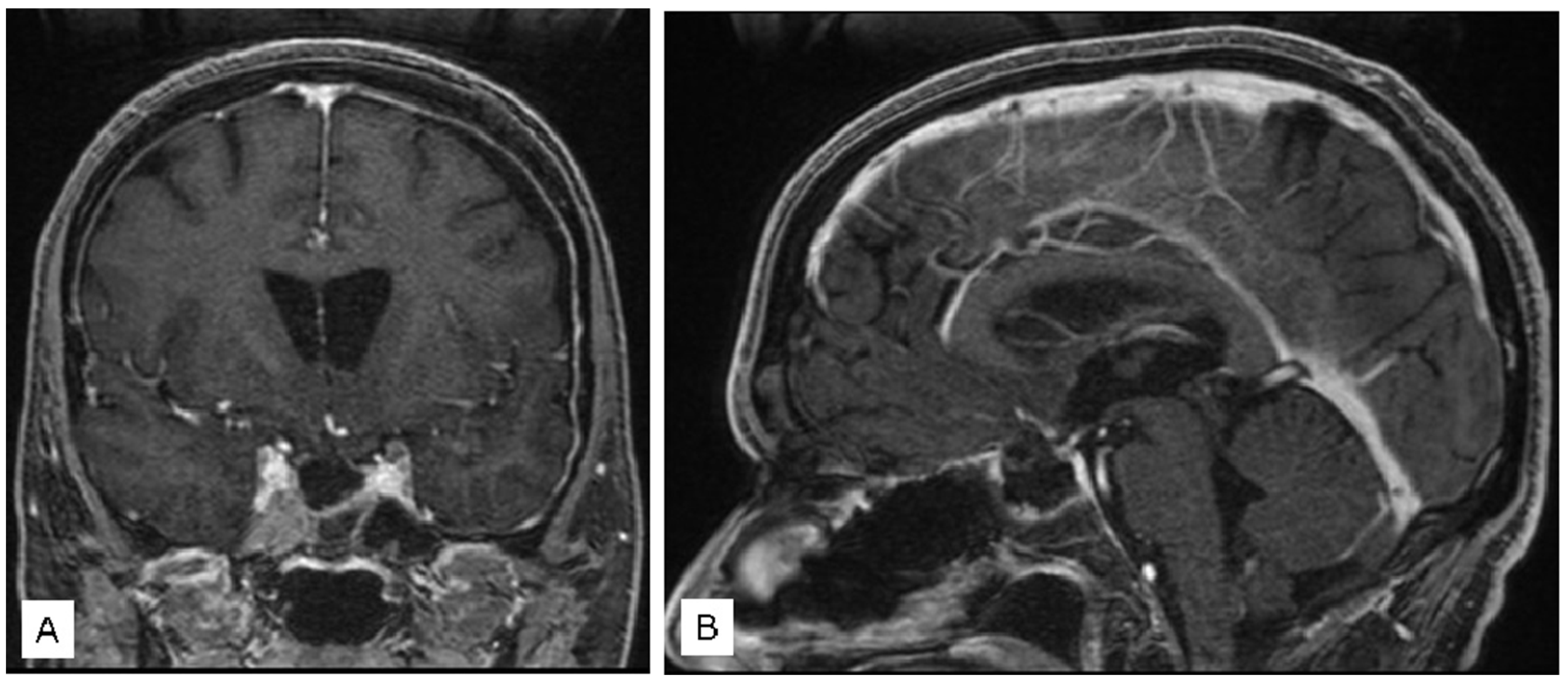
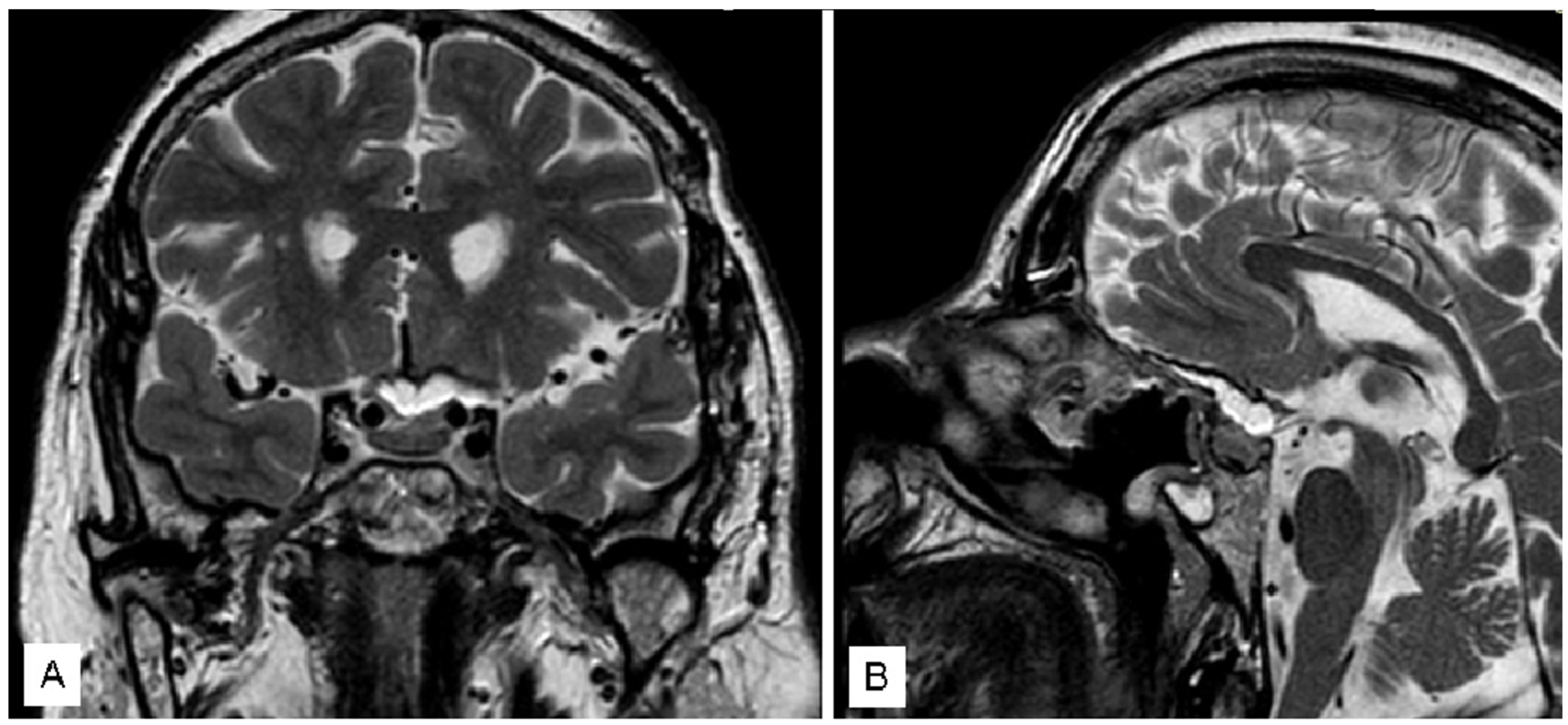
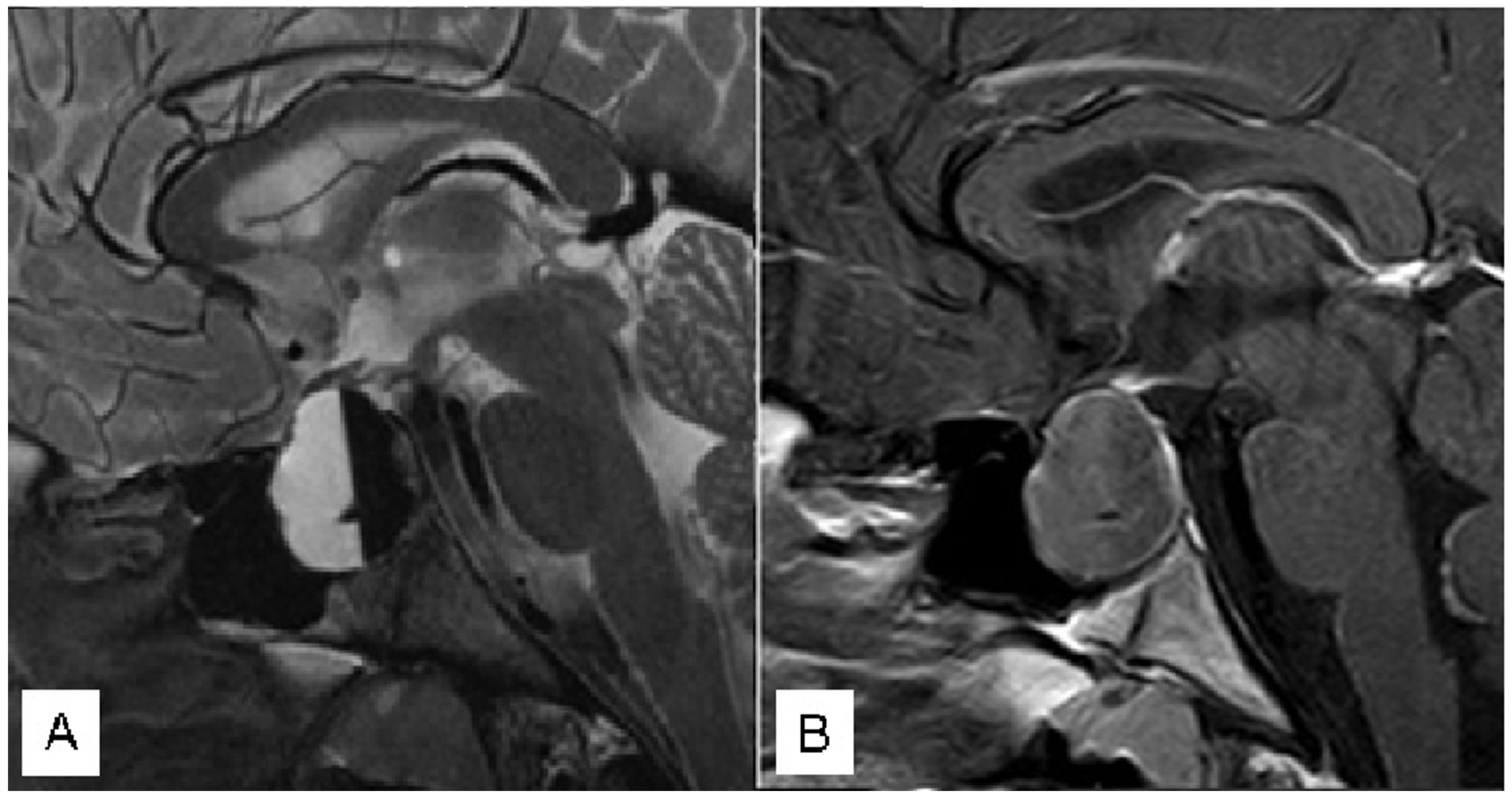
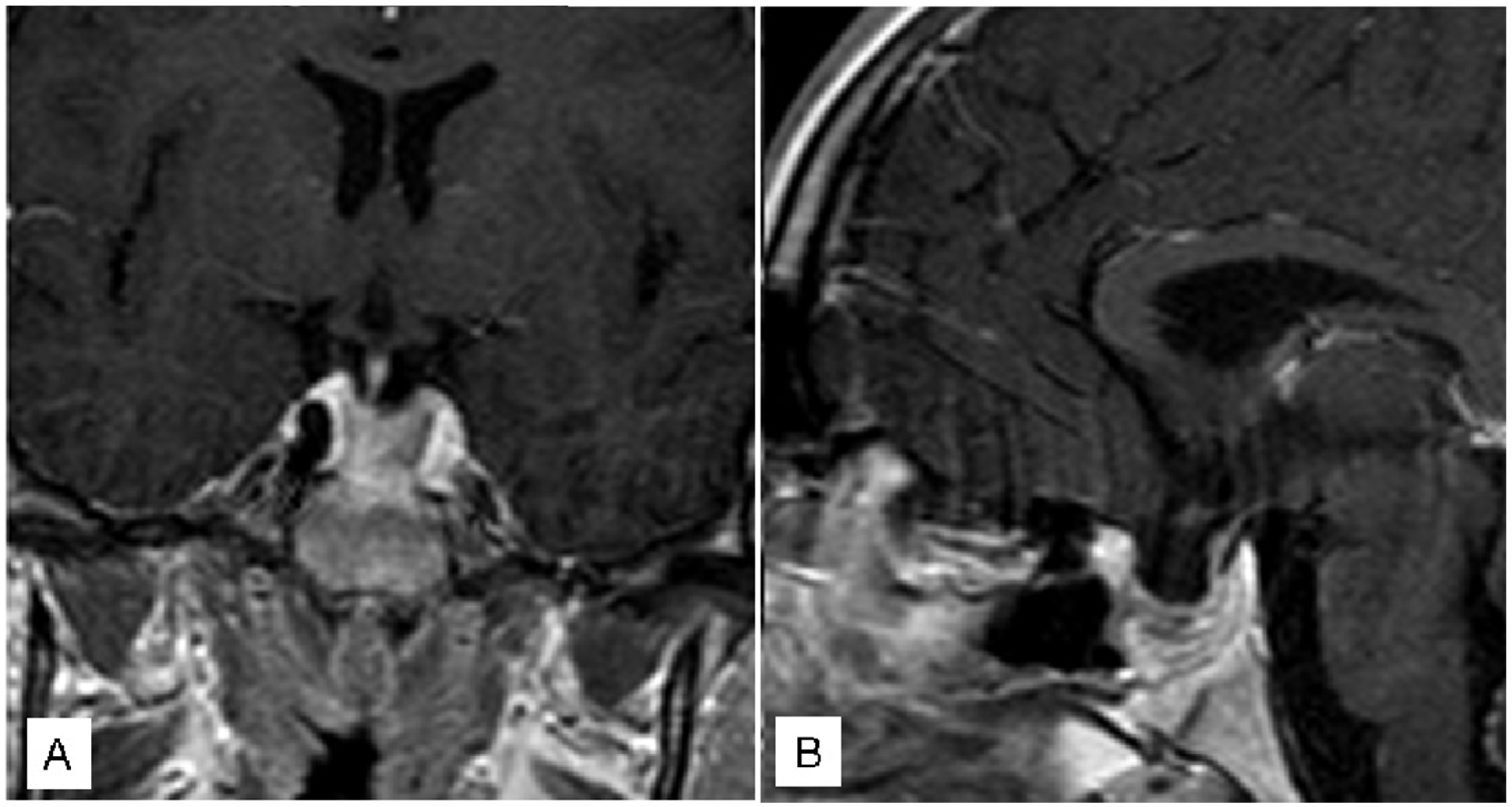
3.1. Illustrative Cases
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bresson, D.; Herman, P.; Polivka, M.; Froelich, S. Sellar Lesions/Pathology. Otolaryngol. Clin. N. Am. 2016, 49, 63–93. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Risso, A.; Zoia, C.; Gianformaggio, C.; Pagella, F.; Pusateri, A.; Benazzo, M.; Gaetani, P. Tension pneumocephalus secondary to osteoradionecrosis of the clivus. Rep. Pract. Oncol. Radiother. 2016, 21, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Emergency Surgery. Available online: https://medical-dictionary.thefreedictionary.com/emergency+surgery (accessed on 9 February 2021).
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Kühne, C.A.; Mand, C.; Lefering, R.; Lendemans, S.; Ruchholtz, S. Urgency of neurosurgical interventions for severe traumatic brain injury. Unfallchirurg 2013, 116, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Sollini, G.; Milanese, L.; La Corte, E.; Rustici, A.; Guaraldi, F.; Asioli, S.; Cirillo, L.; Pasquini, E.; Mazzatenta, D. Endoscopic approaches to orbital lesions: Case series and systematic literature review. J. Neurosurg. 2020, 134, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Zoia, C.; Bongetta, D.; Gaetani, P. Endoscopic transorbital surgery for spheno-orbital lesions: How I do it. Acta Neurochir. 2018, 160, 1231–1233. [Google Scholar] [CrossRef]
- Wang, E.W.; Zanation, A.M.; Gardner, P.A.; Schwartz, T.H.; Eloy, J.A.; Adappa, N.D.; Bettag, M.; Bleier, B.S.; Cappabianca, P.; Carrau, R.L.; et al. ICAR: Endoscopic skull-base surgery. Int. Forum Allergy Rhinol. 2019, 9 (Suppl. S3), S145–S365. [Google Scholar] [CrossRef]
- Cossu, G.; Jouanneau, E.; Cavallo, L.M.; Elbabaa, S.K.; Giammattei, L.; Starnoni, D.; Barges-Coll, J.; Cappabianca, P.; Benes, V.; Baskaya, M.K.; et al. Surgical management of craniopharyngiomas in adult patients: A systematic review and consensus statement on behalf of the EANS skull base section. Acta Neurochir. 2020, 162, 1159–1177. [Google Scholar] [CrossRef]
- Lofrese, G.; Vigo, V.; Rigante, M.; Grieco, D.L.; Maresca, M.; Anile, C.; Mangiola, A.; De Bonis, P. Learning curve of endoscopic pituitary surgery: Experience of a neurosurgery/ENT collaboration. J. Clin. Neurosci. 2018, 47, 299–303. [Google Scholar] [CrossRef]
- Cappabianca, P.; Cavallo, L.M.; de Divitiis, O.; Solari, D.; Esposito, F.; Colao, A. Endoscopic pituitary surgery. Pituitary 2008, 11, 385–390. [Google Scholar] [CrossRef]
- D’Alessandris, Q.G.; Rigante, M.; Mattogno, P.P.; LARocca, G.; Romanello, M.; Auricchio, A.M.; Bevacqua, G.; Fraschetti, F.; Giordano, M.; DIBonaventura, R.; et al. Impact of 4K ultra-high-definition endoscope in pituitary surgery: Analysis of a comparative institutional case series. J. Neurosurg. Sci. 2022, 66, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Altieri, R.; Tardivo, V.; Pacca, P.; Pennacchietti, V.; Penner, F.; Garbossa, D.; Ducati, A.; Garzaro, M.; Zenga, F. 3D HD Endoscopy in Skull Base Surgery: From Darkness to Light. Surg. Technol. Int. 2016, 29, 359–365. [Google Scholar] [PubMed]
- Sondag, L.; Schreuder, F.H.; Pegge, S.A.; Coutinho, J.M.; Dippel, D.W.; Janssen, P.M.; Vandertop, W.P.; Boogaarts, H.D.; Dammers, R.; Klijn, C.J.; et al. Safety and technical efficacy of early minimally invasive endoscopy-guided surgery for intracerebral haemorrhage: The Dutch Intracerebral haemorrhage Surgery Trial pilot study. Acta Neurochir. 2023, 165, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Tanikawa, M.; Sakata, T.; Mase, M. Urgent Optic Nerve Decompression via an Endoscopic Endonasal Transsphenoidal Approach for Craniopharyngioma in a 12-Month-Old Infant: A Case Report. Pediatr. Neurosurg. 2018, 53, 182–187. [Google Scholar] [CrossRef]
- Pacca, P.; Marengo, N.; Di Perna, G.; Penner, F.; Ajello, M.; Garbossa, D.; Zenga, F. Endoscopic Endonasal Approach for Urgent Decompression of Craniovertebral Junction in Syringobulbia. World Neurosurg. 2019, 130, 499–505. [Google Scholar] [CrossRef]
- Chuang, C.-C.; Chang, C.-N.; Wei, K.-C.; Liao, C.-C.; Hsu, P.-W.; Huang, Y.-C.; Chen, Y.-L.; Lai, L.-J.; Pai, P.-C. Surgical treatment for severe visual compromised patients after pituitary apoplexy. J. Neuro-Oncol. 2006, 80, 39–47. [Google Scholar] [CrossRef]
- Goshtasbi, K.; Abiri, A.; Sahyouni, R.; Mahboubi, H.; Raefsky, S.; Kuan, E.C.; Hsu, F.P.; Cadena, G. Visual and Endocrine Recovery Following Conservative and Surgical Treatment of Pituitary Apoplexy: A Meta-Analysis. World Neurosurg. 2019, 132, 33–40. [Google Scholar] [CrossRef]
- Kelly, P.D.; Fernando, S.J.; Malenke, J.A.; Chandra, R.K.; Turner, J.H.; Chambless, L.B. The Effect of Timing of Surgery in Pituitary Apoplexy on Continuously Valued Visual Acuity. J. Neurol. Surg. Part B Skull Base 2021, 82 (Suppl. S3), e70–e78. [Google Scholar] [CrossRef]
- Barkhoudarian, G.; Kelly, D.F. Pituitary Apoplexy. Neurosurg. Clin. N. Am. 2019, 30, 457–463. [Google Scholar] [CrossRef]
- Tu, M.; Lu, Q.; Zhu, P.; Zheng, W. Surgical versus non-surgical treatment for pituitary apoplexy: A systematic review and meta-analysis. J. Neurol. Sci. 2016, 370, 258–262. [Google Scholar] [CrossRef]
- Zoli, M.; Milanese, L.; Faustini-Fustini, M.; Guaraldi, F.; Asioli, S.; Zenesini, C.; Righi, A.; Frank, G.; Foschini, M.P.; Sturiale, C.; et al. Endoscopic Endonasal Surgery for Pituitary Apoplexy: Evidence On a 75-Case Series From a Tertiary Care Center. World Neurosurg. 2017, 106, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.; Sanchez, M.M.; Karekezi, C.; Warsi, N.; Fernández-Gajardo, R.; Panwar, J.; Mansouri, A.; Suppiah, S.; Nassiri, F.; Nejad, R.; et al. Pituitary Apoplexy: Results of Surgical and Conservative Management Clinical Series and Review of the Literature. World Neurosurg. 2019, 130, e988–e999. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Vanderpump, M.; Baldeweg, S.; Drake, W.; Reddy, N.; Lanyon, M.; Markey, A.; Plant, G.; Powell, M.; Sinha, S.; et al. UK guidelines for the management of pituitary apoplexy. Clin. Endocrinol. 2011, 74, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, H.A.; Cote, D.J.; Burke, W.T.; Castlen, J.P.; Bi, W.L.; Laws, E.R., Jr.; Dunn, I.F. Time Course of Symptomatic Recovery After Endoscopic Transsphenoidal Surgery for Pituitary Adenoma Apoplexy in the Modern Era. World Neurosurg. 2016, 96, 434–439. [Google Scholar] [CrossRef]
- Muthukumar, N.; Rossette, D.; Soundaram, M.; Senthilbabu, S.; Badrinarayanan, T. Blindness following pituitary apoplexy: Timing of surgery and neuro-ophthalmic outcome. J. Clin. Neurosci. 2008, 15, 873–879. [Google Scholar] [CrossRef]
- Agrawal, D.; Mahapatra, A.K. Visual outcome of blind eyes in pituitary apoplexy after transsphenoidal surgery: A series of 14 eyes. Surg. Neurol. 2005, 63, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Briet, C.; Salenave, S.; Bonneville, J.-F.; Laws, E.R.; Chanson, P. Pituitary apoplexy. Endocr. Rev. 2015, 36, 622–645. [Google Scholar] [CrossRef]
- Takeda, N.; Fujita, K.; Katayama, S.; Akutu, N.; Hayashi, S.; Kohmura, E. Effect of transsphenoidal surgery on decreased visual acuity caused by pituitary apoplexy. Pituitary 2010, 13, 154–159. [Google Scholar] [CrossRef]
- Sahyouni, R.; Goshtasbi, K.; Choi, E.; Mahboubi, H.; Le, R.; Khahera, A.S.; Hanna, G.K.; Hatefi, D.; Hsu, F.P.; Bhandarkar, N.D.; et al. Vision Outcomes in Early versus Late Surgical Intervention of Pituitary Apoplexy: Meta-Analysis. World Neurosurg. 2019, 127, 52–57. [Google Scholar] [CrossRef]
- Bills, D.C.; Meyer, F.B.; Laws, E.R., Jr.; Davis, D.H.; Ebersold, M.J.; Scheithauer, B.W.; Ilstrup, D.M.; Abboud, C.F. A retrospective analysis of pituitary apoplexy. Neurosurgery 1993, 33, 602–608; discussion 608–609. [Google Scholar] [PubMed]
- Seuk, J.-W.; Kim, C.-H.; Yang, M.-S.; Cheong, J.-H.; Kim, J.-M. Visual Outcome after Transsphenoidal Surgery in Patients with Pituitary Apoplexy. J. Korean Neurosurg. Soc. 2011, 49, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.-J.; Hwang, J.-H.; Hwang, S.-K.; Park, Y.-M. Clinical Outcome of Cranial Neuropathy in Patients with Pituitary Apoplexy. J. Korean Neurosurg. Soc. 2010, 48, 213–218. [Google Scholar] [CrossRef]
- Sibal, L.; Ball, S.G.; Connolly, V.; James, R.A.; Kane, P.; Kelly, W.F.; Kendall-Taylor, P.; Mathias, D.; Perros, P.; Quinton, R.; et al. Pituitary Apoplexy: A Review of Clinical Presentation, Management and Outcome in 45 Cases. Pituitary 2004, 7, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, J.; McGregor, E.J.; Mitchell, R.D.; Gittoes, N.J. Acute management of pituitary apoplexy—Surgery or conservative management? Clin. Endocrinol. 2004, 61, 747–752. [Google Scholar] [CrossRef]

| Parameter | Overall (n = 26) |
|---|---|
| Age (years) | 53.9 (±18.4) |
| Sex (Male) | 22 (84.6%) |
| Symptomatology | |
| Headache | 22 (84.6%) |
| Visual field deficits | 18 (69.2%) |
| Visual acuity deficits | 14 (53.8%) |
| Nausea/Vomit | 14 (53.8%) |
| Drowsiness | 12 (46.2%) |
| Rhinorrhoea | 5 (19.2%) |
| Meningitis | 3 (11.5%) |
| Coma | 3 (11.5%) |
| Amaurosis | 3 (11.5%) |
| Oculomotor Deficits | 3 (11.5%) |
| Pneumocephalus | 2 (7.7%) |
| Photophobia | 2 (7.7%) |
| Parameter | Overall (n = 26) | Postop Stable VA (n = 15) | Postop Improving VA (n = 11) | p Value | Postop Stable VF (n = 14) | Postop Improving VF (n = 12) | p Value |
|---|---|---|---|---|---|---|---|
| Preop VA deficits | 14 (53.8%) | 5 (33.3%) | 9 (81.8%) | 0.014 * | 6 (42.9%) | 8 (66.7%) | 0.225 |
| Preop VF deficits | 18 (69.2%) | 8 (53.3%) | 10 (90.9%) | 0.040 * | 7 (50.0%) | 11 (91.7%) | 0.022 * |
| Previous surgery | 7 (26.9%) | 4 (26.7%) | 3 (27.3%) | >0.999 | 3 (21.4%) | 4 (33.3%) | 0.495 |
| Time to op | 5.5 (±2.3) | 6.3 (±2.4) | 4.5 (±1.8) | 0.035 * | 6.2 (±2.4) | 4.8 (±1.9) | 0.111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattogno, P.P.; Zoli, M.; D’Alessandris, Q.G.; Bongetta, D.; Caccavella, V.M.; Rigante, M.; Della Pepa, G.M.; Mazzatenta, D.; Lauretti, L.; Olivi, A.; et al. Ultra-Early Treatment of Neurosurgical Emergencies with Endoscopic Endonasal Approach: Experience from Three Italian Referral Centers. J. Clin. Med. 2023, 12, 5471. https://doi.org/10.3390/jcm12175471
Mattogno PP, Zoli M, D’Alessandris QG, Bongetta D, Caccavella VM, Rigante M, Della Pepa GM, Mazzatenta D, Lauretti L, Olivi A, et al. Ultra-Early Treatment of Neurosurgical Emergencies with Endoscopic Endonasal Approach: Experience from Three Italian Referral Centers. Journal of Clinical Medicine. 2023; 12(17):5471. https://doi.org/10.3390/jcm12175471
Chicago/Turabian StyleMattogno, Pier Paolo, Matteo Zoli, Quintino Giorgio D’Alessandris, Daniele Bongetta, Valerio Maria Caccavella, Mario Rigante, Giuseppe Maria Della Pepa, Diego Mazzatenta, Liverana Lauretti, Alessandro Olivi, and et al. 2023. "Ultra-Early Treatment of Neurosurgical Emergencies with Endoscopic Endonasal Approach: Experience from Three Italian Referral Centers" Journal of Clinical Medicine 12, no. 17: 5471. https://doi.org/10.3390/jcm12175471
APA StyleMattogno, P. P., Zoli, M., D’Alessandris, Q. G., Bongetta, D., Caccavella, V. M., Rigante, M., Della Pepa, G. M., Mazzatenta, D., Lauretti, L., Olivi, A., Spena, G., & Zoia, C. (2023). Ultra-Early Treatment of Neurosurgical Emergencies with Endoscopic Endonasal Approach: Experience from Three Italian Referral Centers. Journal of Clinical Medicine, 12(17), 5471. https://doi.org/10.3390/jcm12175471









