Evaluation of the Humoral and Cellular Immune Response Post COVID-19 Infection in Kidney Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
- -
- A study group composed of 36 adult kidney transplant recipients (the mean age was 35.90 ± 7.68 years and 61.11% were male);
- -
- A control group composed of 35 health-care subjects (the mean age was 34 ± 8.72 years and 45.71% were male), none of whom had comorbidities or had received immunosuppressive treatment before.
2.2. Methods
2.2.1. SARS-CoV-2 Serological Assessment
Measurement of SARS-CoV-2-Specific Antibodies
Detection of Neutralizing Antibodies against SARS-CoV-2
2.2.2. Evaluation of Cellular Immune Response
- -
- Epitopes of Ag1 are derived from the S1 subunit (receptor-binding domain) of the spike protein and stimulate CD4+ T cells.
- -
- Epitopes of Ag2 are obtained from the S1 and S2 subunits that stimulate CD4+ and CD8+ T cells.
- -
- Ag3 epitopes are derived from S1, S2 subunits, from N and M domain and from whole genome. These epitopes activate both CD4+ and CD8+ T cells.
2.3. Statistical Analysis
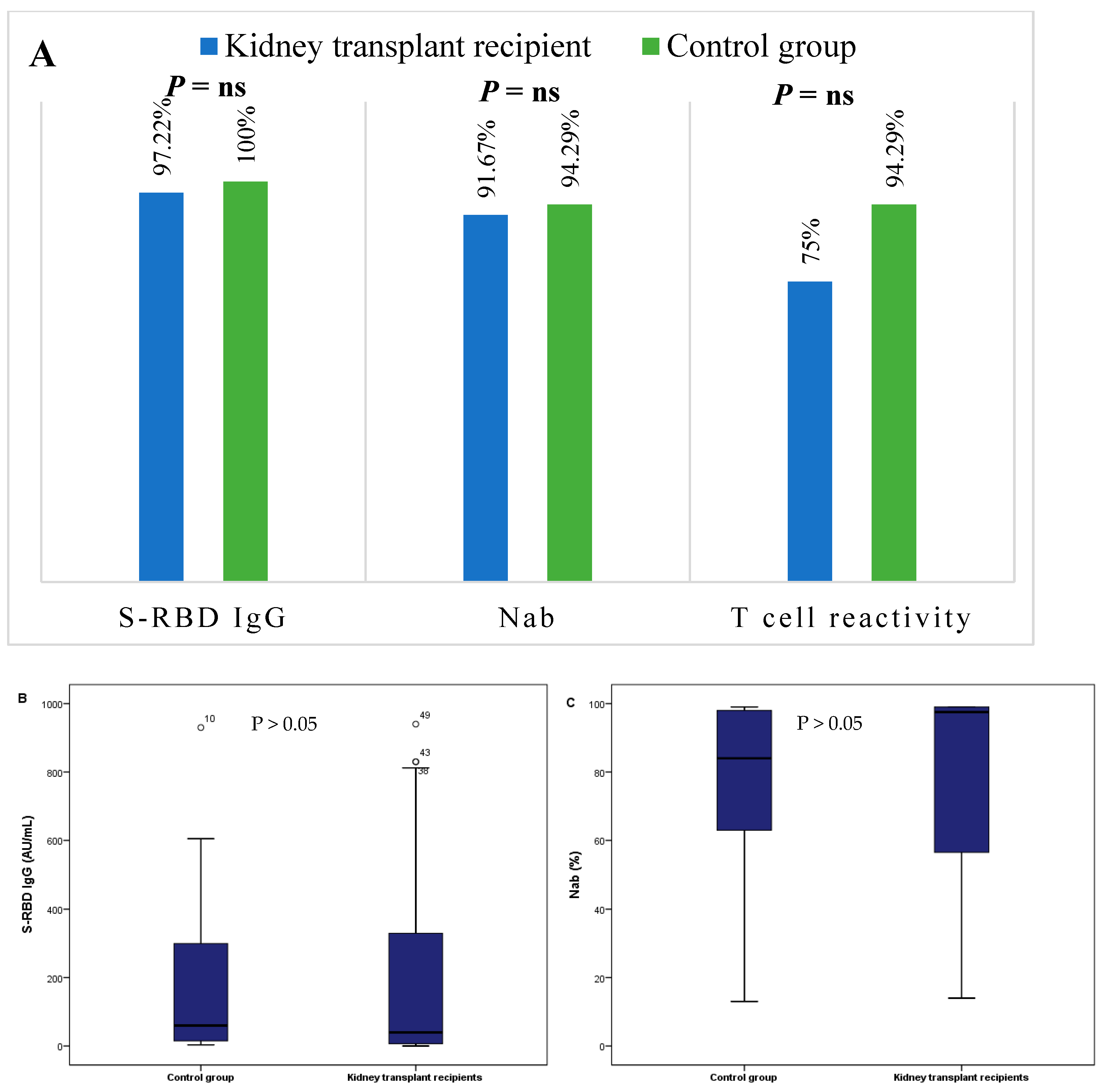
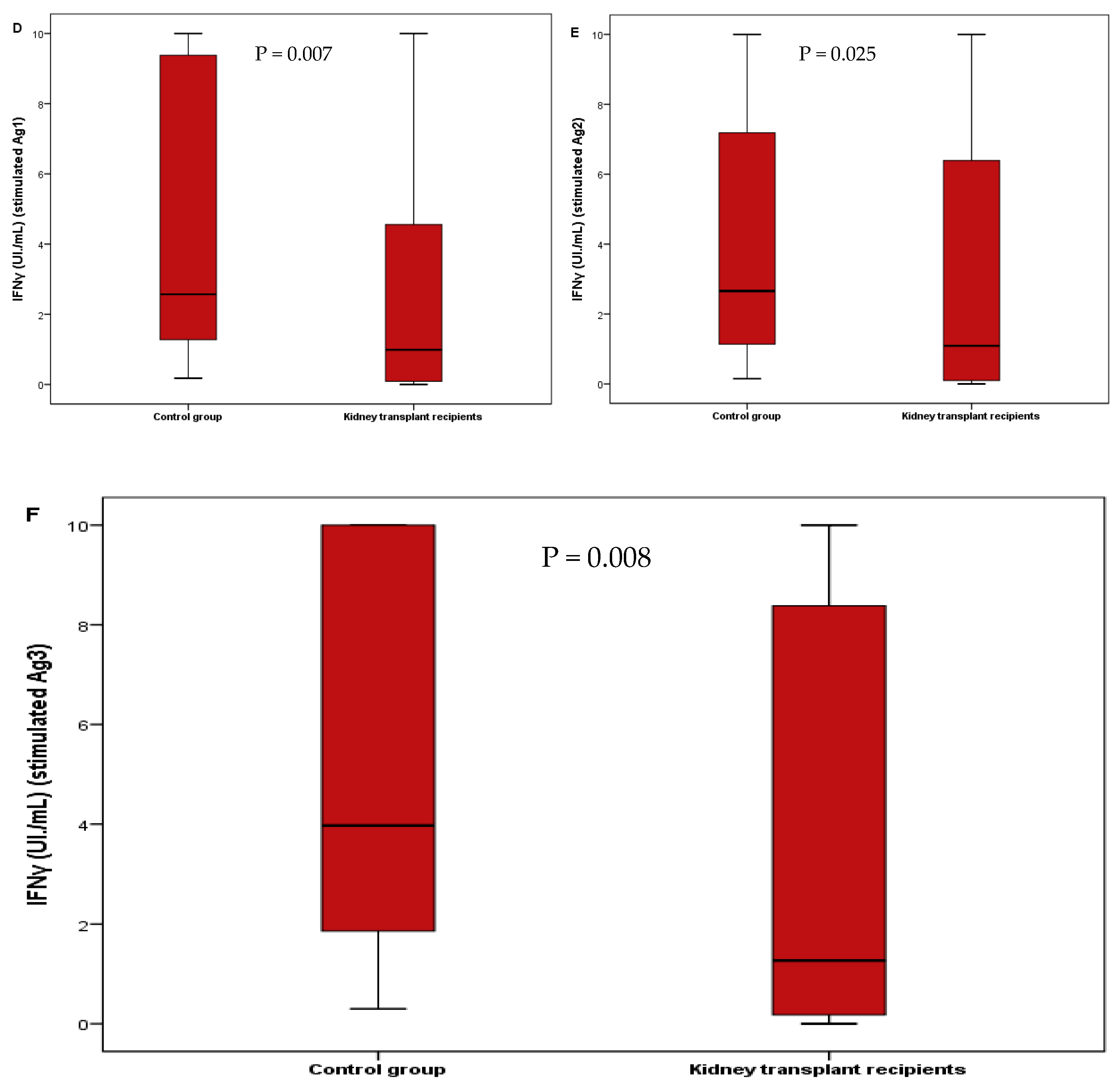
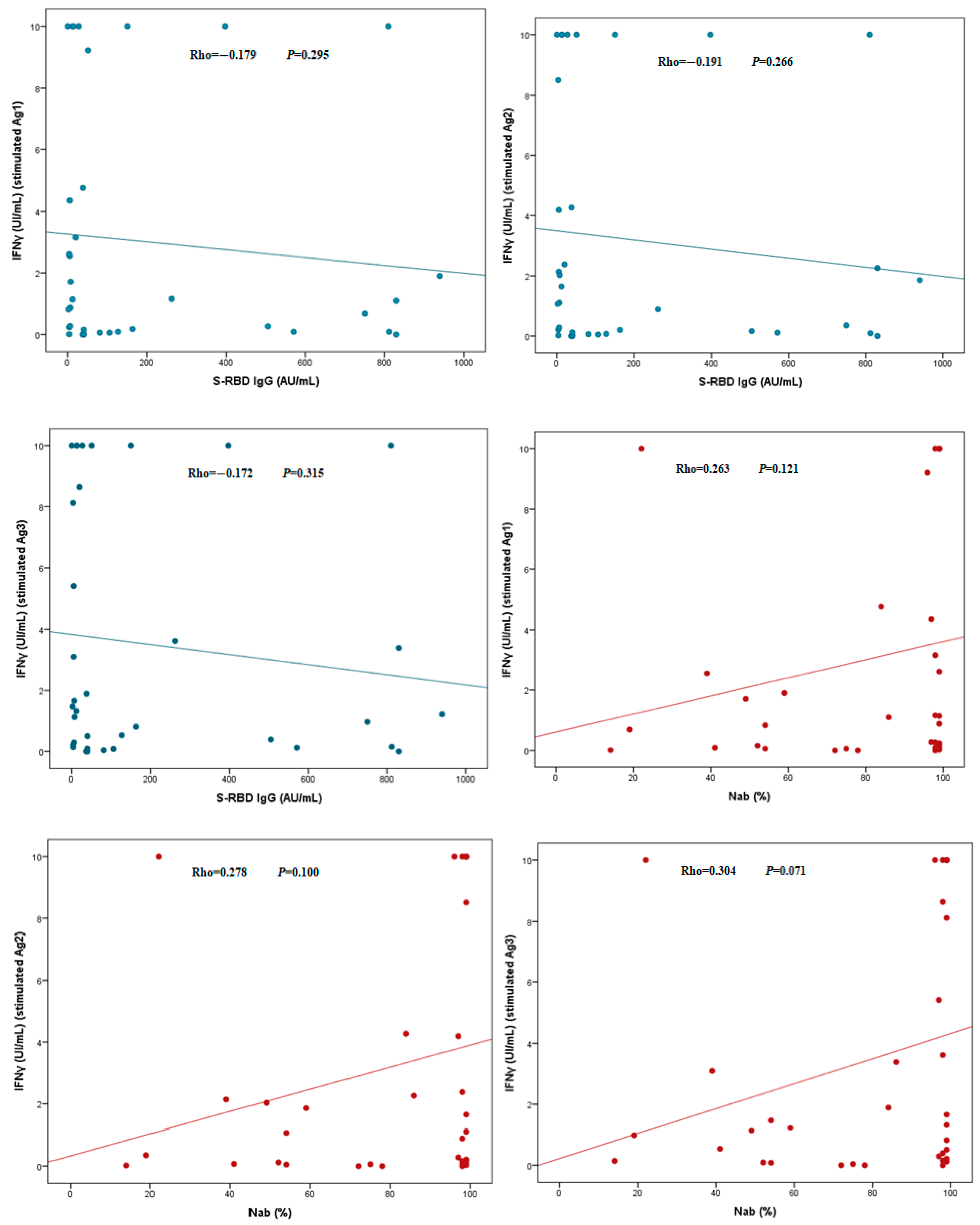
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Gorkhali, R.; Koirala, P.; Rijal, S.; Mainali, A.; Baral, A.; Bhattarai, H.K. Structure and Function of Major SARS-CoV-2 and SARS-CoV Proteins. Bioinform. Biol. Insights 2021, 15, 11779322211025876. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Wang, N.; Shang, J.; Jiang, S.; Du, L. Subunit Vaccines Against Emerging Pathogenic Human Coronaviruses. Front. Microbiol. 2020, 11, 298. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L. SARS-CoV-2 spike protein: A key target for eliciting persistent neutralizing antibodies. Signal Transduct. Target Ther. 2021, 6, 95. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Knies, A.; Ladage, D.; Braun, R.J.; Kimpel, J.; Schneider, M. Persistence of humoral response upon SARS-CoV-2 infection. Rev. Med. Virol. 2022, 32, e2272. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Stellas, D.; Rosati, M.; Sergentanis, T.N.; Hu, X.; Politou, M.; Pappa, V.; Ntanasis-Stathopoulos, I.; Karaliota, S.; Bear, J.; et al. SARS-CoV-2 antibody kinetics eight months from COVID-19 onset: Persistence of spike antibodies but loss of neutralizing antibodies in 24% of convalescent plasma donors. Eur. J. Intern. Med. 2021, 89, 87–96. [Google Scholar] [CrossRef]
- Grandjean, L.; Saso, A.; Ortiz, A.T.; Lam, T.; Hatcher, J.; Thistlethwayte, R.; Harris, M.; Best, T.; Johnson, M.; Wagstaffe, H.; et al. Long-Term Persistence of Spike Protein Antibody and Predictive Modeling of Antibody Dynamics After Infection With Severe Acute Respiratory Syndrome Coronavirus 2. Clin. Infect. Dis. 2022, 74, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.E.; Edwards, E.S.J.; Aui, P.M.; Varese, N.; Stojanovic, S.; McMahon, J.; Peleg, A.Y.; Boo, I.; Drummer, H.E.; Hogarth, P.M.; et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci. Immunol. 2020, 5, eabf8891. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol. Rev. 2022, 310, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Cassaniti, I.; Percivalle, E.; Bergami, F.; Piralla, A.; Comolli, G.; Bruno, R.; Vecchia, M.; Sambo, M.; Colaneri, M.; Zuccaro, V.; et al. SARS-CoV-2 specific T-cell immunity in COVID-19 convalescent patients and unexposed controls measured by ex vivo ELISpot assay. Clin. Microbiol. Infect. 2021, 27, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier-Vargas, G.; Wendling, M.-J.; Perrin, P.; Velay, A.; Bassand, X.; Bedo, D.; Baldacini, C.; Sagnard, M.; Bozman, D.-F.; et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am. J. Transpl. 2020, 20, 3162–3172. [Google Scholar] [CrossRef]
- Gérard, A.O.; Barbosa, S.; Anglicheau, D.; Couzi, L.; Hazzan, M.; Thaunat, O.; Blancho, G.; Caillard, S.; Sicard, A. Association Between Maintenance Immunosuppressive Regimens and COVID-19 Mortality in Kidney Transplant Recipients. Transplantation 2022, 106, 2063–2067. [Google Scholar] [CrossRef]
- Benotmane, I.; Vargas, G.G.; Velay, A.; Wendling, M.-J.; Perrin, P.; Fafi-Kremer, S.; Caillard, S. Persistence of SARS-CoV-2 antibodies in kidney transplant recipients. Am. J. Transpl. 2021, 21, 2307–2310. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.M.; Drouot, L.; Lamulle, J.M.; Hanoy, M.; Edet, S.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; et al. SARS-CoV-2-specific Humoral and Cellular Immunities in Kidney Transplant Recipients and Dialyzed Patients Recovered From Severe and Nonsevere COVID-19. Transpl. Direct 2021, 7, e792. [Google Scholar] [CrossRef] [PubMed]
- Favà, A.; Donadeu, L.; Sabé, N.; Pernin, V.; González-Costello, J.; Lladó, L.; Meneghini, M.; Charmetant, X.; García-Romero, E.; Cachero, A.; et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am. J. Transpl. 2021, 21, 2749–2761. [Google Scholar] [CrossRef] [PubMed]
- Favà, A.; Donadeu, L.; Jouve, T.; Gonzalez-Costello, J.; Lladó, L.; Santana, C.; Toapanta, N.; Lopez, M.; Pernin, V.; Facundo, C.; et al. A comprehensive assessment of long-term SARS-CoV-2-specific adaptive immune memory in convalescent COVID-19 Solid Organ Transplant recipients. Kidney Int. 2022, 101, 1027–1038. [Google Scholar] [CrossRef]
- Magicova, M.; Fialova, M.; Zahradka, I.; Rajnochova-Bloudickova, S.; Hackajlo, D.; Raska, P.; Striz, I.; Viklicky, O. Humoral response to SARS-CoV-2 is well preserved and symptom dependent in kidney transplant recipients. Am. J. Transplant. 2021, 21, 3926–3935. [Google Scholar] [CrossRef]
- Chavarot, N.; Leruez-Ville, M.; Scemla, A.; Burger, C.; Amrouche, L.; Rouzaud, C.; Lebreton, X.; Martinez, F.; Sberro-Soussan, R.; Legendre, C.; et al. Decline and loss of anti-SARS-CoV-2 antibodies in kidney transplant recipients in the 6 months following SARS-CoV-2 infection. Kidney Int. 2021, 99, 486–488. [Google Scholar] [CrossRef]
- Bajpai, D.; Shah, D.; Bose, S.; Deb, S.; Gandhi, C.; Modi, T.; Rao, N.; Kumar, K.; Saxena, N.; Katyal, A.; et al. Development and longevity of antibodies against SARS-CoV-2 in kidney transplant recipients after symptomatic COVID-19. Transpl. Infect. Dis. 2021, 23, e13646. [Google Scholar] [CrossRef]
- Søfteland, J.M.; Gisslén, M.; Liljeqvist, J.; Friman, V.; de Coursey, E.; Karason, K.; Ekelund, J.; Felldin, M.; Magnusson, J.; Baid-Agrawal, S.; et al. Longevity of anti-spike and anti-nucleocapsid antibodies after COVID-19 in solid organ transplant recipients compared to immunocompetent controls. Am. J. Transpl. 2022, 22, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.M.; Olea, B.; Giménez, E.M.; Laguna-Goya, R.M.; Trujillo, H.; Caravaca-Fontán, F.M.; Gutiérrez, E.M.; López-Medrano, F.M.; Remigia, M.J.M.; Almendro-Vazquez, P.B.; et al. SARS-CoV-2-specific Cell-mediated Immunity in Kidney Transplant Recipients Recovered From COVID-19. Transplantation 2021, 105, 1372–1380. [Google Scholar] [CrossRef]
- Kamińska, D.; Augustyniak-Bartosik, H.; Kościelska-Kasprzak, K.; Żabińska, M.; Bartoszek, D.; Poznański, P.; Kuriata-Kordek, M.; Kusztal, M.; Mazanowska, O.; Krajewska, M. Comparing Humoral and Cellular Adaptive Immunity during Convalescent Phase of COVID-19 in Hemodialysis Patients and Kidney Transplant Recipients. J. Clin. Med. 2021, 10, 4833. [Google Scholar] [CrossRef]
- Affeldt, P.; Koehler, F.C.; Brensing, K.A.; Adam, V.; Burian, J.; Butt, L.; Gies, M.; Grundmann, F.; Hinrichs, S.; Johannis, W.; et al. Immune Responses to SARS-CoV-2 Infection and Vaccination in Dialysis Patients and Kidney Transplant Recipients. Microorganisms 2021, 10, 4. [Google Scholar] [CrossRef]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwöbel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021, 9, 100178. [Google Scholar] [CrossRef]
- Kute, V.B.; Bhalla, A.K.; Guleria, S.; Ray, D.S.; Bahadur, M.M.; Shingare, A.; Hegde, U.; Gang, S.; Raju, S.; Patel, H.V.; et al. Clinical Profile and Outcome of COVID-19 in 250 Kidney Transplant Recipients: A Multicenter Cohort Study From India. Transplantation 2021, 105, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Cantarelli, C.; Angeletti, A.; Perin, L.; Russo, L.S.; Sabiu, G.; Podestà, M.A.; Cravedi, P. Immune responses to SARS-CoV-2 in dialysis and kidney transplantation. Clin. Kidney J. 2022, 15, 1816–1828. [Google Scholar] [CrossRef]
- Crespo, M.; Barrilado-Jackson, A.; Padilla, E.; Eguía, J.; Echeverria-Esnal, D.; Cao, H.; Faura, A.; Folgueiras, M.; Solà-Porta, E.; Pascual, S.; et al. Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring. Am. J. Transpl. 2022, 22, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transpl. 2021, 21, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Candon, S.; Guerrot, D.; Drouot, L.; Lemoine, M.; Lebourg, L.; Hanoy, M.; Boyer, O.; Bertrand, D. T cell and antibody responses to SARS-CoV-2: Experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am. J. Transpl. 2021, 21, 854–863. [Google Scholar] [CrossRef]
| Variable | Kidney Transplant Recipients (n = 36) | Control Group (n = 35) |
|---|---|---|
| Age (years) mean ± SD [extremes] | 35.9 ± 7.68 [21–57] | 34 ± 8.72 [20–55] |
| Gender | ||
| 22 (61.11) 14 (38.89) | 16 (45.71) 19 (54.29) |
| Comorbidities | ||
| No, n (%) | 13 (36.11) | 35 (100%) |
| Yes, n (%) | 23 (63.89) | |
| 22 (95.65) 1 (4.35) | |
| Cause of end-stage renal disease | ||
| Hypertension, n (%) | 1 (2.78) | |
| Glomerulonephritis, n (%) | 1 (2.78) | |
| Renal malformation, n (%) | 4 (11.11) | |
| Unknown, n (%) | 30 (83.3) | |
| Time from transplantation (years) mean ± SD [extremes] | 6.14 ± 3.32 [0.6–14] | |
| Immunosuppressive therapy | ||
| CNI only, n (%) | 2 (5.56) | |
| CNI+MMF, n (%) | 12 (33.33) | |
| CNI+CS, n (%) | 6 (16.67) | |
| CNI+MMF+CS, n (%) | 16 (44.44) | |
| History of rejection, n (%) | 9 (25) | |
| Biological parameters | ||
| 5975 ± 1564 7 (19.44) 814.3 ± 63.67 0.45 ± 0.17 15.96 ± 5.54 | |
| Time from COVID-19 disease (months) mean ± SD | 5.22 ± 0.96 |
| Comorbidities | History of Rejection | Lymphopenia | Immunosuppressive Therapy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes n = 23 | No n = 13 | p | Yes n = 9 | No n = 27 | p | Yes n = 7 | No n = 29 | p | CNI+MMF n = 12 | CNI+CS n = 6 | CNI+MMF+CS n = 16 | p | ||
| Humoral immune response | RBD median ± IQR | 27.11 [6.04–150] | 127 [29.75–790] | 0.070 | 7.25 [3.71–660.5] | 39.98 [13.14–262] | 0.391 | 127 [5.96–361.16] | 39.80 [6.88–383.5] | 0.920 | 94.99 [29.51–527.5] | 35.21 [9.1–667.5] | 25.45 [4.40–154] | 0.666 |
| Nab median ± IQR | 98 [78–99] | 75 [46.5–98] | 0.077 | 75 [34–99] | 98 [72–99] | 0.576 | 99 [44.25–99] | 97 [56.5–99] | 0.262 | 98 [82.75–99] | 97 [62.5–98.5] | 79.5 [49.75–99] | 0.543 | |
| Cellular immune response | Ag1 median ± IQR | 1.71 [0.18–10] | 0.27 [0.07–1.5] | 0.098 | 1.29 [0.79–4.45] | 2.85 [0.48–10] | 0.557 | 10 [1.72–10] | 1.71 [0.48–6.98] | 0.236 | 10 [4.35–10] | 5.88 [1.46–9.80] | 0.48 [0.10–1.85] | 0.389 |
| Ag2 median ± IQR | 2.03 [0.2–10] | 1.86 [0.35–2.38] | 0.105 | 1.55 [0.89–8.88] | 2.38 [0.89–10] | 0.713 | 10 [4.79–10] | 2.08 [0.48–8.56] | 0.213 | 10 [4.19–10] | 2.26 [1.15–10] | 1.11 [0.28–4.27] | 0.344 | |
| Ag3 median ± IQR | 3.62 [1.13–10] | 1.22 [0.51–6.01] | 0.145 | 1.56 [1.09–8.59] | 3.24 [0.52–10] | 0.607 | 8.12 [0.53–10] | 1.89 [0.89–9.32] | 0.127 | 10 [4.06–10] | 3.39 [1.74–10] | 0.89 [0.16–1.61] | 0.192 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensaid, K.; Lamara Mahammed, L.; Habchi, K.; Saidani, M.; Allam, I.; Djidjik, R. Evaluation of the Humoral and Cellular Immune Response Post COVID-19 Infection in Kidney Transplant Recipients. J. Clin. Med. 2023, 12, 3900. https://doi.org/10.3390/jcm12123900
Bensaid K, Lamara Mahammed L, Habchi K, Saidani M, Allam I, Djidjik R. Evaluation of the Humoral and Cellular Immune Response Post COVID-19 Infection in Kidney Transplant Recipients. Journal of Clinical Medicine. 2023; 12(12):3900. https://doi.org/10.3390/jcm12123900
Chicago/Turabian StyleBensaid, Kahina, Lydia Lamara Mahammed, Khadidja Habchi, Messaoud Saidani, Ines Allam, and Reda Djidjik. 2023. "Evaluation of the Humoral and Cellular Immune Response Post COVID-19 Infection in Kidney Transplant Recipients" Journal of Clinical Medicine 12, no. 12: 3900. https://doi.org/10.3390/jcm12123900






