Impaired Neutralizing Antibody Activity against B.1.617.2 (Delta) after Anti-SARS-CoV-2 Vaccination in Patients Receiving Anti-CD20 Therapy
Abstract
:1. Introduction
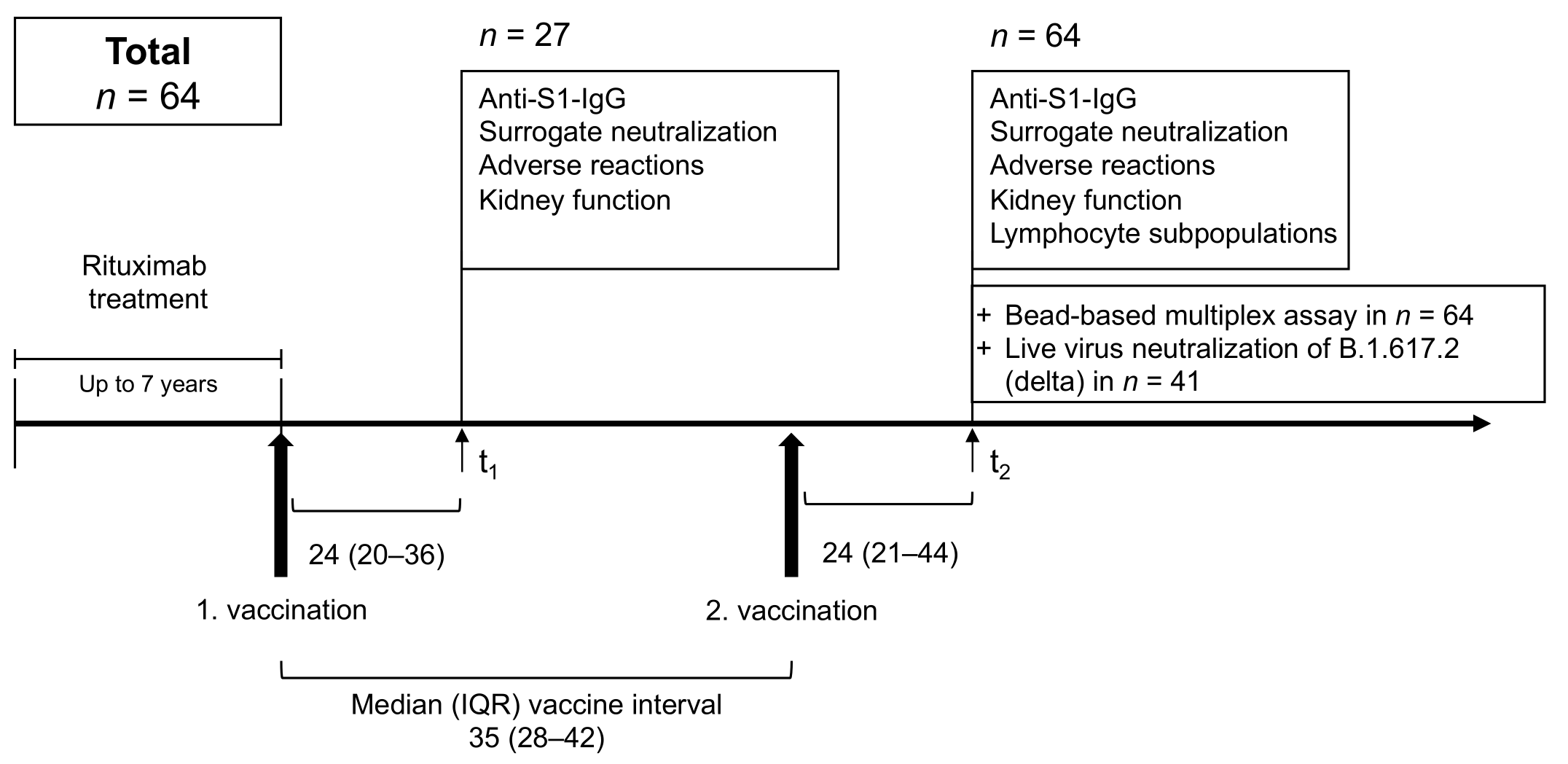
2. Materials and Methods
2.1. Study Design
2.2. IgG Antibodies against SARS-CoV-2 Spike S1 and Nucleocapsid
2.3. SARS-CoV-2 Specific Surrogate Neutralizing Antibodies
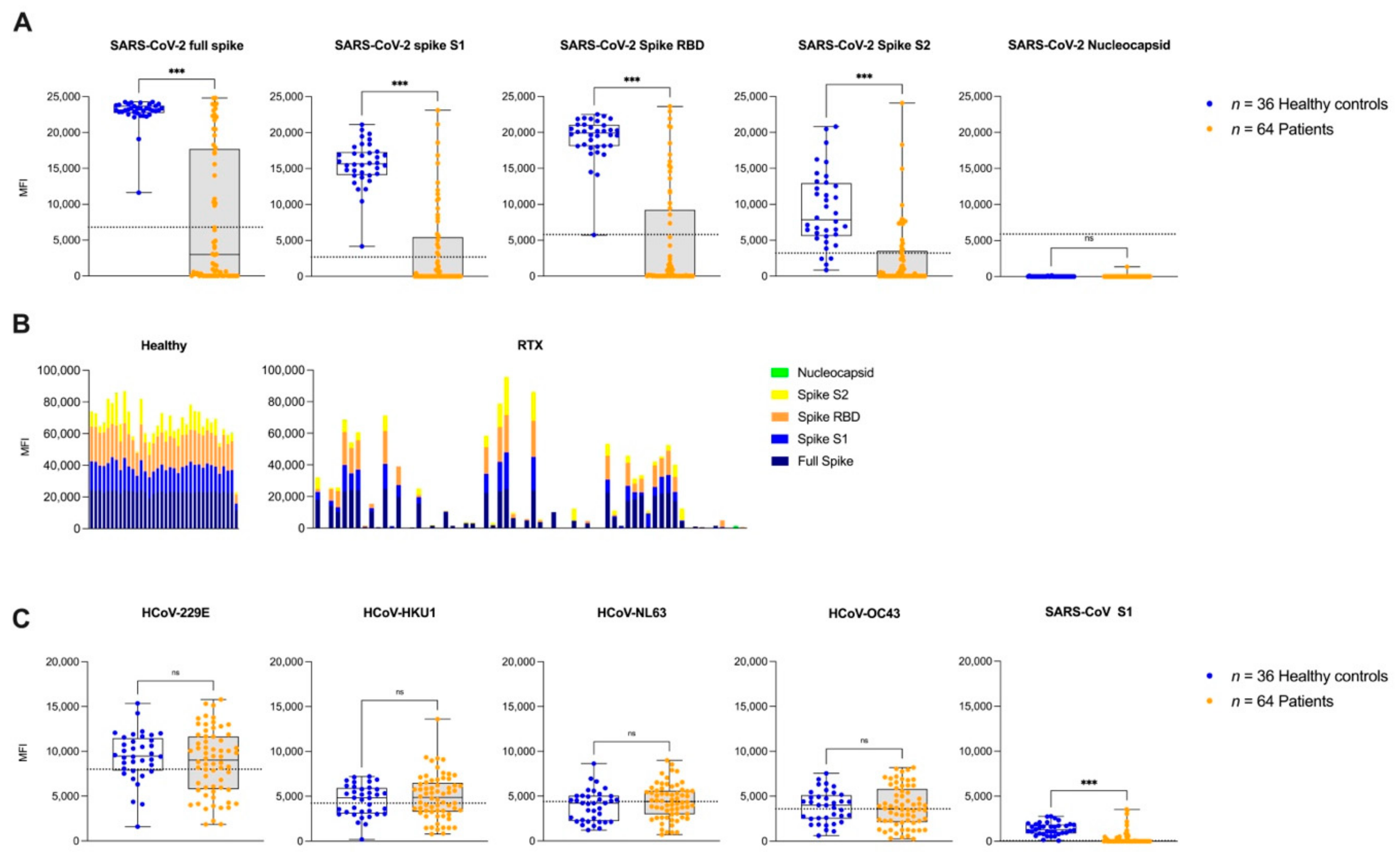
2.4. IgG Antibodies against Different SARS-CoV-2 and Common Cold Coronaviruses Target Epitopes
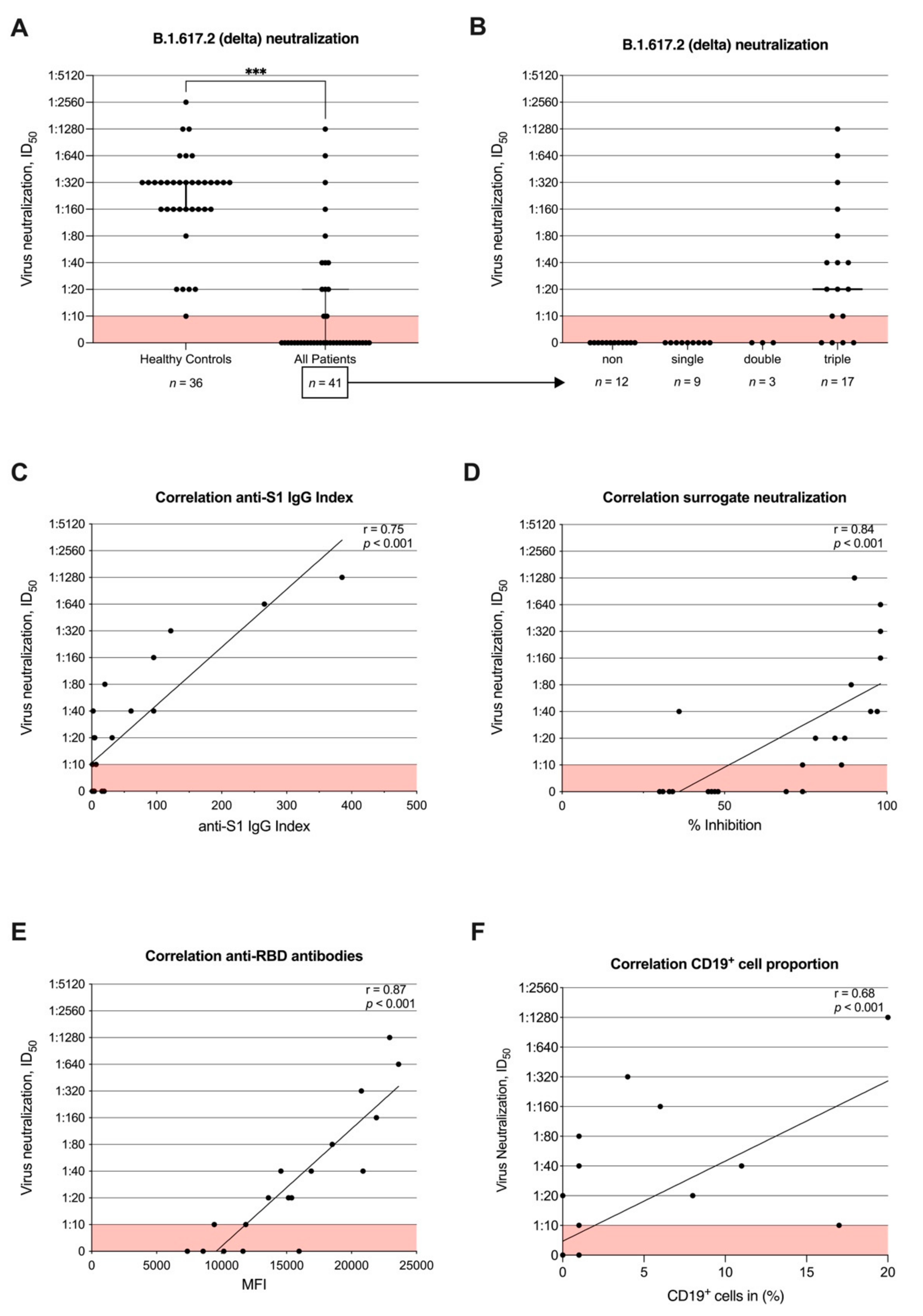
2.5. Neutralization against the B.1.617.2 (Delta) Variant of Concern
2.6. Statistics
3. Results
3.1. Study Population
3.2. Anti-S1 IgG and Surrogate Neutralizing Antibodies after First and Second Anti-SARS-CoV-2 Vaccination
3.3. IgG Antibodies against Different SARS-CoV-2 Epitopes and Other Common Cold Coronaviruses after the Second Vaccination
3.4. Neutralizing Antibody Activity against B.1.617.2 (Delta) after Second Vaccination and Correlation with Commercial Assays and CD19+ Cell Proportion
3.5. Humoral Immune Response Depending on the Last Rituximab Treatment Prior to the First Anti-SARS-CoV-2 Vaccination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenberg, R.A.; Jawad, A.F.; Boyer, J.; Maurer, K.; McDonald, K.; Prak, E.T.; Sullivan, K.E. Rituximab-treated patients have a poor response to influenza vaccination. J. Clin. Immunol. 2013, 33, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, D.; Papadopoulos, E.B.; Young, J.W.; O’Reilly, R.J.; Prockop, S.; Kernan, N.A.; Jakubowski, A.; Boulad, F.; Perales, M.A.; Castro-Malaspina, H.; et al. Immunogenicity of recombinant hepatitis B vaccine (rHBV) in recipients of unrelated or related allogeneic hematopoietic cell (HC) transplants. Blood 2006, 108, 2470–2475. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Mishra, S.; Deepak, P.; Kumar, M.P.; Sharma, A.; Patel, Y.I.; Kennedy, N.A.; Kim, A.H.J.; Sharma, V.; Sebastian, S. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun. Rev. 2022, 21, 102927. [Google Scholar] [CrossRef] [PubMed]
- Spiera, R.; Jinich, S.; Jannat-Khah, D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS-CoV-2 vaccination in patients with rheumatic diseases. Ann. Rheum. Dis. 2021, 80, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Koops, H.; Krueger, K.; Vallbracht, I.; Hasseli, R.; Skapenko, A. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann. Rheum. Dis. 2021, 80, e67. [Google Scholar] [CrossRef] [PubMed]
- Vijenthira, A.; Gong, I.; Betschel, S.D.; Cheung, M.; Hicks, L.K. Vaccine response following anti-CD20 therapy: A systematic review and meta-analysis of 905 patients. Blood Adv. 2021, 5, 2624–2643. [Google Scholar] [CrossRef]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y.; et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Simon, D.; Tascilar, K.; Schmidt, K.; Manger, B.; Weckwerth, L.; Sokolova, M.; Bucci, L.; Fagni, F.; Manger, K.; Schuch, F.; et al. Humoral and Cellular Immune Responses to SARS-CoV-2 Infection and Vaccination in Autoimmune Disease Patients with B Cell Depletion. Arthritis Rheumatol. 2022, 74, 33–37. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Edwards, H.; McIntyre, S.; Mortimer, P.; Gleeson, S.; Martin, P.; Thomson, T.; Randell, P.; Shah, A.; et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 2021, 80, 1322–1329. [Google Scholar] [CrossRef]
- Hadjadj, J.; Planas, D.; Ouedrani, A.; Buffier, S.; Delage, L.; Nguyen, Y.; Bruel, T.; Stolzenberg, M.C.; Staropoli, I.; Ermak, N.; et al. Immunogenicity of BNT162b2 vaccine against the Alpha and Delta variants in immunocompromised patients with systemic inflammatory diseases. Ann. Rheum. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Töllner, M.; Hidmark, A.; Schaier, M.; Nusshag, C.; Kälble, F.; Reichel, P.; Buylaert, M.; Grenz, J.; Ponath, G.; et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 Prime-Boost Vaccination Induces Strong Humoral Responses among Health Care Workers. Vaccines 2021, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.; Benning, L.; Töllner, M.; Nusshag, C.; Kälble, F.; Reichel, P.; Schaier, M.; Bartenschlager, M.; Schnitzler, P.; Zeier, M.; et al. Neutralizing antibody response against variants of concern after vaccination of dialysis patients with BNT162b2. Kidney Int. 2021, 100, 700–702. [Google Scholar] [CrossRef]
- Speer, C.; Göth, D.; Benning, L.; Buylaert, M.; Schaier, M.; Grenz, J.; Nusshag, C.; Kälble, F.; Kreysing, M.; Reichel, P.; et al. Early Humoral Responses of Hemodialysis Patients after COVID-19 Vaccination with BNT162b2. Clin. J. Am. Soc. Nephrol. 2021, 16, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.; Morath, C.; Töllner, M.; Buylaert, M.; Göth, D.; Nusshag, C.; Kälble, F.; Schaier, M.; Grenz, J.; Kreysing, M.; et al. Humoral Responses to Single-Dose BNT162b2 mRNA Vaccination in Dialysis Patients Previously Infected with SARS-CoV-2. Front. Med. 2021, 8, 721286. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Bray, R.A.; Lee, J.H.; Brescia, P.; Kumar, D.; Nong, T.; Shih, R.; Woodle, E.S.; Maltzman, J.S.; Gebel, H.M. Development and Validation of a Multiplex, Bead-based Assay to Detect Antibodies Directed Against SARS-CoV-2 Proteins. Transplantation 2021, 105, 79–89. [Google Scholar] [CrossRef]
- Tönshoff, B.; Müller, B.; Elling, R.; Renk, H.; Meissner, P.; Hengel, H.; Garbade, S.F.; Kieser, M.; Jeltsch, K.; Grulich-Henn, J.; et al. Prevalence of SARS-CoV-2 Infection in Children and Their Parents in Southwest Germany. JAMA Pediatr. 2021, 175, 586–593. [Google Scholar] [CrossRef]
- Mallm, J.-P.; Bundschuh, C.; Kim, H.; Weidner, N.; Steiger, S.; Lander, I.; Börner, K.; Bauer, K.; Hübschmann, D.; Benes, V.; et al. Local emergence and decline of a SARS-CoV-2 variant with mutations L452R and N501Y in the spike protein. medRxiv 2021. [Google Scholar] [CrossRef]
- Simpson-Yap, S.; De Brouwer, E.; Kalincik, T.; Rijke, N.; Hillert, J.A.; Walton, C.; Edan, G.; Moreau, Y.; Spelman, T.; Geys, L.; et al. Associations of Disease-Modifying Therapies with COVID-19 Severity in Multiple Sclerosis. Neurology 2021, 97, e1870–e1885. [Google Scholar] [CrossRef]
- Bingham, C.O., 3rd; Looney, R.J.; Deodhar, A.; Halsey, N.; Greenwald, M.; Codding, C.; Trzaskoma, B.; Martin, F.; Agarwal, S.; Kelman, A. Immunization responses in rheumatoid arthritis patients treated with rituximab: Results from a controlled clinical trial. Arthritis Rheumatol. 2010, 62, 64–74. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, L.E.; Baars, J.W.; Prins, M.H.; van Oers, M.H. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood 2002, 100, 2257–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Krasselt, M.; Wagner, U.; Nguyen, P.; Pietsch, C.; Boldt, A.; Baerwald, C.; Pierer, M.; Seifert, O. Humoral and Cellular Response to COVID-19 Vaccination in Patients with Autoimmune Inflammatory Rheumatic Diseases under Real-life Conditions. Rheumatology 2022, keac089. [Google Scholar] [CrossRef] [PubMed]
- König, M.; Lorentzen, Å.R.; Torgauten, H.M.; Tran, T.T.; Schikora-Rustad, S.; Vaage, E.B.; Mygland, Å.; Wergeland, S.; Aarseth, J.; Aaberge, I.A.S.; et al. Humoral immunity to SARS-CoV-2 mRNA vaccination in multiple sclerosis: The relevance of time since last rituximab infusion and first experience from sporadic revaccinations. J. Neurol. Neurosurg. Psychiatry 2021. [Google Scholar] [CrossRef]
- Disanto, G.; Sacco, R.; Bernasconi, E.; Martinetti, G.; Keller, F.; Gobbi, C.; Zecca, C. Association of Disease-Modifying Treatment and Anti-CD20 Infusion Timing With Humoral Response to 2 SARS-CoV-2 Vaccines in Patients with Multiple Sclerosis. JAMA Neurol. 2021, 78, 1529–1531. [Google Scholar] [CrossRef]
- Palanichamy, A.; Jahn, S.; Nickles, D.; Derstine, M.; Abounasr, A.; Hauser, S.L.; Baranzini, S.E.; Leppert, D.; von Büdingen, H.C. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J. Immunol. 2014, 193, 580–586. [Google Scholar] [CrossRef] [Green Version]
- Leandro, M.J.; Cambridge, G.; Ehrenstein, M.R.; Edwards, J.C. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheumatol. 2006, 54, 613–620. [Google Scholar] [CrossRef]
- Mrak, D.; Tobudic, S.; Koblischke, M.; Graninger, M.; Radner, H.; Sieghart, D.; Hofer, P.; Perkmann, T.; Haslacher, H.; Thalhammer, R.; et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann. Rheum. Dis. 2021, 80, 1345–1350. [Google Scholar] [CrossRef]
- Stefanski, A.-L.; Rincon-Arevalo, H.; Schrezenmeier, E.; Karberg, K.; Szelinski, F.; Ritter, J.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. B cell numbers predict humoral and cellular response upon SARS-CoV-2 vaccination among patients treated with rituximab. medRxiv 2021. [Google Scholar] [CrossRef]
- Maarouf, A.; Rico, A.; Boutiere, C.; Perriguey, M.; Demortiere, S.; Pelletier, J.; Audoin, B. Extending rituximab dosing intervals in patients with MS during the COVID-19 pandemic and beyond? Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e825. [Google Scholar] [CrossRef] [PubMed]
- Thiel, J.; Rizzi, M.; Engesser, M.; Dufner, A.K.; Troilo, A.; Lorenzetti, R.; Voll, R.E.; Venhoff, N. B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: A longitudinal observational study on 120 patients. Arthritis Res. Ther. 2017, 19, 101. [Google Scholar] [CrossRef] [Green Version]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunau, B.; Goldfarb, D.M.; Asamoah-Boaheng, M.; Golding, L.; Kirkham, T.L.; Demers, P.A.; Lavoie, P.M. Immunogenicity of Extended mRNA SARS-CoV-2 Vaccine Dosing Intervals. JAMA 2022, 327, 279–281. [Google Scholar] [CrossRef]
- Moor, M.B.; Suter-Riniker, F.; Horn, M.P.; Aeberli, D.; Amsler, J.; Möller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; Borradori, L.; et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): An investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021, 3, e789–e797. [Google Scholar] [CrossRef]
- Jyssum, I.; Kared, H.; Tran, T.T.; Tveter, A.T.; Provan, S.A.; Sexton, J.; Jørgensen, K.K.; Jahnsen, J.; Kro, G.B.; Warren, D.J.; et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: A prospective, cohort study. Lancet Rheumatol. 2022, 4, E177–E187. [Google Scholar] [CrossRef]
- Achtnichts, L.; Jakopp, B.; Oberle, M.; Nedeltchev, K.; Fux, C.A.; Sellner, J.; Findling, O. Humoral Immune Response after the Third SARS-CoV-2 mRNA Vaccination in CD20 Depleted People with Multiple Sclerosis. Vaccines 2021, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.; Töllner, M.; Benning, L.; Klein, K.; Bartenschlager, M.; Nusshag, C.; Kälble, F.; Reichel, P.; Schnitzler, P.; Zeier, M.; et al. Third COVID-19 vaccine dose with BNT162b2 in patients with ANCA-associated vasculitis. Ann. Rheum. Dis. 2022, 81, 593–595. [Google Scholar] [CrossRef] [PubMed]


| Healthy Controls | Patients | Responders | Non-Responders | |
|---|---|---|---|---|
| (n = 36) | (n = 64) | (n = 13) | (n = 51) | |
| Sex | ||||
| Female | 24 (67%) | 39 (59%) | 9 (69%) | 30 (59%) |
| Male | 12 (33%) | 25 (41%) | 4 (31%) | 21 (41%) |
| Median age, years | 59 (45–62) | 58 (50–69) | 57 (50.5–63.5) | 62 (51–70) |
| Vaccine regimen | ||||
| Homologous BNT162b2 | 33 (92%) | 47 (74%) | 6 (46%) | 41 (80%) |
| Homologous mRNA-1273 | 0 (0%) | 7 (11%) | 6 (46%) | 1 (2%) |
| Heterologous ChAdOx1 nCoV-19/BNT162b2 | 0 (0%) | 3 (5%) | 0 (0%) | 3 (6%) |
| Heterologous ChAdOx1 nCoV-19/mRNA-1273 | 0 (0%) | 1 (1%) | 0 (0%) | 1 (2%) |
| Homologous ChAdOx1 nCoV-19 | 3 (8%) | 6 (9%) | 1 (8%) | 5 (10%) |
| Median (IQR) time since first vaccination, days | 18 (17–21) | 24 (20–36) | 24 (21–32) | 25 (19–37) |
| Median (IQR) time since second vaccination, days | 21 (19–22) | 24 (21–44) | 29 (22–41) | 23 (21–47) |
| Median (IQR) time between both vaccinations, days | 21 (21–21) | 35 (28–42) | 42 (35–42) | 35 (26–42) |
| Median (IQR) time since last RTX therapy, months | - | 5 (4–10) | 21 (9–36) | 5 (4–7) |
| Immunosuppressive co-medication | n = 56 (88%) | n = 13 (100%) | n = 43 (84%) | |
| Glucocorticoids | - | 35/56 (63%) | 5/13 (38%) | 30/43 (70%) |
| Antimetabolites | - | 30/56 (54%) | 6/13 (46%) | 24/43 (56%) |
| Antimalarials | - | 5/56 (9%) | 3/13 (23%) | 2/43 (5%) |
| Methothrexate | - | 10/56 (18%) | 3/13 (23%) | 7/43 (16%) |
| Biologicals | - | 1/56 (2%) | 0/13 (0%) | 1/43 (2%) |
| Other immunosuppression | - | 2/56 (4%) | 0/13 (0%) | 2/43 (5%) |
| Disease | ||||
| ANCA-associated vasculitis | - | 35 (55%) | 8 (61%) | 27 (52%) |
| Systemic lupus erythematosus | - | 7 (11%) | 3 (23%) | 4 (8%) |
| Rheumatoid arthritis | - | 5 (8%) | 5 (10%) | |
| Cryoglobulinemic vasculitis | - | 5 (8%) | 5 (10%) | |
| Myositis | - | 4 (6%) | 1 (8%) | 3 (6%) |
| IgG4-associated disease | - | 3 (5%) | 3 (6%) | |
| Sjögren’s syndrome | - | 2 (3%) | 1 (8%) | 1 (2%) |
| Membranous nephropathy | - | 1 (1%) | 1 (2%) | |
| Systemic sclerosis | - | 1 (1%) | 1 (2%) | |
| Thrombotic thrombocytopenic purpura | - | 1 (1%) | 1 (2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Töllner, M.; Speer, C.; Benning, L.; Bartenschlager, M.; Nusshag, C.; Morath, C.; Zeier, M.; Süsal, C.; Schnitzler, P.; Schmitt, W.; et al. Impaired Neutralizing Antibody Activity against B.1.617.2 (Delta) after Anti-SARS-CoV-2 Vaccination in Patients Receiving Anti-CD20 Therapy. J. Clin. Med. 2022, 11, 1739. https://doi.org/10.3390/jcm11061739
Töllner M, Speer C, Benning L, Bartenschlager M, Nusshag C, Morath C, Zeier M, Süsal C, Schnitzler P, Schmitt W, et al. Impaired Neutralizing Antibody Activity against B.1.617.2 (Delta) after Anti-SARS-CoV-2 Vaccination in Patients Receiving Anti-CD20 Therapy. Journal of Clinical Medicine. 2022; 11(6):1739. https://doi.org/10.3390/jcm11061739
Chicago/Turabian StyleTöllner, Maximilian, Claudius Speer, Louise Benning, Marie Bartenschlager, Christian Nusshag, Christian Morath, Martin Zeier, Caner Süsal, Paul Schnitzler, Wilhelm Schmitt, and et al. 2022. "Impaired Neutralizing Antibody Activity against B.1.617.2 (Delta) after Anti-SARS-CoV-2 Vaccination in Patients Receiving Anti-CD20 Therapy" Journal of Clinical Medicine 11, no. 6: 1739. https://doi.org/10.3390/jcm11061739






