SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure—A Concise Review
Abstract
:1. Introduction
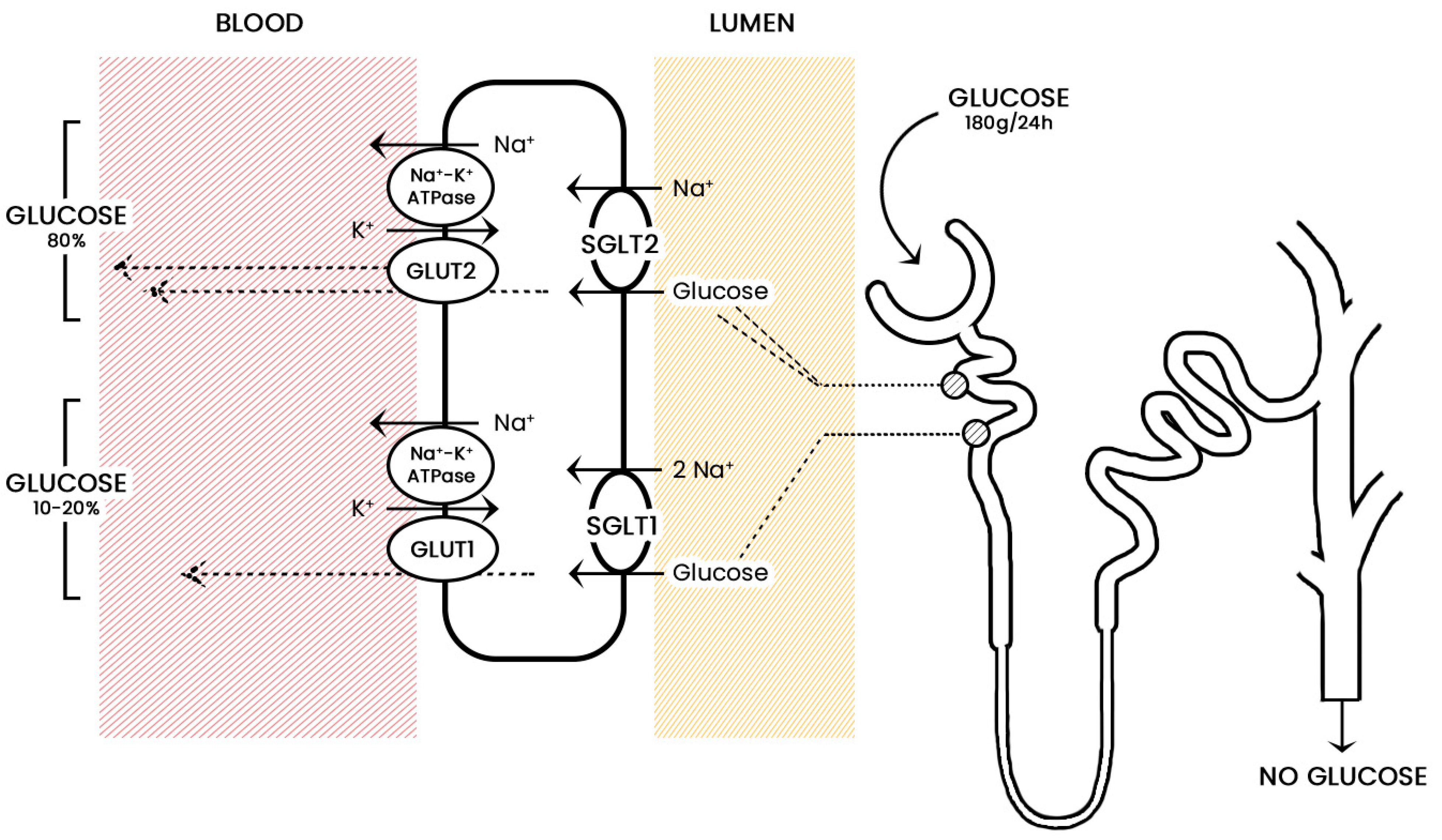
2. Sodium-Glucose Co-Transporters
3. Mechanism of Action of Sodium-Glucose Co-Transporters Inhibitors
4. Clinical Pharmacology
4.1. Affinity
4.2. Dosages
5. Canagliflozin
5.1. Canagliflozin in Monotherapy
5.2. Canagliflozin in Combination with Metformin
5.3. Canagliflozin vs. Sitagliptin as an Add-On Therapy to Metformin
5.4. Canagliflozin in Combination with Metformin and Pioglitazone
5.5. Canagliflozin in Combination with Insulin
6. Dapagliflozin
6.1. Dapagliflozin in Monotherapy
6.2. Dapagliflozin and Metformin
6.3. Dapagliflozin vs. Glipizide as an Add-On Therapy to Metformin
7. Empagliflozin
7.1. Empagliflozin in Monotherapy
7.2. Empagliflozin as an Add-On Therapy to Metformin
7.3. Empagliflozin as an Adjunctive to Therapy with Insulin in Diabetes Mellitus Type 1
8. Adverse Effects
8.1. Genitourinary Tract Infections
8.2. Diabetic Ketoacidosis
8.3. Hypotension
8.4. Hypoglycemia
8.5. Lower Limb Amputations
8.6. Bone Fractures
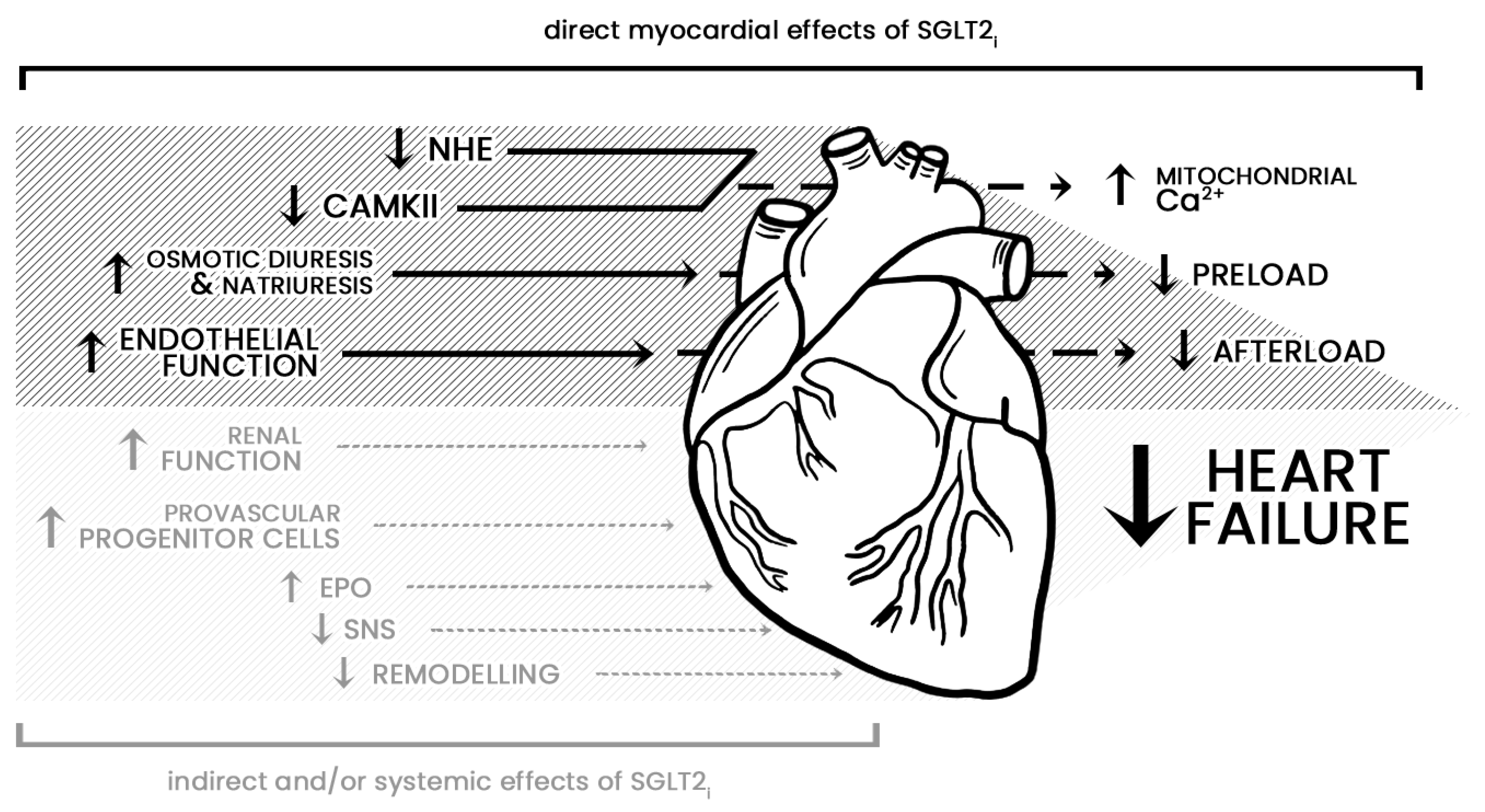
9. Cardiovascular Outcomes of SGLT2i
9.1. EMPA-REG OUTCOME Trial (2015): Empagliflozin—Cardiovascular Outcomes and Mortality in Diabetic Patients
9.2. CANVAS Program (2017): Canagliflozin—Cardiovascular and Renal Outcomes in Diabetic Patients
9.3. DECLARE-TIMI 58 Trial (2018): Dapagliflozin—Cardiovascular Safety Profile in Diabetic Patients
9.4. CREDENCE Trial (2019): Canagliflozin—Renal Outcomes in Diabetes and Nephropathy
9.5. DAPA-HF Trial (2019): Dapagliflozin—Impact on Patients with Heart Failure and Reduced Ejection Fraction Regardless of Diabetes
9.6. EMPEROR-Reduced Trial (2020): Empagliflozin—Cardiovascular and Renal Outcomes in Heart Failure Patients
9.7. SOLOIST-WHF Trial (2020): Sotagliflozin—Impact on Patients with Decompensated Heart Failure and Diabetes
9.8. EMPEROR-Preserved Trial (2021): Empagliflozin—Cardiovascular Outcomes in Heart Failure Patients
10. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [Green Version]
- Rosano, G.M.; Vitale, C.; Seferovic, P. Heart Failure in Patients with Diabetes Mellitus. Card. Fail. Rev. 2017, 3, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Gerstein, H.C. The Hemoglobin A1c Level as a Progressive Risk Factor for Cardiovascular Death, Hospitalization for Heart Failure, or Death in Patients with Chronic Heart Failure: An Analysis of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Arch. Intern. Med. 2008, 168, 1699. [Google Scholar] [CrossRef] [Green Version]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Albanese, G.; Di Martino, A.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Sasso, F.C. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front. Med. 2021, 8, 695792. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F. Global Epidemiology and Future Trends of Heart Failure. AME Med. J. 2020, 5, 15. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [Green Version]
- Eleftheriadou, I.; Grigoropoulou, P.; Liberopoulos, E.; Liatis, S.; Kokkinos, A.; Tentolouris, N. Update on Cardiovascular Effects of Older and Newer Anti-Diabetic Medications. Curr. Med. Chem. 2018, 25, 1549–1566. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, Metabolic and Cardiovascular Considerations of SGLT2 Inhibition. Nat. Rev. Nephrol. 2017, 13, 11–26. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Norton, L.; DeFronzo, R.A. Role of Sodium-Glucose Cotransporter 2 (SGLT 2) Inhibitors in the Treatment of Type 2 Diabetes. Endocr. Rev. 2011, 32, 515–531. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A.; Hompesch, M.; Kasichayanula, S.; Liu, X.; Hong, Y.; Pfister, M.; Morrow, L.A.; Leslie, B.R.; Boulton, D.W.; Ching, A.; et al. Characterization of Renal Glucose Reabsorption in Response to Dapagliflozin in Healthy Subjects and Subjects with Type 2 Diabetes. Diabetes Care 2013, 36, 3169–3176. [Google Scholar] [CrossRef] [Green Version]
- Himsworth, H.P. The Relation of Glycosuria to Glycaemia and the Determination of the Renal Threshold for Glucose. Biochem. J. 1931, 25, 1128–1146. [Google Scholar] [CrossRef] [Green Version]
- Christensen, E.I.; Wagner, C.A.; Kaissling, B. Uriniferous Tubule: Structural and Functional Organization. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. c100073. ISBN 978-0-470-65071-4. [Google Scholar]
- Maunsbach, A.B. Observations on the Segmentation of the Proximal Tubule in the Rat Kidney. J. Ultrastruct. Res. 1966, 16, 239–258. [Google Scholar] [CrossRef]
- Brown, G.K. Glucose Transporters: Structure, Function and Consequences of Deficiency. J. Inherit. Metab. Dis. 2000, 23, 237–246. [Google Scholar] [CrossRef]
- Vallon, V.; Platt, K.A.; Cunard, R.; Schroth, J.; Whaley, J.; Thomson, S.C.; Koepsell, H.; Rieg, T. SGLT2 Mediates Glucose Reabsorption in the Early Proximal Tubule. JASN 2011, 22, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.M.; Turk, E. The Sodium/Glucose Cotransport Family SLC5. Pflug. Arch. 2004, 447, 510–518. [Google Scholar] [CrossRef]
- Diez-Sampedro, A.; Hirayama, B.A.; Osswald, C.; Gorboulev, V.; Baumgarten, K.; Volk, C.; Wright, E.M.; Koepsell, H. A Glucose Sensor Hiding in a Family of Transporters. Proc. Natl. Acad. Sci. USA 2003, 100, 11753–11758. [Google Scholar] [CrossRef] [Green Version]
- Helbert, M.J.F.; Dauwe, S.E.H.; Van der Biest, I.; Nouwen, E.J.; De Broe, M.E. Immunodissection of the Human Proximal Nephron: Flow Sorting of S1S2S3, S1S2 and S3 Proximal Tubular Cells. Kidney Int. 1997, 52, 414–428. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Lee, Y.J.; Han, H.J. Regulatory Mechanisms of Na+/Glucose Cotransporters in Renal Proximal Tubule Cells. Kidney Int. 2007, 72, S27–S35. [Google Scholar] [CrossRef] [Green Version]
- Heise, T.; Seewaldt-Becker, E.; Macha, S.; Hantel, S.; Pinnetti, S.; Seman, L.; Woerle, H.J. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics Following 4 Weeks’ Treatment with Empagliflozin Once Daily in Patients with Type 2 Diabetes. Diabetes Obes. Metab. 2013, 15, 613–621. [Google Scholar] [CrossRef]
- Seman, L.; Macha, S.; Nehmiz, G.; Simons, G.; Ren, B.; Pinnetti, S.; Woerle, H.J.; Dugi, K. Empagliflozin (BI 10773), a Potent and Selective SGLT2 Inhibitor, Induces Dose-Dependent Glucosuria in Healthy Subjects: Clinical Pharmacology in Drug Development. Clin. Pharmacol. Drug Dev. 2013, 2, 152–161. [Google Scholar] [CrossRef]
- Al-Jobori, H.; Daniele, G.; Cersosimo, E.; Triplitt, C.; Mehta, R.; Norton, L.; DeFronzo, R.A.; Abdul-Ghani, M. Empagliflozin and Kinetics of Renal Glucose Transport in Healthy Individuals and Individuals with Type 2 Diabetes. Diabetes 2017, 66, 1999–2006. [Google Scholar] [CrossRef] [Green Version]
- Striepe, K.; Jumar, A.; Ott, C.; Karg, M.V.; Schneider, M.P.; Kannenkeril, D.; Schmieder, R.E. Effects of the Selective Sodium-Glucose Cotransporter 2 Inhibitor Empagliflozin on Vascular Function and Central Hemodynamics in Patients with Type 2 Diabetes Mellitus. Circulation 2017, 136, 1167–1169. [Google Scholar] [CrossRef]
- Chilton, R.; Tikkanen, I.; Cannon, C.P.; Crowe, S.; Woerle, H.J.; Broedl, U.C.; Johansen, O.E. Effects of Empagliflozin on Blood Pressure and Markers of Arterial Stiffness and Vascular Resistance in Patients with Type 2 Diabetes. Diabetes Obes. Metab. 2015, 17, 1180–1193. [Google Scholar] [CrossRef]
- Li, H.; Shin, S.E.; Seo, M.S.; An, J.R.; Choi, I.-W.; Jung, W.-K.; Firth, A.L.; Lee, D.-S.; Yim, M.-J.; Choi, G.; et al. The Anti-Diabetic Drug Dapagliflozin Induces Vasodilation via Activation of PKG and Kv Channels. Life Sci. 2018, 197, 46–55. [Google Scholar] [CrossRef]
- Ugusman, A.; Kumar, J.; Aminuddin, A. Endothelial Function and Dysfunction: Impact of Sodium-Glucose Cotransporter 2 Inhibitors. Pharmacol. Ther. 2021, 224, 107832. [Google Scholar] [CrossRef]
- Salvatore, T.; Caturano, A.; Galiero, R.; Di Martino, A.; Albanese, G.; Vetrano, E.; Sardu, C.; Marfella, R.; Rinaldi, L.; Sasso, F.C. Cardiovascular Benefits from Gliflozins: Effects on Endothelial Function. Biomedicines 2021, 9, 1356. [Google Scholar] [CrossRef]
- Garcia-Ropero, A.; Santos-Gallego, C.G.; Zafar, M.U.; Badimon, J.J. Metabolism of the Failing Heart and the Impact of SGLT2 Inhibitors. Expert Opin. Drug Metab. Toxicol. 2019, 15, 275–285. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, J.; Wagemann, O.; Lücht, C.M.; Trum, M.; Hammer, K.P.; Sag, C.M.; Lebek, S.; Tarnowski, D.; Reinders, J.; Perbellini, F.; et al. Empagliflozin Reduces Ca/Calmodulin-Dependent Kinase II Activity in Isolated Ventricular Cardiomyocytes: Empagliflozin Reduces CaMKII Activity. ESC Heart Fail. 2018, 5, 642–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.-M.; Chang, N.-C.; Lin, S.-Z. Dapagliflozin, a Selective SGLT2 Inhibitor, Attenuated Cardiac Fibrosis by Regulating the Macrophage Polarization via STAT3 Signaling in Infarcted Rat Hearts. Free Radic. Biol. Med. 2017, 104, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Bonaca, M.P.; Furtado, R.H.M.; Mosenzon, O.; Kuder, J.F.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.H.; et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients with Type 2 Diabetes Mellitus: Insights from the DECLARE-TIMI 58 Trial. Circulation 2020, 141, 1227–1234. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 Inhibitors: Mechanisms of Cardiovascular Benefit beyond Glycaemic Control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Zheng, C.-M.; Yen, T.-H.; Lu, K.-C. Molecular Mechanisms of SGLT2 Inhibitor on Cardiorenal Protection. Int. J. Mol. Sci. 2020, 21, 7833. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J. Empagliflozin, Calcium, and SGLT1/2 Receptor Affinity: Another Piece of the Puzzle. ESC Heart Fail. 2018, 5, 549–551. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An Update on Sodium-Glucose Co-Transporter-2 Inhibitors for the Treatment of Diabetes Mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 24, 73–79. [Google Scholar] [CrossRef]
- Simes, B.C.; MacGregor, G.G. Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors: A Clinician’s Guide. DMSO 2019, 12, 2125–2136. [Google Scholar] [CrossRef] [Green Version]
- Zurek, A.M.; Yendapally, R.; Urteaga, E.M. A Review of the Efficacy and Safety of Sodium–Glucose Cotransporter 2 Inhibitors: A Focus on Diabetic Ketoacidosis. Diabetes Spectr. 2017, 30, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Stenlöf, K.; Cefalu, W.T.; Kim, K.-A.; Alba, M.; Usiskin, K.; Tong, C.; Canovatchel, W.; Meininger, G. Efficacy and Safety of Canagliflozin Monotherapy in Subjects with Type 2 Diabetes Mellitus Inadequately Controlled with Diet and Exercise. Diabetes Obes. Metab. 2013, 15, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Stenlöf, K.; Cefalu, W.T.; Kim, K.-A.; Jodar, E.; Alba, M.; Edwards, R.; Tong, C.; Canovatchel, W.; Meininger, G. Long-Term Efficacy and Safety of Canagliflozin Monotherapy in Patients with Type 2 Diabetes Inadequately Controlled with Diet and Exercise: Findings from the 52-Week CANTATA-M Study. Curr. Med. Res. Opin. 2014, 30, 163–175. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Charpentier, G.; Hollander, P.; González-Gálvez, G.; Mathieu, C.; Vercruysse, F.; Usiskin, K.; Law, G.; Black, S.; Canovatchel, W.; et al. Efficacy and Safety of Canagliflozin in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Metformin and Sulphonylurea: A Randomised Trial. Int. J. Clin. Pract. 2013, 67, 1267–1282. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, J.; Chuck, L.; González-Ortiz, M.; Merton, K.; Craig, J.; Capuano, G.; Qiu, R. Initial Combination Therapy with Canagliflozin Plus Metformin Versus Each Component as Monotherapy for Drug-Naïve Type 2 Diabetes. Diabetes Care 2016, 39, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Lu, M.; Ma, L.; Zhou, Y.; Cui, Y. Efficacy and Tolerability of Canagliflozin as Add-on to Metformin in the Treatment of Type 2 Diabetes Mellitus: A Meta-Analysis. Eur. J. Clin. Pharmacol. 2015, 71, 1325–1332. [Google Scholar] [CrossRef]
- Lavalle-González, F.J.; Januszewicz, A.; Davidson, J.; Tong, C.; Qiu, R.; Canovatchel, W.; Meininger, G. Efficacy and Safety of Canagliflozin Compared with Placebo and Sitagliptin in Patients with Type 2 Diabetes on Background Metformin Monotherapy: A Randomised Trial. Diabetologia 2013, 56, 2582–2592. [Google Scholar] [CrossRef] [Green Version]
- Forst, T.; Guthrie, R.; Goldenberg, R.; Yee, J.; Vijapurkar, U.; Meininger, G.; Stein, P. Efficacy and Safety of Canagliflozin over 52 Weeks in Patients with Type 2 Diabetes on Background Metformin and Pioglitazone. Diabetes Obes. Metab. 2014, 16, 467–477. [Google Scholar] [CrossRef]
- Inagaki, N.; Harashima, S.; Maruyama, N.; Kawaguchi, Y.; Goda, M.; Iijima, H. Efficacy and Safety of Canagliflozin in Combination with Insulin: A Double-Blind, Randomized, Placebo-Controlled Study in Japanese Patients with Type 2 Diabetes Mellitus. Cardiovasc. Diabetol. 2016, 15, 89. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, E.; Ramos, S.J.; Salsali, A.; Tang, W.; List, J.F. Dapagliflozin Monotherapy in Type 2 Diabetic Patients with Inadequate Glycemic Control by Diet and Exercise: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care 2010, 33, 2217–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.J.; Gross, J.L.; Pieters, A.; Bastien, A.; List, J.F. Effect of Dapagliflozin in Patients with Type 2 Diabetes Who Have Inadequate Glycaemic Control with Metformin: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2010, 375, 2223–2233. [Google Scholar] [CrossRef]
- Henry, R.R.; Murray, A.V.; Marmolejo, M.H.; Hennicken, D.; Ptaszynska, A.; List, J.F. Dapagliflozin, Metformin XR, or Both: Initial Pharmacotherapy for Type 2 Diabetes, a Randomised Controlled Trial: T2DM: Dapagliflozin, Metformin XR, or Both. Int. J. Clin. Pract. 2012, 66, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Del Prato, S.; Meier, J.J.; Durán-García, S.; Rohwedder, K.; Elze, M.; Parikh, S.J. Dapagliflozin versus Glipizide as Add-on Therapy in Patients with Type 2 Diabetes Who Have Inadequate Glycemic Control with Metformin: A Randomized, 52-Week, Double-Blind, Active-Controlled Noninferiority Trial. Diabetes Care 2011, 34, 2015–2022. [Google Scholar] [CrossRef] [Green Version]
- Roden, M.; Weng, J.; Eilbracht, J.; Delafont, B.; Kim, G.; Woerle, H.J.; Broedl, U.C. EMPA-REG MONO trial investigators Empagliflozin Monotherapy with Sitagliptin as an Active Comparator in Patients with Type 2 Diabetes: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Diabetes Endocrinol. 2013, 1, 208–219. [Google Scholar] [CrossRef]
- Häring, H.-U.; Merker, L.; Seewaldt-Becker, E.; Weimer, M.; Meinicke, T.; Broedl, U.C.; Woerle, H.J.; on behalf of the EMPA-REG MET Trial Investigators. Empagliflozin as Add-On to Metformin in Patients with Type 2 Diabetes: A 24-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Care 2014, 37, 1650–1659. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, J.; Marquard, J.; Laffel, L.M.; Neubacher, D.; Kaspers, S.; Cherney, D.Z.; Zinman, B.; Skyler, J.S.; George, J.; Soleymanlou, N.; et al. Empagliflozin as Adjunctive to Insulin Therapy in Type 1 Diabetes: The EASE Trials. Diabetes Care 2018, 41, 2560–2569. [Google Scholar] [CrossRef] [Green Version]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in Patients with Diabetes Mellitus: A Review of Pathogenesis. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. 1), S27–S36. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Brouwer, E.C.; Gaastra, W.; Verhoef, J.; Hoepelman, A.I.M. Effect of Glucose and PH on Uropathogenic and Non-Uropathogenic Escherichia Coli: Studies with Urine from Diabetic and Non-Diabetic Individuals. J. Med. Microbiol. 1999, 48, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Hirji, I.; Guo, Z.; Andersson, S.W.; Hammar, N.; Gomez-Caminero, A. Incidence of Urinary Tract Infection among Patients with Type 2 Diabetes in the UK General Practice Research Database (GPRD). J. Diabetes Complicat. 2012, 26, 513–516. [Google Scholar] [CrossRef]
- Geerlings, S.; Fonseca, V.; Castro-Diaz, D.; List, J.; Parikh, S. Genital and Urinary Tract Infections in Diabetes: Impact of Pharmacologically-Induced Glucosuria. Diabetes Res. Clin. Pract. 2014, 103, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, C.V.; Schneeweiss, S.; Patorno, E. Comparative Risk of Genital Infections Associated with Sodium-Glucose Co-Transporter-2 Inhibitors. Diabetes Obes. Metab. 2019, 21, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Li, S.; Jia, P.; Deng, K.; Chen, W.; Sun, X. Effects of SGLT2 Inhibitors on UTIs and Genital Infections in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 2824. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Ferrannini, E. Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern with SGLT2 Inhibitors. Diabetes Care 2015, 38, 1638–1642. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Barsotti, E.; Clerico, A.; Muscelli, E. Renal Handling of Ketones in Response to Sodium–Glucose Cotransporter 2 Inhibition in Patients with Type 2 Diabetes. Diabetes Care 2017, 40, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Palmiero, G.; Cesaro, A.; Vetrano, E.; Pafundi, P.C.; Galiero, R.; Caturano, A.; Moscarella, E.; Gragnano, F.; Salvatore, T.; Rinaldi, L.; et al. Impact of SGLT2 Inhibitors on Heart Failure: From Pathophysiology to Clinical Effects. Int. J. Mol. Sci. 2021, 22, 5863. [Google Scholar] [CrossRef]
- Nielsen, R.; Møller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frøkiær, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment with the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141. [Google Scholar] [CrossRef]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The Failing Heart Utilizes 3-Hydroxybutyrate as a Metabolic Stress Defense. JCI Insight 2019, 4, e124079. [Google Scholar] [CrossRef] [Green Version]
- Monami, M.; Nreu, B.; Zannoni, S.; Lualdi, C.; Mannucci, E. Effects of SGLT-2 Inhibitors on Diabetic Ketoacidosis: A Meta-Analysis of Randomised Controlled Trials. Diabetes Res. Clin. Pract. 2017, 130, 53–60. [Google Scholar] [CrossRef]
- Fitchett, D. A Safety Update on Sodium Glucose Co-transporter 2 Inhibitors. Diabetes Obes. Metab. 2019, 21, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Liakos, A.; Karagiannis, T.; Athanasiadou, E.; Sarigianni, M.; Mainou, M.; Papatheodorou, K.; Bekiari, E.; Tsapas, A. Efficacy and Safety of Empagliflozin for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 2014, 16, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.L.; Smyth, L.R.; Riche, D.M.; Bourret, E.M.; Chamberlin, K.W.; White, W.B. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Blood Pressure: A Systematic Review and Meta-Analysis. J. Am. Soc. Hypertens. 2014, 8, 262–275.e9. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [Green Version]
- Fralick, M.; Kim, S.C.; Schneeweiss, S.; Everett, B.M.; Glynn, R.J.; Patorno, E. Risk of Amputation with Canagliflozin across Categories of Age and Cardiovascular Risk in Three US Nationwide Databases: Cohort Study. BMJ 2020, 370, m2812. [Google Scholar] [CrossRef]
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes: Results from the CANVAS Program Randomised Clinical Trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Packer, M.; Butler, J.; Filippatos, G.S.; Jamal, W.; Salsali, A.; Schnee, J.; Kimura, K.; Zeller, C.; George, J.; Brueckmann, M.; et al. Evaluation of the Effect of Sodium-Glucose Co-Transporter 2 Inhibition with Empagliflozin on Morbidity and Mortality of Patients with Chronic Heart Failure and a Reduced Ejection Fraction: Rationale for and Design of the EMPEROR-Reduced Trial. Eur. J. Heart Fail. 2019, 21, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 Inhibitors in Patients with Heart Failure with Reduced Ejection Fraction: A Meta-Analysis of the EMPEROR-Reduced and DAPA-HF Trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Verma, S.; McGuire, D.K.; Kosiborod, M.N. Two Tales: One Story: EMPEROR-Reduced and DAPA-HF. Circulation 2020, 142, 2201–2204. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 Inhibitors for Primary and Secondary Prevention of Cardiovascular and Renal Outcomes in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes: A Meta-Analysis. JAMA Cardiol. 2021, 6, 148. [Google Scholar] [CrossRef]
- Giugliano, D.; Esposito, K. Class Effect for SGLT-2 Inhibitors: A Tale of 9 Drugs. Cardiovasc. Diabetol. 2019, 18, 94. [Google Scholar] [CrossRef]
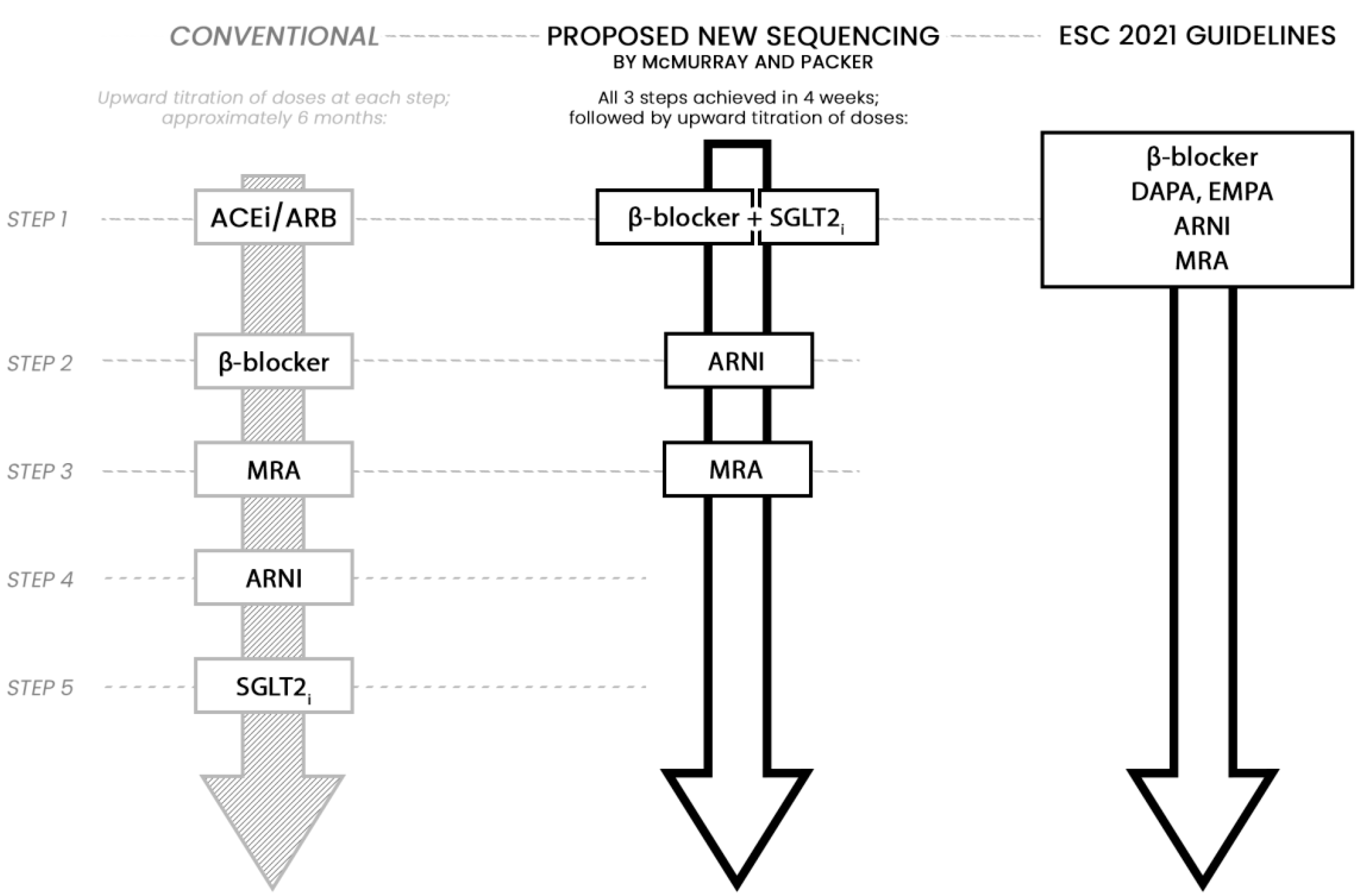
- McMurray, J.J.V.; Packer, M. How Should We Sequence the Treatments for Heart Failure and a Reduced Ejection Fraction?: A Redefinition of Evidence-Based Medicine. Circulation 2021, 143, 875–877. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Prosperi, S.; Costi, B.; Angotti, D.; Birtolo, L.I.; Chimenti, C.; Lavalle, C.; Maestrini, V.; Mancone, M.; et al. Sodium-Glucose Cotransporter 2 Inhibitors and Heart Failure: The Best Timing for the Right Patient. Heart Fail. Rev. 2021. ahead of print. [Google Scholar] [CrossRef]

| Generic Name | Brand Name | Starting Dose (mg) Once Daily | Maintenance Dose (mg) Once Daily |
|---|---|---|---|
| Canagliflozin | Invokana | 100 | 100–300 |
| Dapagliflozin | Farxiga Forxiga | 10 | 10 |
| Empagliflozin | Jardiance | 10 | 10–25 |
| Ertugliflozin | Steglatro | 5 | 5–15 |
| Dose (mg) | eGFR Cutoffs (mL/min/1.73 m2) for Patients with T2DM | Recommendations for Patients with T2DM | eGFR Cutoffs (mL/min/1.73 m2) for Patients with HF | Recommendations for Patients with HF | |
|---|---|---|---|---|---|
| Canagliflozin | 100–300 | 60 | Max 300 mg | - | - |
| 30–59 | Max 100 mg | ||||
| <30 | Max 100 mg for patients already taking CANA, otherwise should not be initiated | ||||
| Dapagliflozin | 10 | ≥45 | 10 mg | ≥25 | 10 mg |
| <45 | Additional glucose-lowering treatment may be needed | <25 | 10 mg for patients already taking DAPA, otherwise should not be initiated | ||
| <25 | 10 mg for patients already taking DAPA, otherwise contraindicated | ||||
| Empagliflozin | 10–25 | ≥60 | Max 25 mg | ≥20 | 10 mg |
| 45–59 | Max 10 mg, only if eGFR before treatment ≥60 | <20 | Contraindicated | ||
| <45 | Contraindicated | ||||
| Ertugliflozin | 5–15 | <60 | Should not be initiated | - | - |
| <45 | Contraindicated |
| EMPA-REG OUTCOME [75] | CANVAS Program [77] | DECLARE-TIMI-58 [36] | CREDENCE [77] | DAPA-HF [78] | EMPEROR- Reduced [81] | SOLOIST- WHF [79] | EMPEROR- Preserved [80] | |
|---|---|---|---|---|---|---|---|---|
| Year | 2015 | 2017 | 2018 | 2019 | 2019 | 2020 | 2020 | 2021 |
| Randomized | yes | yes | yes | yes | yes | yes | yes | yes |
| Double-blind | yes | yes | yes | yes | yes | yes | yes | yes |
| Placebo-controlled | yes | yes | yes | yes | yes | yes | yes | yes |
| SGLT2i | EMPA | CANA | DAPA | CANA | DAPA | EMPA | SOTA | EMPA |
| Study drug doses | 10, 25 mg | 100, 300 mg | 10 mg | 100 mg | 10 mg | 10 mg | 200, 400 mg (uptitrated if tolerated) | 10 mg |
| Number of randomized patients | 7.020 | 10.142 | 17.160 | 4.401 | 4.744 | 3.730 | 1.222 | 5.988 |
| Median observation time | 3.1 years | 2.4 years | 4.2 years | 2.6 years | 18.2 months | 16 months | 9 months | 26.2 months |
| Key inclusion criteria | ||||||||
| Age | ≥18 y.o | ≥30 y.o and a history of symptomatic ASCVD or ≥50 y.o with ≥2 CV risk factors | ≥40 y.o and a history of ASCVD or ≥55 y.o (men) or ≥60 y.o (women) without known ASCVD, but ≥1 CV risk factor | ≥30 y.o | ≥18 y.o | ≥18 y.o | 18–85 y.o | ≥18 y.o |
| T2DM | yes | yes | yes | yes | yes/no | yes/no | yes | yes/no |
| HbA1C |
| 7–10.5% | 6.5–12% | 6.5–12% | - | - | ≥6.5% | - |
| ASCVD |
| as above |
| - | - | - | - | - |
| Heart failure | - | - | - | - |
≥400 pg/mL (if hospitalization for HF within 1 year) ≥600 (in neither of the above) |
LVEF 31–35%: ≥1000 pg/mL (≥2000 pg/mL, if AF) LVEF 36–40%: ≥2500 pg/mL (≥5000 pg/mL, if AF) |
|
>900 pg/mL (if AF) |
| eGFR [mL/min/1.73 m2] | ≥30 | ≥30 | ≥60 (creatinine clearance) | 30–<90 (30–60 in 60% of study population) + albuminuria—UACR: >300–5000 mg/g | ≥30 | ≥20 | ≥30 | ≥20 |
| Other | - | - | - | Stable maximum tolerated daily dose of ACEi or ARB | GDMT for HF for and cardiac device therapy as indicated | GDMT for HF for and cardiac device therapy as indicated | - | - |
| Key exclusion criteria | ||||||||
| Cardiovascular |
| - | Acute CV events | Current or history of NYHA class IV | Symptomatic hypotension or SBP <95 mmHg |
|
|
|
| Other |
|
|
|
|
|
| - |
|
| Results | ||||||||
| Primary outcome | CV death, MI, stroke | CV death, MI, stroke | CV death, MI, stroke | ESKD, doubling of serum creatinine, death from renal or CV causes | Worsening HF (hospitalization or urgent visit resulting in IV therapy), CV death | CV death, HF hospitalization | CV death, HF hospitalizations and urgent visits (first and subsequent) | CV death, hospitalization for HF |
| 10.5% (EMPA) vs. 12.1% (placebo); (HR 0.86; 95% CI 0.74–0.99) | 26.9/1000 patient-years (CANA) vs. 31.5/1000 patient-years (placebo); (HR 0.86; 95% CI 0.75–0.97) | 8.8% (DAPA) vs. 9.4% (placebo); (HR 0.93; 95% CI 0.84–1.03) | 43.2/1000 patient-years (CANA) vs. 61.2/1000 patient-years (placebo); (HR 0.70; 95% CI 0.59–0.82) | 16.3% (DAPA) vs. 21.2% (placebo); (HR 0.74; 95% CI 0.65–0.85) | 19.4% (EMPA) vs. 24.7% (placebo); (HR 0.75; 95% CI 0.65–0.86) | 51.0/100 patient-years (SOTA) vs. 76.3/100 patient-years (placebo); (HR 0.67; 95% CI 0.52–0.85) | 13.8% (EMPA) vs. 17.1% (placebo); (HR 0.79; 95% CI 0.69–0.9) | |
| Death from CV causes | 3.7% (EMPA) vs. 5.9% (placebo); (HR 0.62; 95% CI 0.49–0.77) | 11.6/1000 patient-years (CANA) vs. 12.8/1000 patient-years (placebo); (HR 0.87; 95% CI 0.72–1.06) | 2.9% (DAPA) vs. 2.9% (placebo); (HR 0.98; 95% CI 0.82–1.17) | 19.0/1000 patient-years (CANA) vs. 24.4/1000 patient-years (placebo); (HR 0.78; 95% CI 0.61–1.00) | 9.6% (DAPA) vs. 11.5% (placebo); (HR 0.82; 95% CI 0.69–0.98) | 10.0% (EMPA) vs. 10.8% (placebo); (HR 0.92; 95% CI 0.75–1.12) | 51.0/100 patient-years (SOTA) vs. 58.0/100 patient-years (placebo); (HR 0.84; 95% CI 0.58–1.22) | 7.3% (EMPA) vs. 8.2% (placebo); (HR 0.91; 95% CI 0.76–1.09) |
| Death from any cause | 5.7% (EMPA) vs. 8.3% (placebo); (HR 0.68; 95% CI 0.57–0.82) | 17.3/1000 patient-years (CANA) vs. 19.5/1000 patient-years (placebo); (HR 0.87; 95% CI 0.74–1.01) | 6.2% (DAPA) vs. 6.6% (placebo); (HR 0.93; 95% CI 0.82–1.04) | 29.0/1000 patient-years (CANA) vs. 35.0/1000 patient-years (placebo); (HR 0.83; 95% CI 0.68–1.02) | 11.6% (DAPA) vs. 13.9% (placebo); (HR 0.83; 95% CI 0.71–0.97) | 13.4% (EMPA) vs. 14.2% (placebo); (HR 0.92; 95% CI 0.77–1.10) | 65.0/100 patient-years (SOTA) vs. 76.0/100 patient-years (placebo); (HR 0.82; 95% CI 0.59–1.14) | 14.1% (EMPA) vs. 14.3% (placebo); (HR 1.00; 95% CI 0.87–1.15) |
| HF hospitalization | 2.7% (EMPA) vs. 4.3% (placebo); (HR 0.65; 95% CI 0.50–0.85) | 5.5/1000 patient-years (CANA) vs. 8.7/1000 patient-years (placebo); (HR 0.67; 95% CI 0.52–0.87) | 2.5% (DAPA) vs. 3.3% (placebo); (HR 0.73; 95% CI 0.61–0.88) | 15.7/1000 patient-years (CANA) vs. 25.3/1000 patient-years (placebo); (HR 0.61; 95% CI 0.47–0.80) | 9.7% (DAPA) vs. 13.4% (placebo); (HR 0.70; 95% CI 0.59–0.83) | 13.2% (EMPA) vs. 18.3% (placebo); (HR 0.69; 95% CI 0.59–0.81) | 194.0/100 patient-years (SOTA) vs. 297.0/100 patient-years (placebo); (HR 0.64; 95% CI 0.49–0.83) (total number of HF hospitalizations and urgent visits) | 8.6% (EMPA) vs. 11.8% (placebo); (HR 0.71; 95% CI 0.60–0.83) |
| Safety | ||||||||
| Serious AE | 38.2% (EMPA) vs. 42.3% (placebo) | 104.3/1000 patient-years (CANA) vs. 120/1000 patient-years (placebo) | 34.1% (DAPA) vs. 36.2% (placebo) | 145.2/1000 patient-years (CANA) vs. 164.4/1000 patient-years (placebo) | 37.8% (DAPA) vs. 42.0% (placebo) | 41.4% (EMPA) vs. 48.1% (placebo) | 3.0% (SOTA) vs. 2.8% (placebo (only events leading to study drug discontinuation) | 47.9% (EMPA) vs. 51.6% (placebo) |
| Genital infections | 6.4% (EMPA) vs. 1.8% (placebo) | 34.9/1000 patient-years (CANA) vs. 10.8/1000 patient-years (placebo) (men) 68.8/1000 patient-years (CANA) vs. 17.5/1000 patient-years (placebo) (women) | 0.9% (DAPA) vs. 0.1% (placebo) | 8.4/1000 patient-years (CANA) vs. 0.9/1000 patient-years (placebo) (men) 12.6/1000 patient-years (CANA) vs. 6.1/1000 patient-years (placebo) (women) | - | 1.7% (EMPA) vs. 0.6% (placebo) | - | 2.2% (EMPA) vs. 0.7% (placebo) |
| Urinary tract infections | 18.0% (EMPA) vs. 18.1% (placebo) | 40.0/1000 patient-years (CANA) vs. 37.0/1000 patient-years (placebo) | 1.5% (DAPA) vs. 1.6% (placebo) | 48.3/1000 patient-years (CANA) vs. 45.1/1000 patient-years (placebo) | 0.5% (DAPA) vs. 0.7% (placebo) | 4.9% (EMPA) vs. 4.5% (placebo) | 4.8% (SOTA) vs. 5.1% (placebo) | 9.9% (EMPA) vs. 8.1% (placebo) |
| Hypoglycemia | 27.8% (EMPA) vs. 27.9% (placebo) | 50.0/1000 patient-years (CANA) vs. 46.4/1000 patient-years (placebo) | 0.7% (DAPA) vs. 1.0% (placebo) | 44.3/1000 patient-years (CANA) vs. 48.9/1000 patient-years (placebo) | 0.2% (DAPA) vs. 0.2% (placebo) | 1.4% (EMPA) vs. 1.5% (placebo) | 1.5% (SOTA) vs. 0.3% (placebo) | 2.4% (EMPA) vs. 2.6% (placebo) |
| DKA | 0.1% (EMPA) vs. <0.1% (placebo) | 0.6/1000 patient-years (CANA) vs. 0.3/1000 patient-years (placebo) | 0.3% (DAPA) vs. 0.1% (placebo) | 2.2/1000 patient-years (CANA) vs. 0.2/1000 patient-years (placebo) | 0.1% (DAPA) vs. 0.0% (placebo) | 0% (EMPA) vs. 0% (placebo) | 0.3% (SOTA) vs. 0.7% (placebo) | 0.1% (EMPA) vs. 0.2% (placebo) |
| Amputations | - | 6.3/1000 patient-years (CANA) vs. 3.4/1000 patient-years (placebo) | 1.4% (DAPA) vs. 1.3% (placebo) | 12.3/1000 patient-years (CANA) vs. 11.2/1000 patient-years (placebo) | 0.5% (DAPA) vs. 0.5% (placebo) | 0.7% (EMPA) vs. 0.5% (placebo) | - | 0.5% (EMPA) vs. 0.8% (placebo) |
| Bottom line | ||||||||
| Summary | Lower rate of the primary composite CV outcome, death from CV and any cause and hospitalizations for HF in patients receiving EMPA in addition to standard care | Lower rate of the primary composite CV outcome and hospitalizations for HF in patients receiving CANA | Noninferior but not superior regarding primary composite CV outcome; however, DAPA reduces risk of hospitalizations for HF | Lower rate of the primary composite CV outcome, death from CV causes and hospitalizations for HF in patients receiving CANA | Lower rate of the primary composite CV outcome, death from CV and any cause and hospitalizations for HF in patients receiving DAPA regardless of presence or absence of T2DM | Lower rate of the primary composite CV outcome, and hospitalizations for HF in patients receiving EMPA regardless of presence or absence of T2DM | Lower rate of the primary composite CV outcome, HF hospitalization and urgent visits in patients receiving SOTA | Lower rate of the primary composite CV outcome, and hospitalizations for HF in patients receiving EMPA regardless of presence or absence of T2DM |
| Additionalconsiderations |
|
|
|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, D.M.; Ahmed, N.; Tariq, H.; Walgamage, M.; Walgamage, T.; Mohammed, A.; Chou, J.T.-T.; Kałużna-Oleksy, M.; Lesiak, M.; Straburzyńska-Migaj, E. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure—A Concise Review. J. Clin. Med. 2022, 11, 1470. https://doi.org/10.3390/jcm11061470
Keller DM, Ahmed N, Tariq H, Walgamage M, Walgamage T, Mohammed A, Chou JT-T, Kałużna-Oleksy M, Lesiak M, Straburzyńska-Migaj E. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure—A Concise Review. Journal of Clinical Medicine. 2022; 11(6):1470. https://doi.org/10.3390/jcm11061470
Chicago/Turabian StyleKeller, Daria M., Natasha Ahmed, Hamza Tariq, Malsha Walgamage, Thilini Walgamage, Azad Mohammed, Jadzia Tin-Tsen Chou, Marta Kałużna-Oleksy, Maciej Lesiak, and Ewa Straburzyńska-Migaj. 2022. "SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure—A Concise Review" Journal of Clinical Medicine 11, no. 6: 1470. https://doi.org/10.3390/jcm11061470
APA StyleKeller, D. M., Ahmed, N., Tariq, H., Walgamage, M., Walgamage, T., Mohammed, A., Chou, J. T.-T., Kałużna-Oleksy, M., Lesiak, M., & Straburzyńska-Migaj, E. (2022). SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure—A Concise Review. Journal of Clinical Medicine, 11(6), 1470. https://doi.org/10.3390/jcm11061470







