Microcirculatory Disease in Patients after Heart Transplantation
Abstract
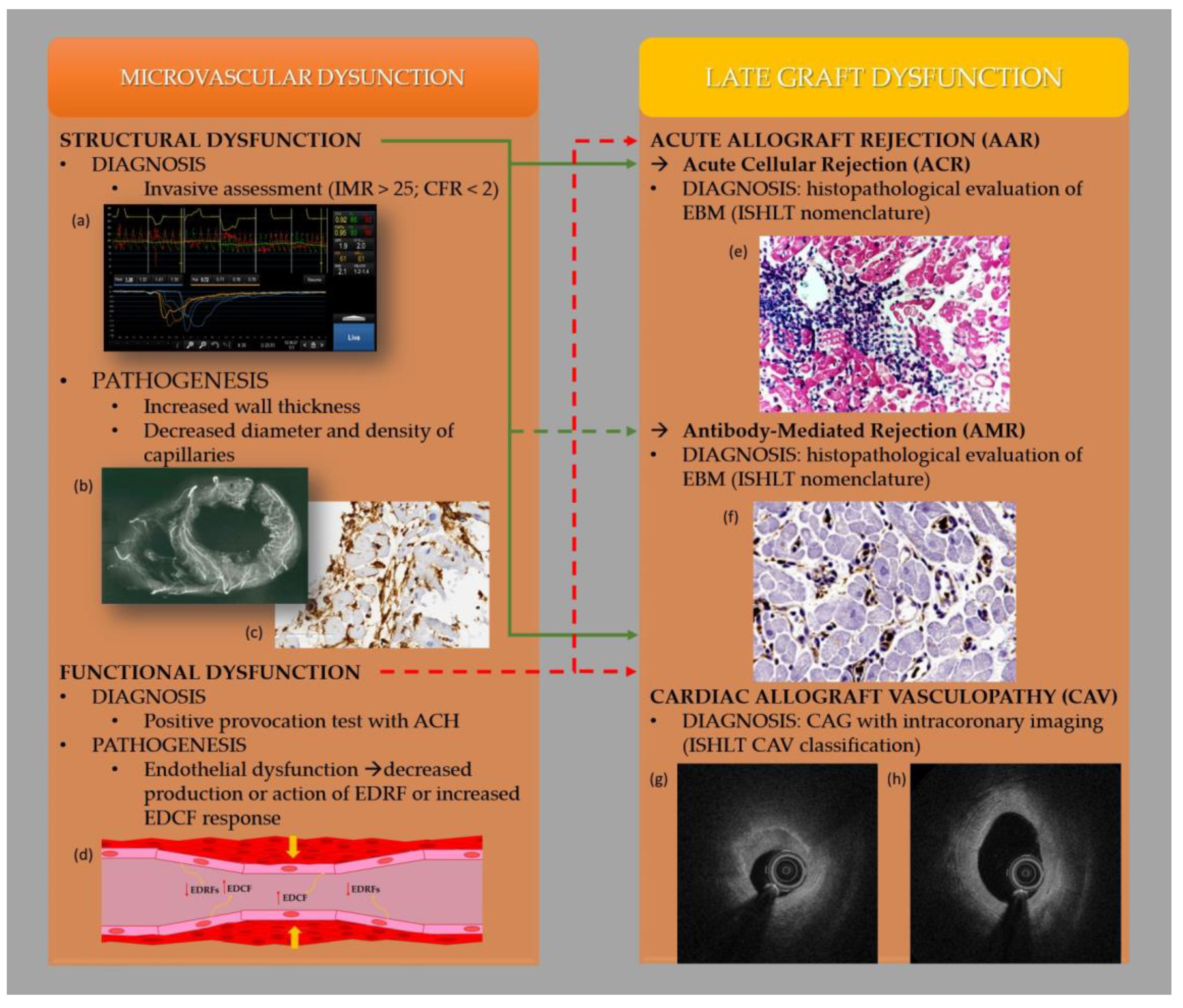
:1. Introduction
2. Coronary Microcirculation Assessment Methods
3. Mechanisms of Heart Transplant Rejection
4. Coronary Microcirculation Disease in Patients after Heart Transplantation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tremblay-Gravel, M.; Racine, N.; de Denus, S.; Ducharme, A.; Pelletier, G.B.; Giraldeau, G.; Liszkowski, M.; Parent, M.-C.; Carrier, M.; Fortier, A.; et al. Changes in Outcomes of Cardiac Allograft Vasculopathy over 30 Years Following Heart Transplantation. JACC Heart Fail. 2017, 5, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, J.; Zuckermann, A.; Macdonald, P.; Leprince, P.; Esmailian, F.; Luu, M.; Mancinni, D.; Patel, J.; Razi, R.; Reichenspurner, H.; et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J. Heart Lung Transplant. 2014, 33, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Piening, B.D.; Dowdell, A.K.; Zhang, M.; Loza, B.L.; Walls, D.; Gao, H.; Mohebnasab, M.; Li, Y.R.; Elftmann, E.; Wei, E.; et al. Whole transcriptome profiling of prospective endomyocardial biopsies reveals prognostic and diagnostic signatures of cardiac allograft rejection. J. Heart Lung Transplant. 2022, 41, 840–848. [Google Scholar] [CrossRef]
- Otunla, A.A.; Shanmugarajah, K.; Madariaga, M.L.; Davies, A.H.; Shalhoub, J. Chronic Rejection and Atherosclerosis in Post-Transplant Cardiovascular Mortality: Two Sides of the Same Coin. Heart Lung Circ. 2022, 31, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report—2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Majmudar, M.D.; Murthy, V.L.; Shah, R.V.; Kolli, S.; Mousavi, N.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Sitek, A.; et al. Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Neglia, D.; Michelassi, C.; Giovanna Trivieri, M.; Sambuceti, G.; Giorgetti, A.; Pratali, L.; Gallopin, M.; Salvadori, P.; Sorace, O.; Carpeggiani, C.; et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002, 105, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Taqueti, V.R.; Everett, B.M.; Murthy, V.L.; Gaber, M.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Di Carli, M.F. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015, 131, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Taqueti, V.R.; Hachamovitch, R.; Murthy, V.L.; Naya, M.; Foster, C.R.; Hainer, J.; Dorbala, S.; Blankstein, R.; Di Carli, M.F. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015, 131, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Klotzka, A.; Iwańczyk, S.; Ropacka-Lesiak, M.; Misan, N.; Lesiak, M. Anthracycline-induced microcirculation disorders: AIM PILOT Study. Kardiol. Pol. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Crea, F.; Camici, P.G.; Merz, C.N.B. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Shimokawa, H. Coronary Vasomotion Abnormalities; Springer Nature: Singapore, 2021. [Google Scholar]
- Shimokawa, H. 2014 Williams Harvey Lecture: Importance of coronary vasomotion abnormalities-from bench to bedside. Eur. Heart J. 2014, 35, 3180–3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar]
- Fearon, W.F.; Hirohata, A.; Nakamura, M.; Luikart, H.; Lee, D.P.; Vagelos, R.H.; Hunt, S.A.; Valantine, H.A.; Fitzgerald, P.J.; Yock, P.G.; et al. Discordant changes in epicardial and microvascular coronary physiology after cardiac transplantation: Physiologic Investigation for Transplant Arteriopathy II (PITA II) study. J. Heart Lung Transplant. 2006, 25, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Iwańczyk, S.; Smukowska-Gorynia, A.; Woźniak, P.; Grygier, M.; Lesiak, M.; Araszkiewicz, A. Invasive assessment of the microvascular coronary circulation in patients with coronary artery aneurysmal disease. Pol. Arch. Intern. Med. 2023, 33, 16392. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.; Bergmark, B.A.; Vita, T.; Taqueti, V.R.; Gupta, A.; Seidelmann, S.; Christensen, T.E.; Osborne, M.T.; Shah, N.R.; Ghosh, N.; et al. Diagnostic and prognostic value of myocardial blood flow quantification as non-invasive indicator of cardiac allograft vasculopathy. Eur. Heart J. 2018, 39, 316–323. [Google Scholar] [CrossRef]
- Sen, S.; Petraco, R.; Mayet, J.; Davies, J. Wave intensity analysis in the human coronary circulation in health and disease. Curr. Cardiol. Rev. 2014, 10, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Cuculi, F.; De Maria, G.L.; Meier, P.; Dall’Armellina, E.; De Caterina, A.R.; Channon, K.M.; Prendergast, B.D.; Choudhury, R.C.; Forfar, J.C.; Kharbanda, R.K.; et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2014, 64, 1894–1904. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, W.; Shaukat, A.; Linsday, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef]
- Berry, G.J.; Burke, M.M.; Andersen, C.; Bruneval, P.; Fedrigo, M.; Fishbein, M.C.; Goddard, M.; Hammond, E.H.; Leone, O.; Marboe, C.; et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J. Heart Lung Transplant. 2013, 32, 1147–1162. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Choi, J.O.; Shin, D.; Park, Y.; Kim, J.; Lee, S.H.; Kim, D.; Yang, J.H.; Cho, Y.K.; et al. Coronary Microcirculatory Dysfunction and Acute Cellular Rejection After Heart Transplantation. Circulation 2021, 144, 1459–1472. [Google Scholar] [CrossRef]
- Rossano, J.W.; Singh, T.P.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric heart transplantation report—2019; Focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Chih, S.; Chong, A.Y.; Erthal, F.; deKemp, R.A.; Davies, R.A.; Stadnick, E.; So, D.Y.; Overgaard, C.; Wells, G.; Mielniczuk, L.M.; et al. PET Assessment of Epicardial Intimal Disease and Microvascular Dysfunction in Cardiac Allograft Vasculopathy. J. Am. Coll. Cardiol. 2018, 71, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Fearon, W.F. Invasive coronary microcirculation assessment--current status of index of microcirculatory resistance. Circ. J. 2014, 78, 1021–1028. [Google Scholar] [CrossRef] [Green Version]
- Haddad, F.; Khazanie, P.; Deuse, T.; Weisshaar, D.; Zhou, J.; Nam, C.W.; Vu, T.A.; Gomari, F.A.; Skhiri, M.; Simos, A.; et al. Clinical and functional correlates of early microvascular dysfunction after heart transplantation. Circ. Heart Fail. 2012, 5, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.G.; Cooper, D.K. A histopathologic grading system of hyperacute (humoral, antibody-mediated) cardiac xenograft and allograft rejection. J. Heart Lung Transplant. 1996, 15, 804–817. [Google Scholar] [PubMed]
- Ludhwani, D.; Abraham, J.; Kanmanthareddy, A. Heart Transplantation Rejection; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ramzy, D.; Rao, V.; Brahm, J.; Miriuka, S.; Delgado, D.; Ross, H.J. Cardiac allograft vasculopathy: A review. Can. J. Surg. 2005, 48, 319. [Google Scholar] [PubMed]
- Pober, J.S.; Chih, S.; Kobashigawa, J.; Madsen, J.C.; Tellides, G. Cardiac allograft vasculopathy: Current review and future research directions. Cardiovasc. Res. 2021, 117, 2624–2638. [Google Scholar] [CrossRef]
- Guddeti, R.R.; Matsuo, Y.; Matsuzawa, Y.; Aoki, T.; Lerman, L.O.; Kushwaha, S.S.; Lerman, A. Clinical implications of intracoronary imaging in cardiac allograft vasculopathy. Circ. Cardiovasc. Imaging 2014, 8, e002636. [Google Scholar] [CrossRef]
- Ahn, J.M.; Zimmermann, F.M.; Gullestad, L.; Angerås, O.; Karason, K.; Russell, K.; Lunde, K.; Okada, K.; Luikart, H.; Khush, K.K.; et al. Microcirculatory Resistance Predicts Allograft Rejection and Cardiac Events After Heart Transplantation. J. Am. Coll. Cardiol. 2021, 78, 2425–2435. [Google Scholar] [CrossRef]
- Yang, H.M.; Khush, K.; Luikart, H.; Okada, K.; Lim, H.S.; Kobayashi, Y.; Honda, Y.; Yeung, A.C.; Valantine, H.; Fearon, W.F. Invasive Assessment of Coronary Physiology Predicts Late Mortality After Heart Transplantation. Circulation 2016, 133, 1945–1950. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Honda, Y.; Luikart, H.; Yock, P.G.; Fitzgerald, P.J.; Yeung, A.C.; Valantine, A.H.; Khush, K.K.; Fearon, W.F. Early invasive assessment of the coronary microcirculation predicts subsequent acute rejection after heart transplantation. Int. J. Cardiol. 2019, 290, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Song, G.; Bai, X. Predictive Efficacy of the Index of Microcirculatory Resistance for Acute Allograft Rejection and Cardiac Events After Heart Transplantation: A Systematic Review and Meta-Analysis. Heart Surg. Forum. 2022, 25, E784–E792. [Google Scholar] [CrossRef] [PubMed]
- Afzali, B.; Chapman, E.; Racapé, M.; Adam, B.; Bruneval, P.; Gil, F.; Kim, D.; Hidalgo, L.; Campbell, P.; Sis, B.; et al. Molecular Assessment of Microcirculation Injury in Formalin-Fixed Human Cardiac Allograft Biopsies with Antibody-Mediated Rejection. Am. J. Transplant. 2017, 17, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Colvin-Adams, M.; Harcourt, N.; Duprez, D. Endothelial dysfunction and cardiac allograft vasculopathy. J. Cardiovasc. Transl. Res. 2013, 6, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Klein, L.W.; Parrillo, J.E.; Scherer, M.; Burns, D.; Tamburro, P.; Oberoi, M.; Johnson, M.R.; Costanzo, M.R. Coronary Endothelial Dysfunction After Heart Transplantation Predicts Allograft Vasculopathy and Cardiac Death. Circulation 2001, 104, 3091–3096. [Google Scholar] [CrossRef] [Green Version]
- Escaned, J.; Flores, A.; García-Pavía, P.; Segovia, J.; Jimenez, J.; Aragoncillo, P.; Salas, C.; Alfonso, F.; Hernandez, R.; Angiolillo, D.J.; et al. Assessment of microcirculatory remodeling with intracoronary flow velocity and pressure measurements: Validation with endomyocardial sampling in cardiac allografts. Circulation 2009, 120, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Okada, K.; Khush, K.; Kobayashi, Y.; Sinha, S.; Luikart, H.; Valantine, H.; Yeung, A.C.; Honda, Y.; Fearon, W.F. Coronary Endothelial Dysfunction and the Index of Microcirculatory Resistance as a Marker of Subsequent Development of Cardiac Allograft Vasculopathy. Circulation 2017, 135, 1093. [Google Scholar] [CrossRef] [Green Version]
- Chih, S.; Chong, A.Y.; Džavík, V.; So, D.Y.; Aleksova, N.; Wells, G.A.; Bernick, J.; Overgaard, C.B.; Stadnick, E.; Mielniczuk, L.M.; et al. Fibrotic Plaque and Microvascular Dysfunction Predict Early Cardiac Allograft Vasculopathy Progression After Heart Transplantation: The Early Post Transplant Cardiac Allograft Vasculopathy Study. Circ. Heart Fail. 2023, e010173. [Google Scholar] [CrossRef]
- Flyer, J.N.; Zuckerman, W.A.; Richmond, M.E.; Anderson, B.R.; Mendelsberg, T.G.; McAllister, J.M.; Liberman, L.; Addonizio, L.J.; Silver, E.S. Prospective Study of Adenosine on Atrioventricular Nodal Conduction in Pediatric and Young Adult Patients after Heart Transplantation. Circulation 2017, 135, 2485–2493. [Google Scholar] [CrossRef]
- Cheung, A.; Menkis, A.H. Cyclosporine heart transplantation. Transplant. Proc. 1998, 30, 1881–1884. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Miller, G.G.; Shakar, S.F.; Zolty, R.; Lowes, B.D.; Wolfel, E.E.; Mestroni, L.; Pagell, R.L.; Kobashigawa, J. Drug therapy in the heart transplant recipient: Part II. Immunosuppressive drugs. Circulation 2004, 110, 3858–3865. [Google Scholar] [CrossRef] [PubMed]
- Benzimra, M.; Calligaro, G.L.; Glanville, A.R. Acute rejection. J. Thorac. Dis. 2017, 9, 5440–5457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwańczyk, S.; Woźniak, P.; Smukowska-Gorynia, A.; Araszkiewicz, A.; Nowak, A.; Jankowski, M.; Konwerska, A.; Urbanowicz, T.; Lesiak, M. Microcirculatory Disease in Patients after Heart Transplantation. J. Clin. Med. 2023, 12, 3838. https://doi.org/10.3390/jcm12113838
Iwańczyk S, Woźniak P, Smukowska-Gorynia A, Araszkiewicz A, Nowak A, Jankowski M, Konwerska A, Urbanowicz T, Lesiak M. Microcirculatory Disease in Patients after Heart Transplantation. Journal of Clinical Medicine. 2023; 12(11):3838. https://doi.org/10.3390/jcm12113838
Chicago/Turabian StyleIwańczyk, Sylwia, Patrycja Woźniak, Anna Smukowska-Gorynia, Aleksander Araszkiewicz, Alicja Nowak, Maurycy Jankowski, Aneta Konwerska, Tomasz Urbanowicz, and Maciej Lesiak. 2023. "Microcirculatory Disease in Patients after Heart Transplantation" Journal of Clinical Medicine 12, no. 11: 3838. https://doi.org/10.3390/jcm12113838




