Biased Quantification of Rat Liver Fibrosis—Meta-Analysis with Practical Recommendations and Clinical Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Statistical Analysis
2.3.1. ANOVA—The Denominator
2.3.2. ANOVA—The Numerator
2.3.3. Detection of Significant Results
3. Results
3.1. Literature Search
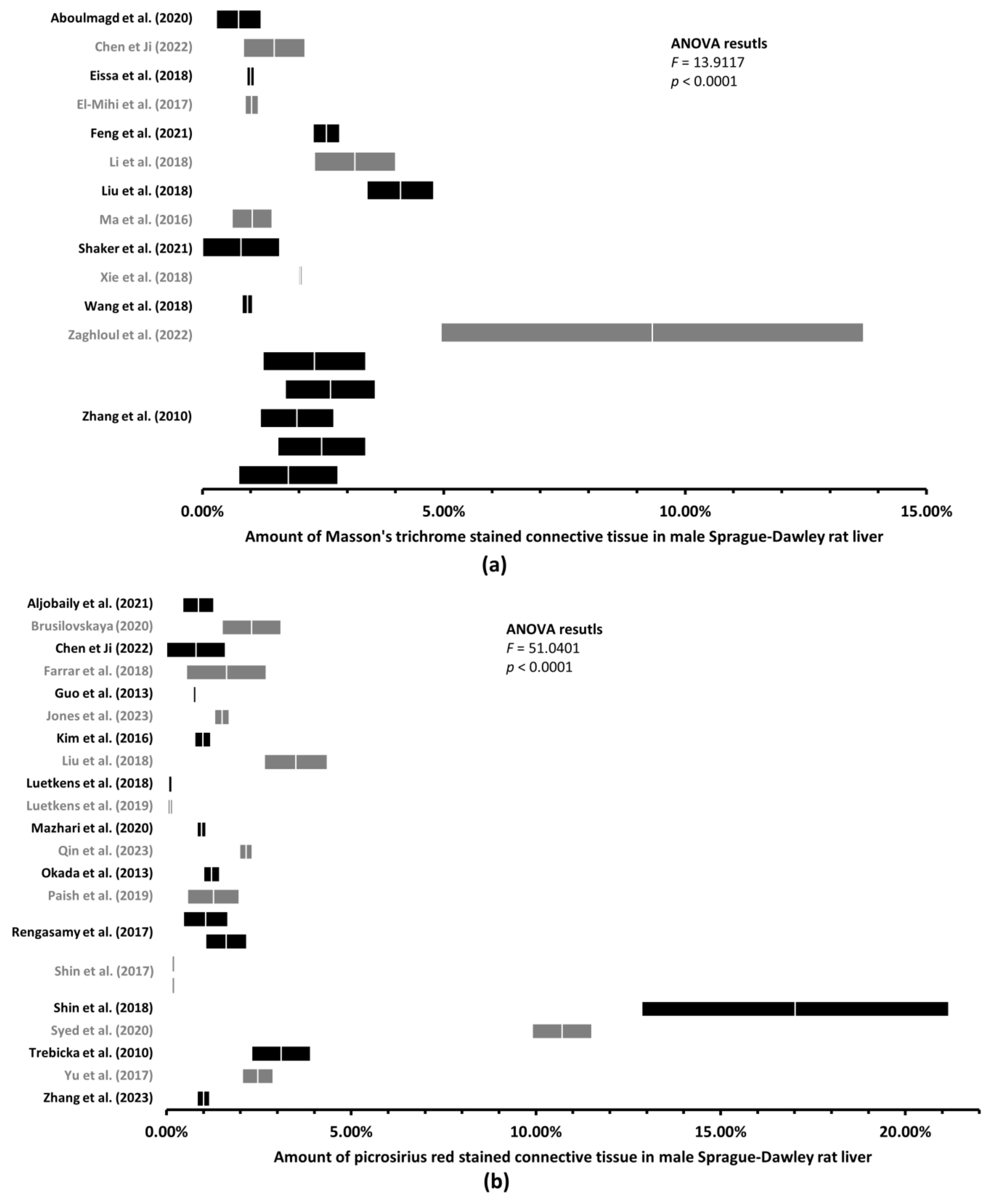
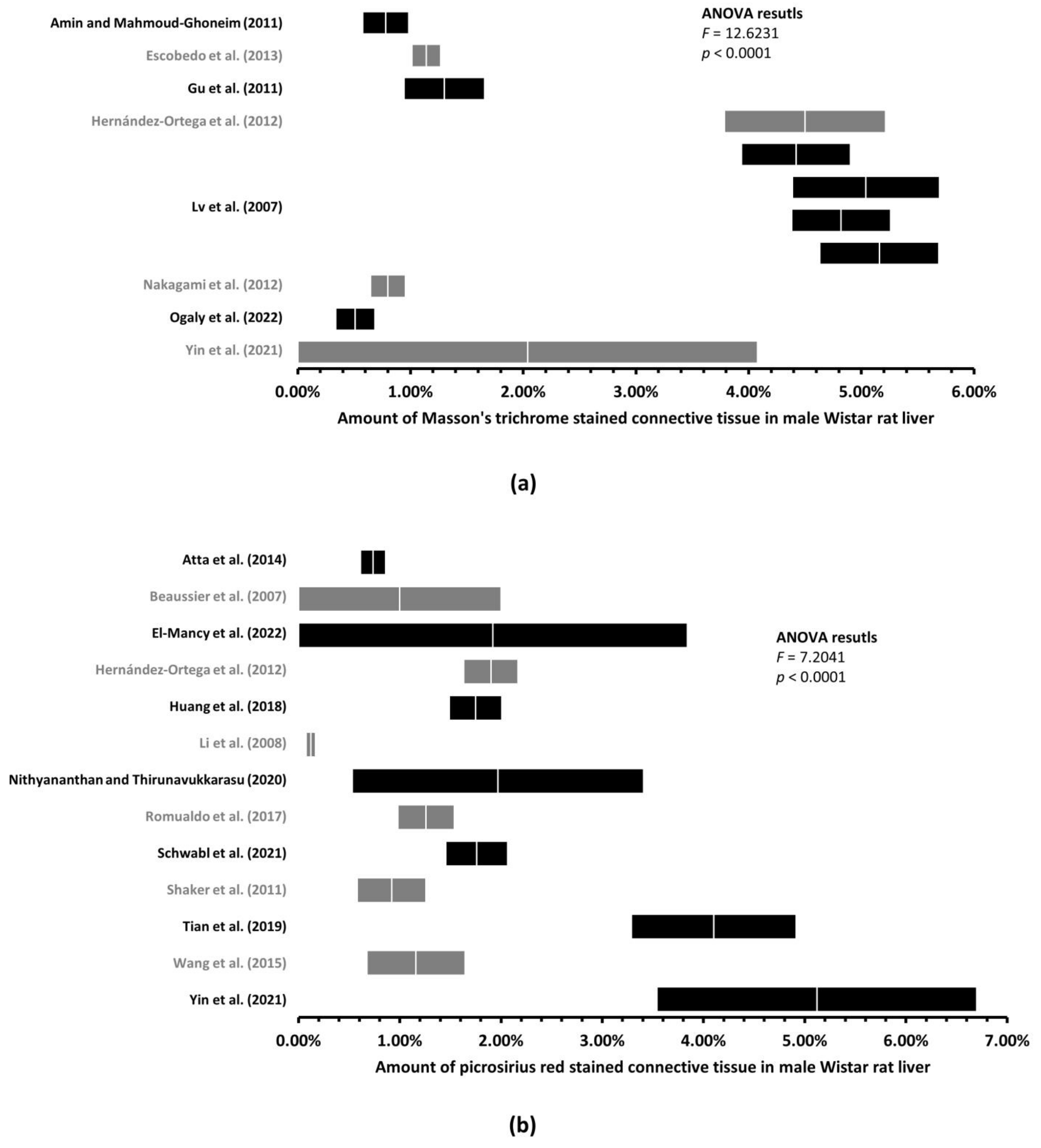
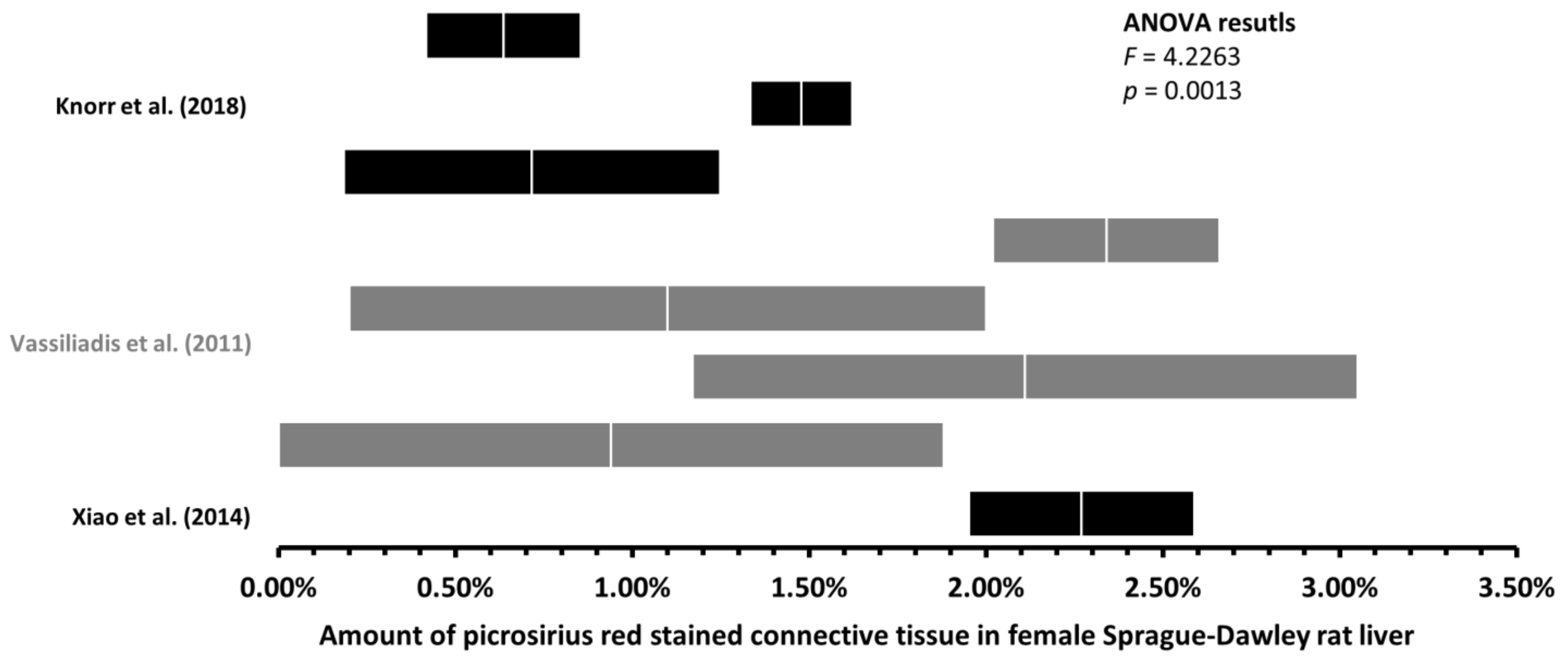
3.2. Up to 170-Fold Differences in the Amount of Healthy Rat Liver Connective Tissue
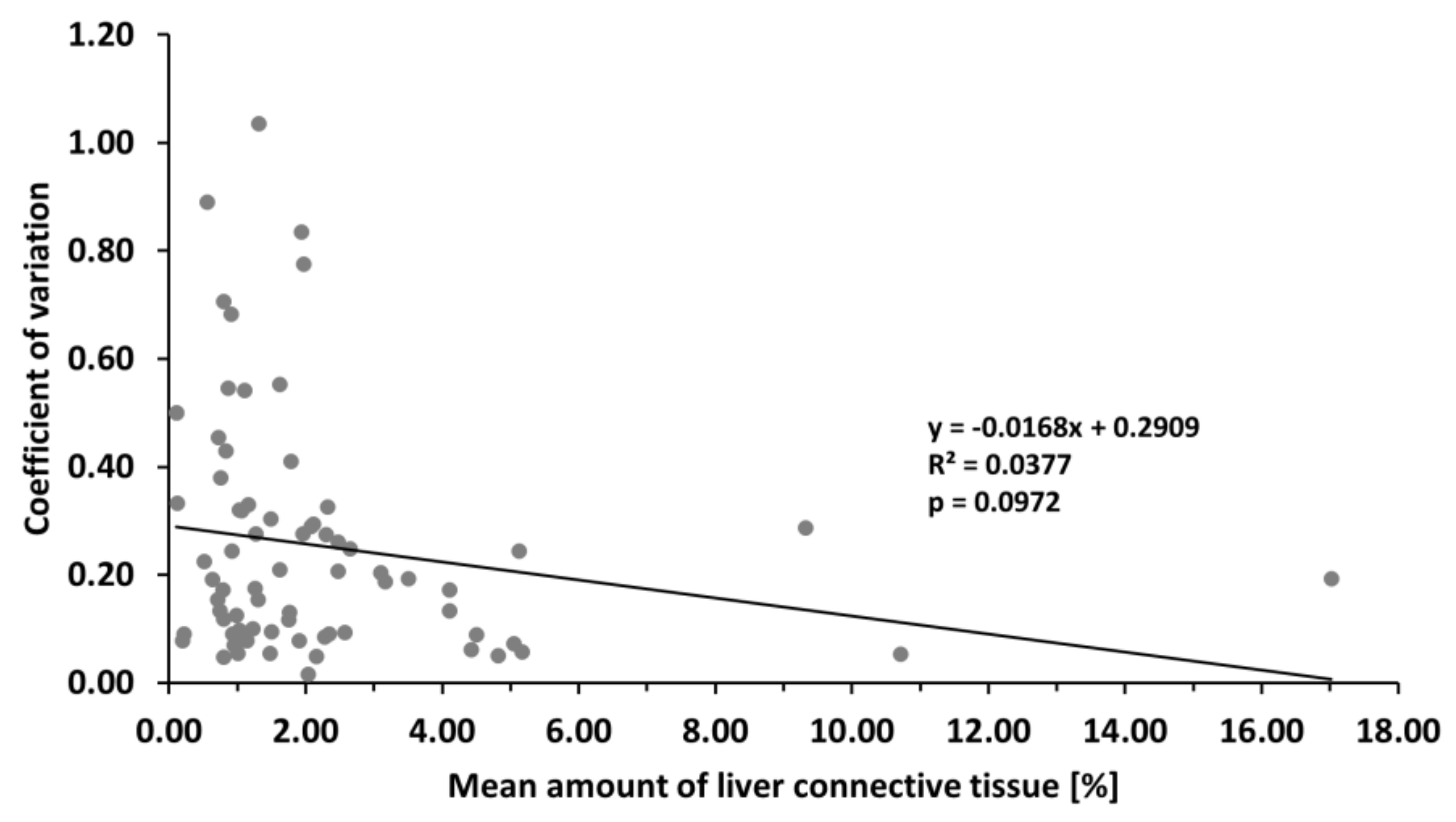
3.3. With More Connective Tissue, Tendency of the Variability of the Estimated Amount of Connective Tissue to Decrease
4. Discussion
4.1. Biased Sampling and Quantification of Rat Liver Connective Tissue
4.2. Reliability and Validity of the Assessment of Liver Fibrosis
4.3. Fibrosis Stage Is Not a Continuous Variable
4.4. Practical Recommendations
4.5. Clinical Application
4.6. Minimum Detectable Effect
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalifa, A.; Rockey, D.C. The Utility of Liver Biopsy in 2020. Curr. Opin. Gastroenterol. 2020, 36, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. 1500 Scientists Lift the Lid on Reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannheimer, E.G.; Quintanilha, L.F.; Carvalho, A.B.; Paredes, B.D.; Gonçalves de Carvalho, F.; Takyia, C.M.; Resende, C.M.C.; Ferreira da Motta Rezende, G.; Carlos Campos de Carvalho, A.; Schanaider, A.; et al. Bone Marrow Cells Obtained from Cirrhotic Rats Do Not Improve Function or Reduce Fibrosis in a Chronic Liver Disease Model. Clin. Transplant. 2011, 25, 54–60. [Google Scholar] [CrossRef]
- Brusilovskaya, K.; Königshofer, P.; Lampach, D.; Szodl, A.; Supper, P.; Bauer, D.; Beer, A.; Stift, J.; Timelthaler, G.; Oberhuber, G.; et al. Soluble Guanylyl Cyclase Stimulation and Phosphodiesterase-5 Inhibition Improve Portal Hypertension and Reduce Liver Fibrosis in Bile Duct–ligated Rats. United Eur. Gastroenterol. J. 2020, 8, 1174–1185. [Google Scholar] [CrossRef]
- Denga, T.M.; Gunter, S.; Fourie, S.; le Roux, R.; Manilall, A.; Millen, A.M.E.; Mokotedi, L. Interleukin-6 Blockers Improve Inflammation-Induced Lipid Metabolism Impairments but Induce Liver Fibrosis in Collagen-Induced Arthritis. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Mahmoud-Ghoneim, D. Texture Analysis of Liver Fibrosis Microscopic Images: A Study on the Effect of Biomarkers. Acta Biochim. Biophys. Sin. 2011, 43, 193–203. [Google Scholar] [CrossRef] [Green Version]
- French, S.W.; Miyamoto, K.; Wong, K.; Jui, L.; Briere, L. Role of the Ito Cell in Liver Parenchymal Fibrosis in Rats Fed Alcohol and a High Fat-Low Protein Diet. Am. J. Pathol. 1988, 132, 73–85. [Google Scholar]
- Wilczynski, E.; Sasson, E.; Eliav, U.; Navon, G.; Nevo, U. Quantitative Magnetization EXchange MRI Measurement of Liver Fibrosis Model in Rodents. J. Magn. Reson. Imaging 2023, 57, 285. [Google Scholar] [CrossRef]
- Setyaningsih, W.A.W.; Sari, D.C.R.; Romi, M.M.; Arfian, N. Liver Fibrosis Associated with Adipose Tissue and Liver Inflammation in an Obesity Model. Med. J. Malaysia 2021, 76, 304–310. [Google Scholar]
- Farrar, C.T.; DePeralta, D.K.; Day, H.; Rietz, T.A.; Wei, L.; Lauwers, G.Y.; Keil, B.; Subramaniam, A.; Sinskey, A.J.; Tanabe, K.K.; et al. 3D Molecular MR Imaging of Liver Fibrosis and Response to Rapamycin Therapy in a Bile Duct Ligation Rat Model. J. Hepatol. 2015, 63, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Gu, K.; Zhao, J.-D.; Ren, Z.-G.; Ma, N.-Y.; Lai, S.-T.; Wang, J.; Liu, J.; Jiang, G.-L. A Natural Process of Cirrhosis Resolution and Deceleration of Liver Regeneration after Thioacetamide Withdrawal in a Rat Model. Mol. Biol. Rep. 2011, 38, 1687–1696. [Google Scholar] [CrossRef]
- Hernández-Ortega, L.D.; Alcántar-Díaz, B.E.; Ruiz-Corro, L.A.; Sandoval-Rodriguez, A.; Bueno-Topete, M.; Armendariz-Borunda, J.; Salazar-Montes, A.M. Quercetin Improves Hepatic Fibrosis Reducing Hepatic Stellate Cells and Regulating Pro-Fibrogenic/Anti-Fibrogenic Molecules Balance. J. Gastroenterol. Hepatol. 2012, 27, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, J.; Qian, J.; Wu, G.; Ma, Z. Emodin Alleviates CCl4-induced Liver Fibrosis by Suppressing Epithelial-mesenchymal Transition and Transforming Growth Factor-β1 in Rats. Mol. Med. Rep. 2018, 18, 3262–3270. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.-G.; Lv, X.-D.; Zhan, L.-L.; Chen, L.; Zou, Q.-Y.; Xiang, J.-Q.; Qin, J.-L.; Zhang, W.-W.; Zeng, Z.-J.; Jin, H.; et al. Human Urokinase-Type Plasminogen Activator Gene-Modified Bone Marrow-Derived Mesenchymal Stem Cells Attenuate Liver Fibrosis in Rats by down-Regulating the Wnt Signaling Pathway. World J. Gastroenterol. 2016, 22, 2092–2103. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.; Berry, G.; Matthews, J.N.S. Statistical Methods in Medical Research; Wiley: Hoboken, NJ, USA, 2002; ISBN 978-0-632-05257-8. [Google Scholar]
- Zhang, C.-G.; Zhang, B.; Deng, W.-S.; Duan, M.; Chen, W.; Wu, Z.-Y. Role of Estrogen Receptor β Selective Agonist in Ameliorating Portal Hypertension in Rats with CCl4-Induced Liver Cirrhosis. World J. Gastroenterol. 2016, 22, 4484–4500. [Google Scholar] [CrossRef] [PubMed]
- Knorr, A.; Hirth-Dietrich, C.; Alonso-Alija, C.; Härter, M.; Hahn, M.; Keim, Y.; Wunder, F.; Stasch, J.-P. Nitric Oxide-Independent Activation of Soluble Guanylate Cyclase by BAY 60-2770 in Experimental Liver Fibrosis. Arzneimittelforschung 2008, 58, 71–80. [Google Scholar] [CrossRef]
- Vassiliadis, E.; Vang Larsen, D.; Clausen, R.E.; Veidal, S.S.; Barascuk, N.; Larsen, L.; Simonsen, H.; Silvestre, T.S.; Hansen, C.; Overgaard, T.; et al. Measurement of CO3-610, a Potential Liver Biomarker Derived from Matrix Metalloproteinase-9 Degradation of Collagen Type III, in a Rat Model of Reversible Carbon-Tetrachloride-Induced Fibrosis. Biomark. Insights 2011, 6, BMI.S6347. [Google Scholar] [CrossRef]
- Aboulmagd, Y.M.; El-Bahy, A.A.Z.; Menze, E.T.; Azab, S.S.; El-Demerdash, E. Role of Linagliptin in Preventing the Pathological Progression of Hepatic Fibrosis in High Fat Diet and Streptozotocin-Induced Diabetic Obese Rats. Eur. J. Pharmacol. 2020, 881, 173224. [Google Scholar] [CrossRef]
- Atta, H.; El-Rehany, M.; Hammam, O.; Abdel-Ghany, H.; Ramzy, M.; Roderfeld, M.; Roeb, E.; Al-Hendy, A.; Raheim, S.A.; Allam, H.; et al. Mutant MMP-9 and HGF Gene Transfer Enhance Resolution of CCl4-Induced Liver Fibrosis in Rats: Role of ASH1 and EZH2 Methyltransferases Repression. PLoS ONE 2014, 9, e112384. [Google Scholar] [CrossRef] [Green Version]
- Luetkens, J.A.; Klein, S.; Träber, F.; Block, W.; Schmeel, F.C.; Sprinkart, A.M.; Kuetting, D.L.R.; Uschner, F.E.; Schierwagen, R.; Thomas, D.; et al. Quantification of Liver Fibrosis: Extracellular Volume Fraction Using an MRI Bolus-Only Technique in a Rat Animal Model. Eur. Radiol. Exp. 2019, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Nithyananthan, S.; Thirunavukkarasu, C. Arsenic Trioxide, a Cancer Chemo Drug Hampers Fibrotic Liver Regeneration by Interrupting Oxidative Stress Rekindling and Stellate Cell Rejuvenation. J. Cell. Physiol. 2020, 235, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wen, R.; Lin, Q.; Wang, N.; Lu, P.; Zhu, X. Wogonoside Shows Antifibrotic Effects in an Experimental Regression Model of Hepatic Fibrosis. Dig. Dis. Sci. 2015, 60, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Li, X.; Luo, X.; Sha, Y.; Gong, P.; Gu, J.; Tan, R. Hepatoprotective Effect and Potential Mechanism of Aqueous Extract from Phyllanthus Emblica on Carbon-Tetrachloride-Induced Liver Fibrosis in Rats. Evid. Based Complement. Alternat. Med. 2021, 2021, e5345821. [Google Scholar] [CrossRef] [PubMed]
- Mazhari, S.; Gitiara, A.; Baghaei, K.; Hatami, B.; Rad, R.E.; Asadirad, A.; Joharchi, K.; Tokhanbigli, S.; Hashemi, S.M.; Łos, M.J.; et al. Therapeutic Potential of Bone Marrow-Derived Mesenchymal Stem Cells and Imatinib in a Rat Model of Liver Fibrosis. Eur. J. Pharmacol. 2020, 882, 173263. [Google Scholar] [CrossRef]
- Shaker, M.E.; Eisa, N.H.; Elgaml, A.; El-Mesery, A.; El-Shafey, M.; El-Dosoky, M.; El-Mowafy, M.; El-Mesery, M. Ingestion of Mannose Ameliorates Thioacetamide-Induced Intrahepatic Oxidative Stress, Inflammation and Fibrosis in Rats. Life Sci. 2021, 286, 120040. [Google Scholar] [CrossRef]
- Aljobaily, N.; Viereckl, M.J.; Hydock, D.S.; Aljobaily, H.; Wu, T.-Y.; Busekrus, R.; Jones, B.; Alberson, J.; Han, Y. Creatine Alleviates Doxorubicin-Induced Liver Damage by Inhibiting Liver Fibrosis, Inflammation, Oxidative Stress, and Cellular Senescence. Nutrients 2021, 13, 41. [Google Scholar] [CrossRef]
- Beaussier, M.; Wendum, D.; Schiffer, E.; Dumont, S.; Rey, C.; Lienhart, A.; Housset, C. Prominent Contribution of Portal Mesenchymal Cells to Liver Fibrosis in Ischemic and Obstructive Cholestatic Injuries. Lab. Investig. 2007, 87, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Eissa, L.A.; Kenawy, H.I.; El-Karef, A.; Elsherbiny, N.M.; El-Mihi, K.A. Antioxidant and Anti-Inflammatory Activities of Berberine Attenuate Hepatic Fibrosis Induced by Thioacetamide Injection in Rats. Chem. Biol. Interact. 2018, 294, 91–100. [Google Scholar] [CrossRef]
- El-Mihi, K.A.; Kenawy, H.I.; El-Karef, A.; Elsherbiny, N.M.; Eissa, L.A. Naringin Attenuates Thioacetamide-Induced Liver Fibrosis in Rats through Modulation of the PI3K/Akt Pathway. Life Sci. 2017, 187, 50–57. [Google Scholar] [CrossRef]
- Escobedo, G.; Arjona-Román, J.L.; Meléndez-Pérez, R.; Suárez-Álvarez, K.; Guzmán, C.; Aguirre-García, J.; Gutiérrez-Reyes, G.; Vivas, O.; Varela-Fascinetto, G.; Rodríguez-Romero, A.; et al. Liver Exhibits Thermal Variations According to the Stage of Fibrosis Progression: A Novel Use of Modulated-Differential Scanning Calorimetry for Research in Hepatology. Hepatol. Res. 2013, 43, 785–794. [Google Scholar] [CrossRef]
- Farrar, C.T.; Gale, E.M.; Kennan, R.; Ramsay, I.; Masia, R.; Arora, G.; Looby, K.; Wei, L.; Kalpathy-Cramer, J.; Bunzel, M.M.; et al. CM-101: Type I Collagen–targeted MR Imaging Probe for Detection of Liver Fibrosis. Radiology 2018, 287, 581–589. [Google Scholar] [CrossRef]
- Feng, S.; Tong, H.; Gao, J.-H.; Tang, S.-H.; Yang, W.-J.; Wang, G.-M.; Zhou, H.-Y.; Wen, S.-L. Anti-inflammation Treatment for Protection of Hepatocytes and Amelioration of Hepatic Fibrosis in Rats. Exp. Ther. Med. 2021, 22, 1213. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-L.; Liang, B.; Wang, X.-W.; Fan, F.-G.; Jin, J.; Lan, R.; Yang, J.-H.; Wang, X.-C.; Jin, L.; Cao, Q. Glycyrrhizic Acid Attenuates CCl4-Induced Hepatocyte Apoptosis in Rats via a P53-Mediated Pathway. World J. Gastroenterol. 2013, 19, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-R.; Wei, S.-J.; Huang, Y.-Q.; Xing, W.; Wang, L.-Y.; Liang, L.-L. Mechanism of Combined Use of Vitamin D and Puerarin in Anti-Hepatic Fibrosis by Regulating the Wnt/β-Catenin Signalling Pathway. World J. Gastroenterol. 2018, 24, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, J.I.; Paik, Y.-H.; Yun, C.-O.; Chang, H.Y.; Lee, S.Y.; Lee, K.S. A Single Adenovirus-Mediated Relaxin Delivery Attenuates Established Liver Fibrosis in Rats. J. Gene Med. 2016, 18, 16–26. [Google Scholar] [CrossRef]
- Li, C.-H.; Pan, L.-H.; Yang, Z.-W.; Li, C.-Y.; Xu, W.-X. Preventive Effect of Qianggan-Rongxian Decoction on Rat Liver Fibrosis. World J. Gastroenterol. 2008, 14, 3569–3573. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Zhang, Z.; Zheng, J.; Shi, Y.; Liu, J.; Cao, Y.; Yuan, X.; Chu, Y. Recombinant Truncated TGF-β Receptor II Attenuates Carbon Tetrachloride-induced Epithelial-mesenchymal Transition and Liver Fibrosis in Rats. Mol. Med. Rep. 2018, 17, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Luetkens, J.A.; Klein, S.; Träber, F.; Schmeel, F.C.; Sprinkart, A.M.; Kuetting, D.L.R.; Block, W.; Uschner, F.E.; Schierwagen, R.; Hittatiya, K.; et al. Quantification of Liver Fibrosis at T1 and T2 Mapping with Extracellular Volume Fraction MRI: Preclinical Results. Radiology 2018, 288, 748–754. [Google Scholar] [CrossRef]
- Lv, X.-H.; Zhou, L.-P.; Liu, D.-P.; Wang, Y.; Wang, B.-Y.; Fu, B.-Y.; Song, M.; Liu, C.-R. Traditional Chinese Medicine Kang Xian Fu Fang I Is Effective for Prophylaxis and Treatment of Alcoholic Liver Disease in Rats. Hepatobiliary Pancreat. Dis. Int. 2007, 6, 182–187. [Google Scholar]
- Nakagami, H.; Shimamura, M.; Miyake, T.; Shimosato, T.; Minobe, N.; Moritani, T.; Kiomy Osako, M.; Nakagami, F.; Koriyama, H.; Kyutoku, M.; et al. Nifedipine Prevents Hepatic Fibrosis in a Non-Alcoholic Steatohepatitis Model Induced by an L-Methionine-and Choline-Deficient Diet. Mol. Med. Rep. 2012, 5, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Okada, Y.; Yamaguchi, K.; Nakajima, T.; Nishikawa, T.; Jo, M.; Mitsumoto, Y.; Kimura, H.; Nishimura, T.; Tochiki, N.; Yasui, K.; et al. Rosuvastatin Ameliorates High-Fat and High-Cholesterol Diet-Induced Nonalcoholic Steatohepatitis in Rats. Liver Int. 2013, 33, 301–311. [Google Scholar] [CrossRef]
- Paish, H.L.; Reed, L.H.; Brown, H.; Bryan, M.C.; Govaere, O.; Leslie, J.; Barksby, B.S.; Garcia Macia, M.; Watson, A.; Xu, X.; et al. A Bioreactor Technology for Modeling Fibrosis in Human and Rodent Precision-Cut Liver Slices. Hepatology 2019, 70, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, M.; Singh, G.; Fakharuzi, N.A.; Balasubramanian, S.; Swamynathan, P.; Thej, C.; Sasidharan, G.; Gupta, P.K.; Das, A.K.; Rahman, A.Z.A.; et al. Transplantation of Human Bone Marrow Mesenchymal Stromal Cells Reduces Liver Fibrosis More Effectively than Wharton’s Jelly Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2017, 8, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romualdo, G.R.; Grassi, T.F.; Goto, R.L.; Tablas, M.B.; Bidinotto, L.T.; Fernandes, A.A.H.; Cogliati, B.; Barbisan, L.F. An Integrative Analysis of Chemically-Induced Cirrhosis-Associated Hepatocarcinogenesis: Histological, Biochemical and Molecular Features. Toxicol. Lett. 2017, 281, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwabl, P.; Hambruch, E.; Budas, G.R.; Supper, P.; Burnet, M.; Liles, J.T.; Birkel, M.; Brusilovskaya, K.; Königshofer, P.; Peck-Radosavljevic, M.; et al. The Non-Steroidal FXR Agonist Cilofexor Improves Portal Hypertension and Reduces Hepatic Fibrosis in a Rat NASH Model. Biomedicines 2021, 9, 60. [Google Scholar] [CrossRef]
- Shaker, M.E.; Zalata, K.R.; Mehal, W.Z.; Shiha, G.E.; Ibrahim, T.M. Comparison of Imatinib, Nilotinib and Silymarin in the Treatment of Carbon Tetrachloride-Induced Hepatic Oxidative Stress, Injury and Fibrosis. Toxicol. Appl. Pharmacol. 2011, 252, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.K.; Kwon, O.S.; Lee, J.J.; Park, Y.H.; Choi, C.S.; Jeong, S.H.; Choi, D.J.; Kim, Y.S.; Kim, J.H. Effect of Rifaximin on Hepatic Fibrosis in Bile Duct-Ligated Rat Model. Korean J. Gastroenterol. 2017, 70, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Shin, G.-M.; Koppula, S.; Chae, Y.-J.; Kim, H.-S.; Lee, J.-D.; Kim, M.-K.; Song, M. Anti-Hepatofibrosis Effect of Allium Senescens in Activated Hepatic Stellate Cells and Thioacetamide-Induced Fibrosis Rat Model. Pharm. Biol. 2018, 56, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Syed, A.A.; Reza, M.I.; Shafiq, M.; Kumariya, S.; Singh, P.; Husain, A.; Hanif, K.; Gayen, J.R. Naringin Ameliorates Type 2 Diabetes Mellitus-Induced Steatohepatitis by Inhibiting RAGE/NF-ΚB Mediated Mitochondrial Apoptosis. Life Sci. 2020, 257, 118118. [Google Scholar] [CrossRef]
- Tian, H.; Liu, L.; Li, Z.; Liu, W.; Sun, Z.; Xu, Y.; Wang, S.; Liang, C.; Hai, Y.; Feng, Q.; et al. Chinese Medicine CGA Formula Ameliorates Liver Fibrosis Induced by Carbon Tetrachloride Involving Inhibition of Hepatic Apoptosis in Rats. J. Ethnopharmacol. 2019, 232, 227–235. [Google Scholar] [CrossRef]
- Trebicka, J.; Hennenberg, M.; Odenthal, M.; Shir, K.; Klein, S.; Granzow, M.; Vogt, A.; Dienes, H.-P.; Lammert, F.; Reichen, J.; et al. Atorvastatin Attenuates Hepatic Fibrosis in Rats after Bile Duct Ligation via Decreased Turnover of Hepatic Stellate Cells. J. Hepatol. 2010, 53, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ho, C.T.; Liong, E.C.; Nanji, A.A.; Leung, T.M.; Lau, T.Y.H.; Fung, M.L.; Tipoe, G.L. Epigallocatechin Gallate Attenuates Fibrosis, Oxidative Stress, and Inflammation in Non-Alcoholic Fatty Liver Disease Rat Model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-Kappa B Pathways. Eur. J. Nutr. 2014, 53, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Song, T.; Huo, M.; Zhang, Y.; Zhang, Y.-Y.; Ma, Z.-H.; Wang, N.; Zhang, J.-P.; Chu, L. Fasudil Alleviates Hepatic Fibrosis in Type 1 Diabetic Rats: Involvement of the Inflammation and RhoA/ROCK Pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5665–5677. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hao, G.; Wang, D.; Liu, J.; Dong, X.; Sun, Y.; Pan, Q.; Li, Y.; Shi, X.; Li, L.; et al. Therapeutic Effect and Location of GFP-Labeled Placental Mesenchymal Stem Cells on Hepatic Fibrosis in Rats. Stem Cells Int. 2017, 2017, e1798260. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, P.; Gao, X.; Qian, W.; Xu, K. RAAV2-TGF-Β3 Decreases Collagen Synthesis and Deposition in the Liver of Experimental Hepatic Fibrosis Rat. Dig. Dis. Sci. 2010, 55, 2821–2830. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Jiao, F.-Z.; Zhang, W.-B.; Chen, Q.; Gong, Z.-J. Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid Alleviates Liver Fibrosis by Suppressing the Transforming Growth Factor-Β1 Signal Pathway. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 423–429. [Google Scholar] [CrossRef]
- Chen, X.-F.; Ji, S. Sorafenib Attenuates Fibrotic Hepatic Injury Through Mediating Lysine Crotonylation. Drug Des. Devel. Ther. 2022, 16, 2133–2144. [Google Scholar] [CrossRef]
- El-Mancy, E.M.; Elsherbini, D.M.A.; Al-Serwi, R.H.; El-Sherbiny, M.; Ahmed Shaker, G.; Abdel-Moneim, A.-M.H.; Enan, E.T.; Elsherbiny, N.M. α-Lipoic Acid Protects against Cyclosporine A-Induced Hepatic Toxicity in Rats: Effect on Oxidative Stress, Inflammation, and Apoptosis. Toxics 2022, 10, 442. [Google Scholar] [CrossRef]
- Jones, A.K.; Chen, H.; Ng, K.J.; Villalona, J.; McHugh, M.; Zeveleva, S.; Wilks, J.; Brilisauer, K.; Bretschneider, T.; Qian, H.S.; et al. Soluble Guanylyl Cyclase Activator BI 685509 Reduces Portal Hypertension and Portosystemic Shunting in a Rat Thioacetamide-Induced Cirrhosis Model. J. Pharmacol. Exp. Ther. 2023, 386, 70–79. [Google Scholar] [CrossRef]
- Ogaly, H.A.; Aldulmani, S.A.A.; Al-Zahrani, F.A.M.; Abd-Elsalam, R.M. D-Carvone Attenuates CCl4-Induced Liver Fibrosis in Rats by Inhibiting Oxidative Stress and TGF-ß 1/SMAD3 Signaling Pathway. Biology 2022, 11, 739. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Y.; Liang, Y.; Li, Q.; Xie, X.; Zhang, H. Astragaloside IV Alleviates Atorvastatin-Induced Hepatotoxicity via AMPK/SIRT1 Pathway. Pharmacology 2023, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, R.A.; Zaghloul, A.M.; El-Kashef, D.H. Hepatoprotective Effect of Baicalin against Thioacetamide-Induced Cirrhosis in Rats: Targeting NOX4/NF-ΚB/NLRP3 Inflammasome Signaling Pathways. Life Sci. 2022, 295, 120410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, F.; Li, P.; Li, H. ARRDC3 Inhibits Liver Fibrosis and Epithelial-to-Mesenchymal Transition via the ITGB4/PI3K/Akt Signaling Pathway. Immunopharmacol. Immunotoxicol. 2023, 45, 160–171. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Bargi, R.; Beheshti, F.; Hosseini, M.; Farzadnia, M.; Khazaei, M. Thymoquinone Restores Liver Fibrosis and Improves Oxidative Stress Status in a Lipopolysaccharide-Induced Inflammation Model in Rats. Avicenna J. Phytomed. 2017, 7, 502–510. [Google Scholar]
- Marcos, R.; Monteiro, R.A.; Rocha, E. The Use of Design-Based Stereology to Evaluate Volumes and Numbers in the Liver: A Review with Practical Guidelines. J. Anat. 2012, 220, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-S.; Lee, T.-H.; Chou, W.-Y.; Wu, C.-L.; Cho, C.-L.; Lu, C.-N.; Jawan, B.; Wang, C.-H. Interleukin-10 Gene Therapy Reverses Thioacetamide-Induced Liver Fibrosis in Mice. Biochem. Biophys. Res. Commun. 2005, 336, 324–331. [Google Scholar] [CrossRef]
- Erstad, D.J.; Farrar, C.T.; Ghoshal, S.; Masia, R.; Ferreira, D.S.; Chen, Y.-C.I.; Choi, J.-K.; Wei, L.; Waghorn, P.A.; Rotile, N.J.; et al. Molecular Magnetic Resonance Imaging Accurately Measures the Antifibrotic Effect of EDP-305, a Novel Farnesoid X Receptor Agonist. Hepatol. Commun. 2018, 2, 821–835. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Feng, Y.; Zhang, Y.; Yuan, F.; Zhang, J.; Ren, H.; Jia, L. Mycelium Polysaccharides from Termitomyces Albuminosus Attenuate CCl4-Induced Chronic Liver Injury Via Inhibiting TGFβ1/Smad3 and NF-ΚB Signal Pathways. Int. J. Mol. Sci. 2019, 20, 4872. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Zhou, M.; Cheng, S.; Hu, Y.; Gao, M.; Ma, Y.; Limpanont, Y.; Zhou, H.; Dekumyoy, P.; Cheng, Y.; et al. Myricetin Possesses Anthelmintic Activity and Attenuates Hepatic Fibrosis via Modulating TGFβ1 and Akt Signaling and Shifting Th1/Th2 Balance in Schistosoma Japonicum-Infected Mice. Front. Immunol. 2020, 11, 593. [Google Scholar] [CrossRef] [Green Version]
- Masubuchi, S.; Takai, S.; Jin, D.; Tashiro, K.; Komeda, K.; Li, Z.-L.; Otsuki, Y.; Okamura, H.; Hayashi, M.; Uchiyama, K. Chymase Inhibitor Ameliorates Hepatic Steatosis and Fibrosis on Established Non-Alcoholic Steatohepatitis in Hamsters Fed a Methionine- and Choline-Deficient Diet. Hepatol. Res. 2013, 43, 970–978. [Google Scholar] [CrossRef]
- Serna-Salas, S.A.; Navarro-González, Y.D.; Martínez-Hernández, S.L.; Barba-Gallardo, L.F.; Sánchez-Alemán, E.; Aldaba-Muruato, L.R.; Macías-Pérez, J.R.; Ventura-Juárez, J.; Muñoz-Ortega, M.H. Doxazosin and Carvedilol Treatment Improves Hepatic Regeneration in a Hamster Model of Cirrhosis. BioMed Res. Int. 2018, 2018, 4706976. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.-L.; Yang, Z.; Zang, Y.-J.; Li, D.-J.; Liang, Z.-P.; Shen, Z.-Y. Inhibitory Effects of Prostaglandin E1 on Activation of Hepatic Stellate Cells in Rabbits with Schistosomiasis. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2007, 6, 176–181. [Google Scholar] [PubMed]
- Wang, W.; Zhao, C.; Zhou, J.; Zhen, Z.; Wang, Y.; Shen, C. Simvastatin Ameliorates Liver Fibrosis via Mediating Nitric Oxide Synthase in Rats with Non-Alcoholic Steatohepatitis-Related Liver Fibrosis. PLoS ONE 2013, 8, e76538. [Google Scholar] [CrossRef] [Green Version]
- Yarpuzlu, B.; Ayyildiz, M.; Tok, O.E.; Aktas, R.G.; Basdogan, C. Correlation between the Mechanical and Histological Properties of Liver Tissue. J. Mech. Behav. Biomed. Mater. 2014, 29, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atmaca, H.T.; Gazyagci, A.N.; Terzi, O.S.; Sumer, T. Role of Stellate Cells in Hepatic Echinococcosis in Cattle. J. Parasit. Dis. Off. Org. Indian Soc. Parasitol. 2019, 43, 576–582. [Google Scholar] [CrossRef]
- Clapper, J.R.; Hendricks, M.D.; Gu, G.; Wittmer, C.; Dolman, C.S.; Herich, J.; Athanacio, J.; Villescaz, C.; Ghosh, S.S.; Heilig, J.S.; et al. Diet-Induced Mouse Model of Fatty Liver Disease and Nonalcoholic Steatohepatitis Reflecting Clinical Disease Progression and Methods of Assessment. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 305, G483–G495. [Google Scholar] [CrossRef] [Green Version]
- Fallowfield, J.A.; Hayden, A.L.; Snowdon, V.K.; Aucott, R.L.; Stutchfield, B.M.; Mole, D.J.; Pellicoro, A.; Gordon-Walker, T.T.; Henke, A.; Schrader, J.; et al. Relaxin Modulates Human and Rat Hepatic Myofibroblast Function and Ameliorates Portal Hypertension in Vivo. Hepatology 2014, 59, 1492–1504. [Google Scholar] [CrossRef]
- Marcos, R.; Correia-Gomes, C. Liver and Gender: Are There Differences in Fibrous Tissue before the Onset of Fibrosis? Hepatology 2015, 61, 1093–1094. [Google Scholar] [CrossRef]
- Marcos, R.; Bragança, B.; Fontes-Sousa, A.P. Image Analysis or Stereology. J. Histochem. Cytochem. 2015, 63, 734–736. [Google Scholar] [CrossRef] [Green Version]
- Hoy, A.M.; McDonald, N.; Lennen, R.J.; Milanesi, M.; Herlihy, A.H.; Kendall, T.J.; Mungall, W.; Gyngell, M.; Banerjee, R.; Janiczek, R.L.; et al. Non-Invasive Assessment of Liver Disease in Rats Using Multiparametric Magnetic Resonance Imaging: A Feasibility Study. Biol. Open 2018, 7, bio033910. [Google Scholar] [CrossRef] [Green Version]
- Mik, P.; Tonar, Z.; Malečková, A.; Eberlová, L.; Liška, V.; Pálek, R.; Rosendorf, J.; Jiřík, M.; Mírka, H.; Králíčková, M.; et al. Distribution of Connective Tissue in the Male and Female Porcine Liver: Histological Mapping and Recommendations for Sampling. J. Comp. Pathol. 2018, 162, 1–13. [Google Scholar] [CrossRef]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver Biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.S.; Hastah, F.; Galan, M.V.; Gordon, S.C. Fibrosis Heterogeneity in Nonalcoholic Steatohepatitis and Hepatitis C Virus Needle Core Biopsy Specimens. Am. J. Clin. Pathol. 2005, 123, 382–387. [Google Scholar] [CrossRef]
- Guido, M.; Rugge, M. Liver Biopsy Sampling in Chronic Viral Hepatitis. Semin. Liver Dis. 2004, 24, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Dargère, D.; Paradis, V. Sampling Variability of Liver Fibrosis in Chronic Hepatitis C. Hepatology 2003, 38, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Merriman, R.B.; Ferrell, L.D.; Patti, M.G.; Weston, S.R.; Pabst, M.S.; Aouizerat, B.E.; Bass, N.M. Correlation of Paired Liver Biopsies in Morbidly Obese Patients with Suspected Nonalcoholic Fatty Liver Disease. Hepatology 2006, 44, 874–880. [Google Scholar] [CrossRef]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling Variability of Liver Biopsy in Nonalcoholic Fatty Liver Disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef]
- Regev, A.; Berho, M.; Jeffers, L.J.; Milikowski, C.; Molina, E.G.; Pyrsopoulos, N.T.; Feng, Z.-Z.; Reddy, K.R.; Schiff, E.R. Sampling Error and Intraobserver Variation in Liver Biopsy in Patients with Chronic HCV Infection. Am. J. Gastroenterol. 2002, 97, 2614–2618. [Google Scholar] [CrossRef]
- Brunt, E.M. Liver Biopsy Reliability in Clinical Trials: Thoughts from a Liver Pathologist. J. Hepatol. 2020, 73, 1310–1312. [Google Scholar] [CrossRef]
- Forlano, R.; Mullish, B.H.; Maurice, J.B.; Thursz, M.R.; Goldin, R.D.; Manousou, P. NAFLD: Time to Apply Quantitation in Liver Biopsies as Endpoints in Clinical Trials. J. Hepatol. 2021, 74, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Lubel, J.S.; Herath, C.B.; Tchongue, J.; Grace, J.; Jia, Z.; Spencer, K.; Casley, D.; Crowley, P.; Sievert, W.; Burrell, L.M.; et al. Angiotensin-(1–7), an Alternative Metabolite of the Renin–angiotensin System, Is up-Regulated in Human Liver Disease and Has Antifibrotic Activity in the Bile-Duct-Ligated Rat. Clin. Sci. 2009, 117, 375–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvaruso, V.; Di Marco, V.; Bavetta, M.G.; Cabibi, D.; Conte, E.; Bronte, F.; Simone, F.; Burroughs, A.K.; Craxì, A. Quantification of Fibrosis by Collagen Proportionate Area Predicts Hepatic Decompensation in Hepatitis C Cirrhosis. Aliment. Pharmacol. Ther. 2015, 41, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Forlano, R.; Mullish, B.H.; Giannakeas, N.; Maurice, J.B.; Angkathunyakul, N.; Lloyd, J.; Tzallas, A.T.; Tsipouras, M.; Yee, M.; Thursz, M.R.; et al. High-Throughput, Machine Learning-Based Quantification of Steatosis, Inflammation, Ballooning, and Fibrosis in Biopsies From Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 18, 2081–2090.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.H.; Shin, H.P.; Lee, S.; Lim, Y.J.; Hwang, S.H.; Han, H.; Park, H.K.; Chung, J.-H.; Yim, S.-V. Effect of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells in a Cirrhotic Rat Model. Liver Int. 2009, 29, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Tschanz, S.; Schneider, J.P.; Knudsen, L. Design-Based Stereology: Planning, Volumetry and Sampling Are Crucial Steps for a Successful Study. Ann. Anat. Anat. Anz. 2014, 196, 3–11. [Google Scholar] [CrossRef]
- Junatas, K.L.; Tonar, Z.; Kubíková, T.; Liška, V.; Pálek, R.; Mik, P.; Králíčková, M.; Witter, K. Stereological Analysis of Size and Density of Hepatocytes in the Porcine Liver. J. Anat. 2017, 230, 575–588. [Google Scholar] [CrossRef] [Green Version]
- Gundersen, H.J.G.; Jensen, E.B.V.; Kiêu, K.; Nielsen, J. The Efficiency of Systematic Sampling in Stereology—Reconsidered. J. Microsc. 1999, 193, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Heo, M.; Zhang, S. Sample Size Calculations for Clustered and Longitudinal Outcomes in Clinical Research; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4665-5626-3. [Google Scholar]
- Vittinghoff, E.; Glidden, D.V.; Shiboski, S.C.; McCulloch, C.E. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4614-1353-0. [Google Scholar]
- Debnath, T.; Mallarpu, C.S.; Chelluri, L.K. Development of Bioengineered Organ Using Biological Acellular Rat Liver Scaffold and Hepatocytes. Organogenesis 2020, 16, 61–72. [Google Scholar] [CrossRef]
- Rojkind, M.; Giambrone, M.-A.; Biempica, L. Collagen Types in Normal and Cirrhotic Liver. Gastroenterology 1979, 76, 710–719. [Google Scholar] [CrossRef]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and Application of a Numerical Scoring System for Assessing Histological Activity in Asymptomatic Chronic Active Hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef]
- Scheuer, P.J. Classification of Chronic Viral Hepatitis: A Need for Reassessment. J. Hepatol. 1991, 13, 372–374. [Google Scholar] [CrossRef]
- Batts, K.P.; Ludwig, J. Chronic Hepatitis. An Update on Terminology and Reporting. Am. J. Surg. Pathol. 1995, 19, 1409–1417. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N. Histological Grading and Staging of Chronic Hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Poynard, T. An Algorithm for the Grading of Activity in Chronic Hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Natta, M.V.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]

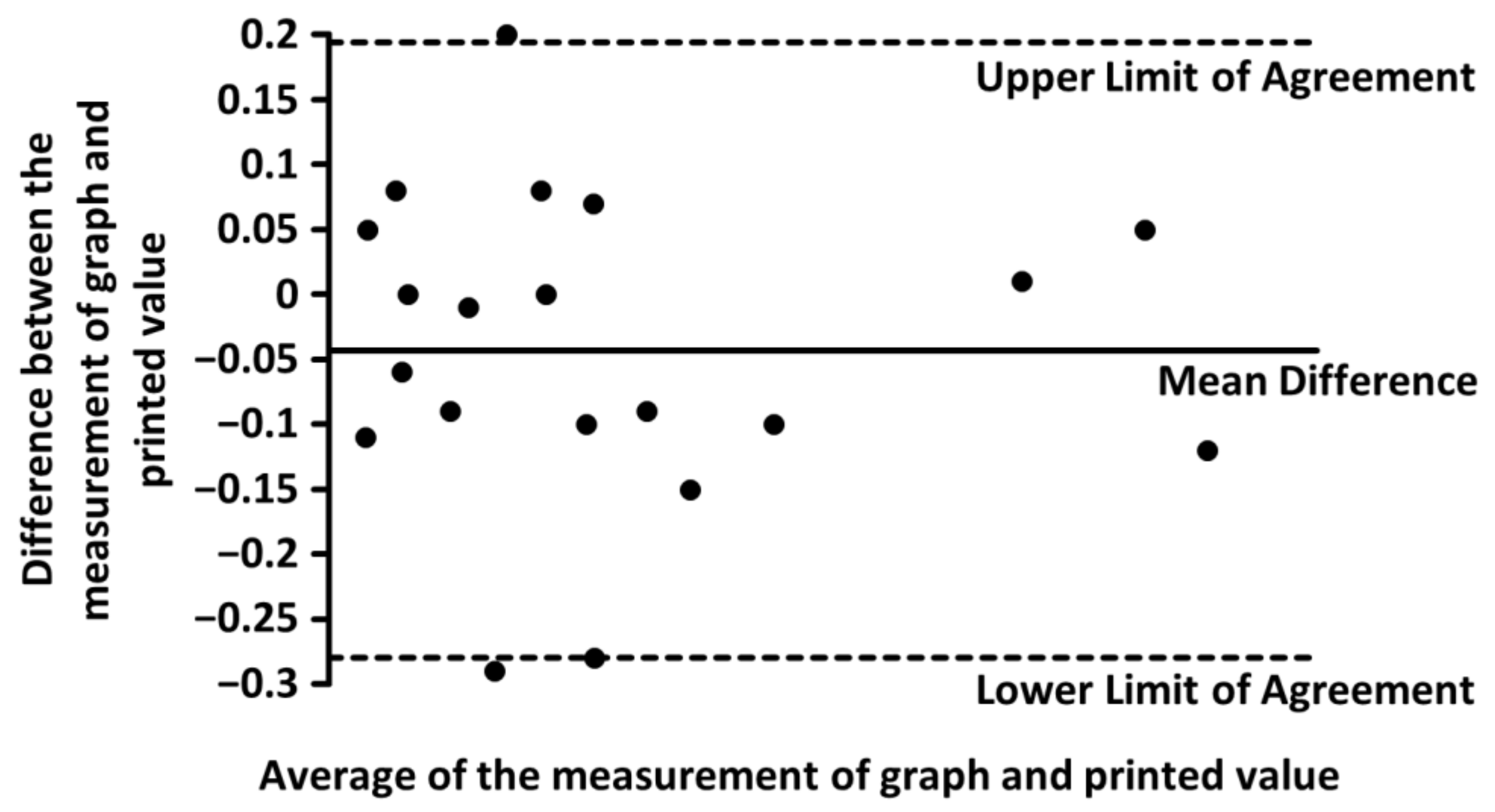
| Parameter | Measurement of Graph | Printed Value |
|---|---|---|
| N | 20 | |
| Mean | 1.40% | 1.44% |
| Standard Deviation | 1.26% | 1.26% |
| Mean Difference | −0.043 ± 0.24% | |
| +/− Limits of Agreement | ||
| Rat Strain | Sex | Staining Method | N | Mean Amount of Liver Connective Tissue | ||
|---|---|---|---|---|---|---|
| Pooled Mean | Jackknife Estimate of Pooled Mean | Bias | ||||
| Wistar | M | Masson’s trichrome | 11 | 2.76% | 3.04% | 0.00% |
| Picrosirius red | 13 | 1.76% | 1.91% | 0.00% | ||
| F | Masson’s trichrome | No data | ||||
| Picrosirius red | 1 | One study—Mannheimer et al. [3], 0.71% | ||||
| Sprague–Dawley | M | Masson’s trichrome | 17 | 2.30% | 2.45% | 0.00% |
| Picrosirius red | 23 | 2.41% | 2.52% | 0.00% | ||
| F | Masson’s trichrome | 1 | One study—Zhang et al. [16], 2.08% | |||
| Picrosirius red | 8 | 1.45% | 1.65% | 0.00% | ||
| Rat Strain | Sex | Staining Method | Number of Rat Groups | Mean Amount of Liver Connective Tissue | |||
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Pooled Mean | Pooled Standard Error | ||||
| Wistar | M | Masson’s trichrome | 11 | 0.51% | 5.16% | 2.76% | 0.27% |
| Picrosirius red | 13 | 0.12% | 5.12% | 1.76% | 0.34% | ||
| F | Masson’s trichrome | No data | |||||
| Picrosirius red | 1 | 0.71% | 0.11% | ||||
| One study—Mannheimer et al. [3] | |||||||
| Sprague–Dawley | M | Masson’s trichrome | 17 | 0.55% | 9.32% | 2.30% | 0.40% |
| Picrosirius red | 23 | 0.10% | 17.02% | 2.41% | 0.29% | ||
| F | Masson’s trichrome | 1 | 2.08% | 0.54% | |||
| One study—Zhang et al. [16] | |||||||
| Picrosirius red | 8 | 0.64% | 2.34% | 1.45% | 0.27% | ||
| Laboratory Animal | Sex | Staining Method | Amount of Liver Connective Tissue (Mean ± SD) | Reference |
|---|---|---|---|---|
| Mouse | M | Picrosirius red | 1.45 ± 0.07% | Clapper et al. [77] |
| Rat | M | Picrosirius red | 1.50% | Fallowfield et al. [78] |
| M | Picrosirius red | 0.50% | Hoy et al. [79] | |
| M | Picrosirius red | 2.0 ± 0.3% | Marcos et al. [80] | |
| M | Picrosirius red | 3.2 ± 0.2% | ||
| F | Picrosirius red | 2.00% | Marcos a Correia-Gomes [81] | |
| Pig | M | Aniline blue | 4.7 ± 2.4% | Mik et al. [82] |
| F | Aniline blue | 3.6 ± 2.2% |
| Rat Strain | Sex | Staining Method | Mean Amount of LCT | MDI in LCT | Expected Increase in LCT/Minimal Sample Size for the Detection of Such Increase | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.25-Fold | Size | 1.5-Fold | Size | 2-Fold | Size | |||||
| Wistar | M | Masson’s trichrome | 2.76% | 0.59% | 3.45% | 19 | 4.14% | 5 | 5.52% | 1 |
| M | Picrosirius red | 1.76% | 0.81% | 2.20% | 48 | 2.64% | 12 | 3.52% | 3 | |
| SD | M | Masson’s trichrome | 2.30% | 0.74% | 2.88% | 28 | 3.45% | 7 | 4.60% | 2 |
| M | Picrosirius red | 2.41% | 0.51% | 3.01% | 25 | 3.62% | 6 | 4.82% | 2 | |
| F | Picrosirius red | 1.45% | 0.69% | 1.81% | 70 | 2.18% | 18 | 2.90% | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mik, P.; Barannikava, K.; Surkova, P. Biased Quantification of Rat Liver Fibrosis—Meta-Analysis with Practical Recommendations and Clinical Implications. J. Clin. Med. 2023, 12, 5072. https://doi.org/10.3390/jcm12155072
Mik P, Barannikava K, Surkova P. Biased Quantification of Rat Liver Fibrosis—Meta-Analysis with Practical Recommendations and Clinical Implications. Journal of Clinical Medicine. 2023; 12(15):5072. https://doi.org/10.3390/jcm12155072
Chicago/Turabian StyleMik, Patrik, Katsiaryna Barannikava, and Polina Surkova. 2023. "Biased Quantification of Rat Liver Fibrosis—Meta-Analysis with Practical Recommendations and Clinical Implications" Journal of Clinical Medicine 12, no. 15: 5072. https://doi.org/10.3390/jcm12155072





