Associations between Periodontitis and COPD: An Artificial Intelligence-Based Analysis of NHANES III
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Handling and Statistical Analysis
- CPU: AMD Ryzen 9 5950X 16-Core Processor (Santa Clara, CA, USA);
- RAM: 64 GB;
- GPU: NVIDIA Geforce RTX 3090 (Santa Clara, CA, USA);
- Python version: 3.10.4 (64-bit) (Wilmington, DE, USA);
- OS: Windows 10 (Redmond, WA, USA).
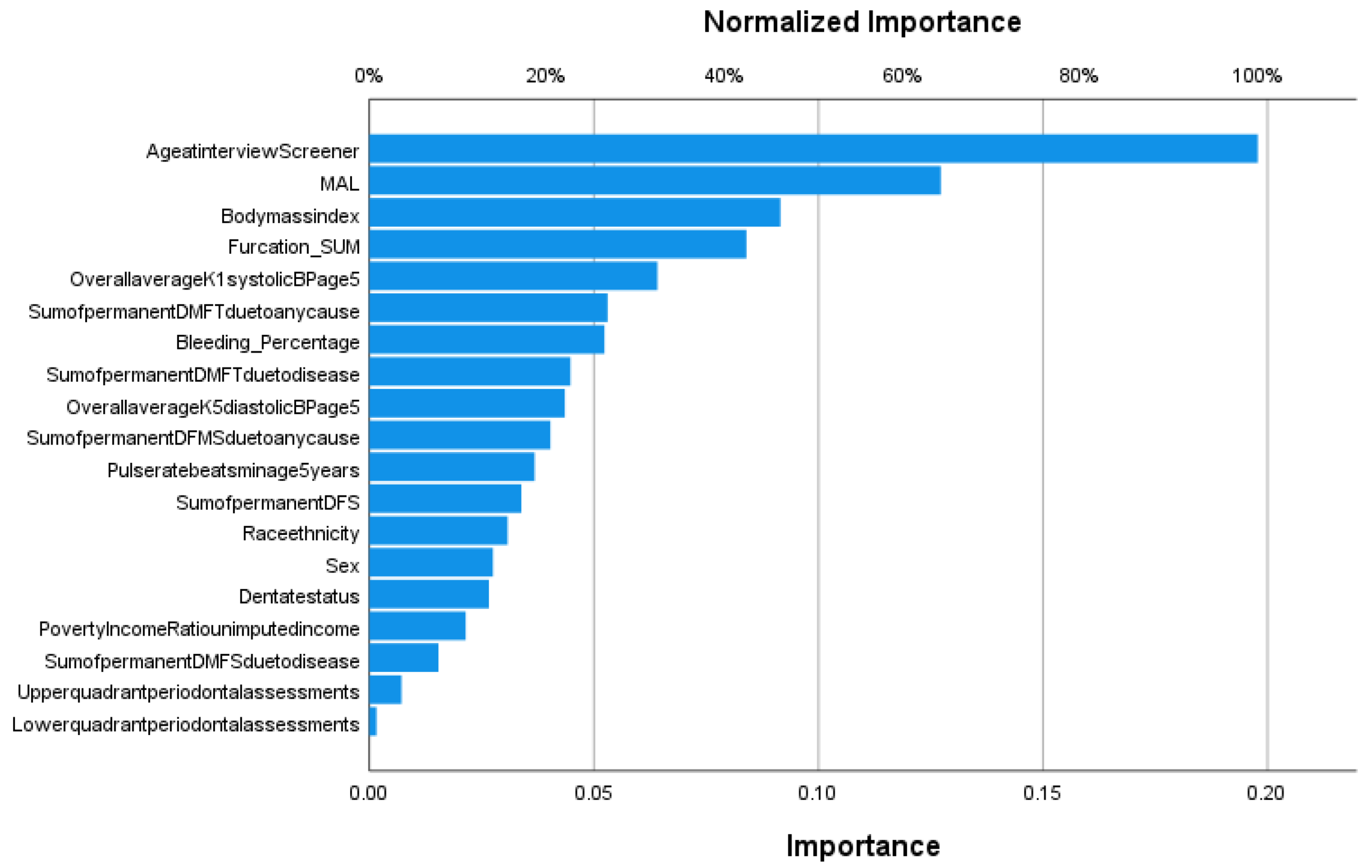
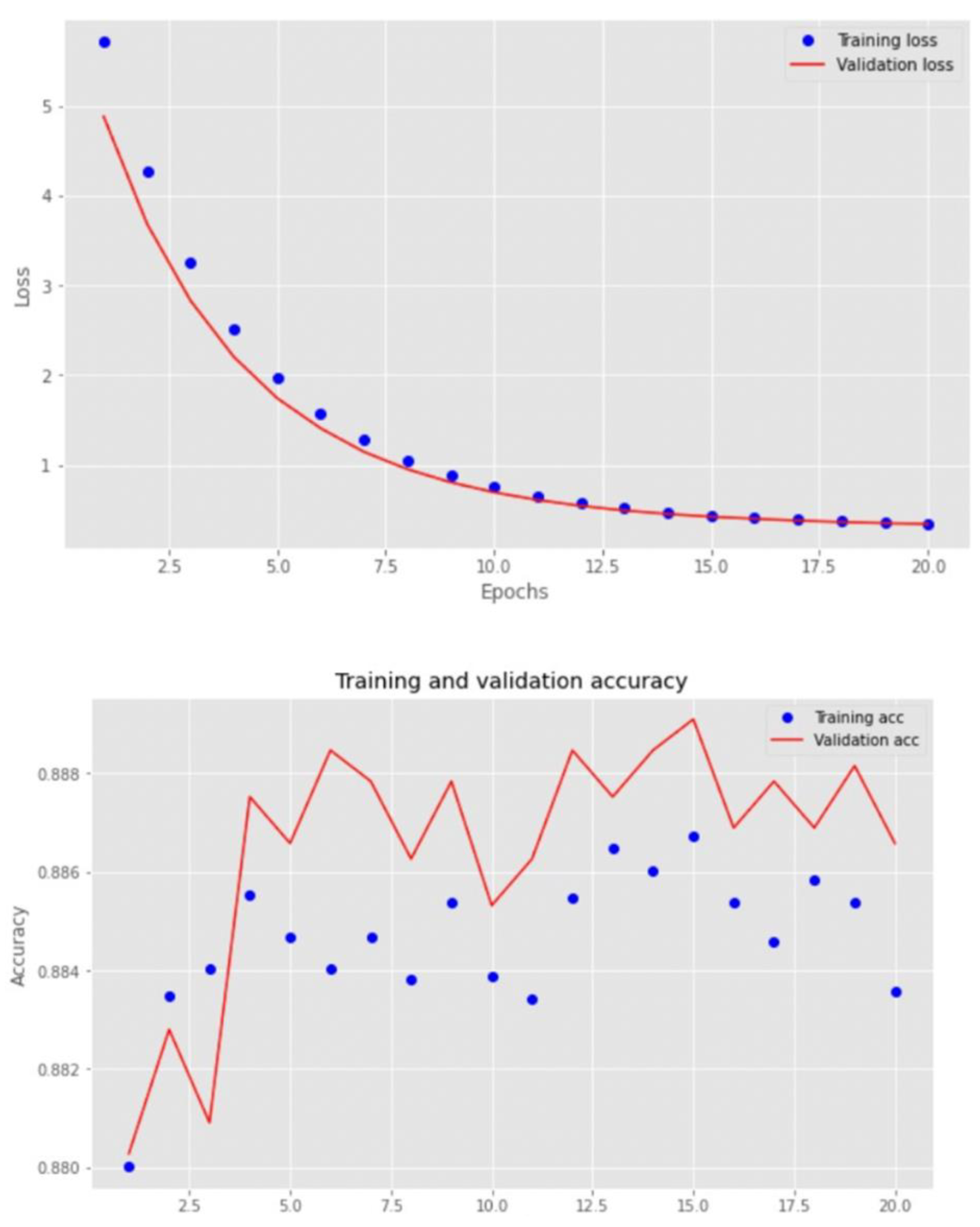
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, P.E.; Ogawa, H. The Global Burden of Periodontal Disease: Towards Integration with Chronic Disease Prevention and Control. Periodontology 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Hegde, R.; Awan, K.H. Effects of Periodontal Disease on Systemic Health. Dis.-A-Mon. 2019, 65, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sudhakara, P.; Gupta, A.; Bhardwaj, A.; Wilson, A. Oral Dysbiotic Communities and Their Implications in Systemic Diseases. Dent. J. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Gupta, A.; Verma, A.K.; Pathak, A.; Verma, S.; Chaudhary, S.C.; Kaushal, S.; Lal, N.; Kant, S.; Verma, U.P. Impact of Non-Surgical Periodontal Therapy on Pulmonary Functions, Periodontal Health and Salivary Matrix Metalloproteinase-8 of COPD Patients with Chronic Periodontitis: A Clinico-Biochemical Study. Turk. Thorac. J. 2021, 22, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Monsarrat, P.; Blaizot, A.; Kémoun, P.; Ravaud, P.; Nabet, C.; Sixou, M.; Vergnes, J.-N. Clinical Research Activity in Periodontal Medicine: A Systematic Mapping of Trial Registers. J. Clin. Periodontol. 2016, 43, 390–400. [Google Scholar] [CrossRef]
- Okuda, K.; Ishihara, K.; Nakagawa, T.; Hirayama, A.; Inayama, Y.; Okuda, K. Detection of Treponema Denticola in Atherosclerotic Lesions. J. Clin. Microbiol. 2001, 39, 1114–1117. [Google Scholar] [CrossRef] [Green Version]
- Białowąs, K.; Radwan-Oczko, M.; Duś-Ilnicka, I.; Korman, L.; Świerkot, J. Periodontal Disease and Influence of Periodontal Treatment on Disease Activity in Patients with Rheumatoid Arthritis and Spondyloarthritis. Rheumatol. Int. 2020, 40, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.A. Prevalence of Periodontal Disease, Its Association with Systemic Diseases and Prevention. Int. J. Health Sci. (Qassim) 2017, 11, 72–80. [Google Scholar]
- Soriano, J.B.; Rodríguez-Roisin, R. Chronic Obstructive Pulmonary Disease Overview: Epidemiology, Risk Factors, and Clinical Presentation. Proc. Am. Thorac. Soc. 2011, 8, 363–367. [Google Scholar] [CrossRef]
- 2020 Gold Reports. Available online: https://goldcopd.org/gold-reports/ (accessed on 10 September 2022).
- Su, Y.-C.; Jalalvand, F.; Thegerström, J.; Riesbeck, K. The Interplay between Immune Response and Bacterial Infection in COPD: Focus Upon Non-Typeable Haemophilus Influenzae. Front. Immunol. 2018, 9, 2530. [Google Scholar] [CrossRef]
- Huertas, A.; Palange, P. COPD: A Multifactorial Systemic Disease. Adv. Respir. Dis. 2011, 5, 217–224. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Luppi, F.; Beghé, B.; Rabe, K.F. Complex Chronic Comorbidities of COPD. Eur. Respir. J. 2008, 31, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Zhang, B.; Xing, H.; Yang, S.; Xu, J.; Liu, H. Patients with Chronic Obstructive Pulmonary Disease Suffer from Worse Periodontal Health—Evidence from a Meta-Analysis. Front. Physiol. 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes-Filho, I.S.; Cruz, S.S.D.; Trindade, S.C.; Passos-Soares, J.S.; Carvalho-Filho, P.C.; Figueiredo, A.C.M.G.; Lyrio, A.O.; Hintz, A.M.; Pereira, M.G.; Scannapieco, F. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Dis. 2020, 26, 446. [Google Scholar] [CrossRef]
- Ouyang, Y.; Liu, J.; Wen, S.; Xu, Y.; Zhang, Z.; Pi, Y.; Chen, D.; Su, Z.; Liang, Z.; Wang, Y.; et al. Association between Chronic Obstructive Pulmonary Disease and Periodontitis: The Common Role of Innate Immune Cells? Cytokine 2022, 158, 155982. [Google Scholar] [CrossRef]
- Sapey, E.; Yonel, Z.; Edgar, R.; Parmar, S.; Hobbins, S.; Newby, P.; Crossley, D.; Usher, A.; Johnson, S.; Walton, G.M.; et al. The Clinical and Inflammatory Relationships between Periodontitis and Chronic Obstructive Pulmonary Disease. J. Clin. Periodontol. 2020, 47, 1040–1052. [Google Scholar] [CrossRef]
- Punceviciene, E.; Rovas, A.; Puriene, A.; Stuopelyte, K.; Vitkus, D.; Jarmalaite, S.; Butrimiene, I. Investigating the Relationship between the Severity of Periodontitis and Rheumatoid Arthritis: A Cross-Sectional Study. Clin. Rheumatol. 2021, 40, 3153–3160. [Google Scholar] [CrossRef]
- Suzuki, R.; Kamio, N.; Kaneko, T.; Yonehara, Y.; Imai, K. Fusobacterium Nucleatum Exacerbates Chronic Obstructive Pulmonary Disease in Elastase-induced Emphysematous Mice. FEBS Open Bio. 2022, 12, 638–648. [Google Scholar] [CrossRef]
- Apessos, I.; Voulgaris, A.; Agrafiotis, M.; Andreadis, D.; Steiropoulos, P. Effect of Periodontal Therapy on COPD Outcomes: A Systematic Review. BMC Pulm. Med. 2021, 21, 92. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Ho, A.W. Potential Associations between Chronic Respiratory Disease and Periodontal Disease: Analysis of National Health and Nutrition Examination Survey III. J. Periodontol. 2001, 72, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gaeckle, N.T.; Pragman, A.A.; Pendleton, K.M.; Baldomero, A.K.; Criner, G.J. The Oral-Lung Axis: The Impact of Oral Health on Lung Health. Respir. Care 2020, 65, 1211–1220. [Google Scholar] [CrossRef]
- Reddi, K.; Meghji, S.; Wilson, M.; Henderson, B. Comparison of the Osteolytic Activity of Surface-Associated Proteins of Bacteria Implicated in Periodontal Disease. Oral Dis. 1995, 1, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.M.; Reis, C.; Manzanares-Céspedes, M.C. Chronic Periodontitis, Inflammatory Cytokines, and Interrelationship with Other Chronic Diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Birkedal-Hansen, H. Role of Cytokines and Inflammatory Mediators in Tissue Destruction. J. Periodontal Res. 1993, 28, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Jaedicke, K.M.; Preshaw, P.M.; Taylor, J.J. Salivary Cytokines as Biomarkers of Periodontal Diseases. Periodontology 2000 2016, 70, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Zekeridou, A.; Giannopoulou, C.; Cancela, J.; Courvoisier, D.; Mombelli, A. Effect of Initial Periodontal Therapy on Gingival Crevicular Fluid Cytokine Profile in Subjects with Chronic Periodontitis. Clin. Exp. Dent. Res. 2017, 3, 62–68. [Google Scholar] [CrossRef]
- Bruzzaniti, S.; Bocchino, M.; Santopaolo, M.; Calì, G.; Stanziola, A.A.; D’Amato, M.; Esposito, A.; Barra, E.; Garziano, F.; Micillo, T.; et al. An Immunometabolic Pathomechanism for Chronic Obstructive Pulmonary Disease. Proc. Natl. Acad. Sci. USA 2019, 116, 15625–15634. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.S.; Jadhav, P.; Kamath, K.P.; Kumar, S.R.; Vijayalaxmi, S.; Anil, S. A Case-Control Study on the Association between Periodontitis and Coronavirus Disease (COVID-19). J. Periodontol. 2022, 93, 584–590. [Google Scholar] [CrossRef]
- Miller, D.D.; Brown, E.W. Artificial Intelligence in Medical Practice: The Question to the Answer? Am. J. Med. 2018, 131, 129–133. [Google Scholar] [CrossRef]
- Khanagar, S.B.; Al-ehaideb, A.; Maganur, P.C.; Vishwanathaiah, S.; Patil, S.; Baeshen, H.A.; Sarode, S.C.; Bhandi, S. Developments, Application, and Performance of Artificial Intelligence in Dentistry—A Systematic Review. J. Dent. Sci. 2021, 16, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Ian Goodfellow, Yoshua Bengio, and Aaron Courville: Deep Learning|SpringerLink. Available online: https://link.springer.com/article/10.1007/s10710-017-9314-z (accessed on 10 September 2022).
- Waring, J.; Lindvall, C.; Umeton, R. Automated Machine Learning: Review of the State-of-the-Art and Opportunities for Healthcare. Artif. Intell. Med. 2020, 104, 101822. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, M.; Min, J.S.; Bian, J.; Modave, F. Big Data Hurdles in Precision Medicine and Precision Public Health. BMC Med. Inf. Decis. Mak. 2018, 18, 139. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.-C.; Chang, P.-Y.; Lin, C.-L.; Chen, C.-H.; Tu, C.-Y.; Hsia, T.-C.; Shih, C.-M.; Hsu, W.-H.; Sung, F.-C.; Kao, C.-H. Risk of Periodontal Diseases in Patients With Chronic Obstructive Pulmonary Disease. Medicine 2015, 94, e2047. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.P.; Mute, B.R.; Doiphode, S.S.; Bardapurkar, S.J.; Borkar, M.S.; Raje, D.V. Association Between Periodontal Disease and Chronic Obstructive Pulmonary Disease: A Reality or Just a Dogma? J. Periodontol. 2013, 84, 1717–1723. [Google Scholar] [CrossRef] [Green Version]
- Öztekin, G.; Baser, U.; Kucukcoskun, M.; Tanrikulu-Kucuk, S.; Ademoglu, E.; Isik, G.; Ozkan, G.; Yalcin, F.; Kiyan, E. The Association between Periodontal Disease and Chronic Obstructive Pulmonary Disease: A Case Control Study. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 424–430. [Google Scholar] [CrossRef]
- Zeng, X.-T.; Tu, M.-L.; Liu, D.-Y.; Zheng, D.; Zhang, J.; Leng, W. Periodontal Disease and Risk of Chronic Obstructive Pulmonary Disease: A Meta-Analysis of Observational Studies. PLoS ONE 2012, 7, e46508. [Google Scholar] [CrossRef] [Green Version]
- Sample Design: Third National Health and Nutrition Examination Survey Sample Design: Third National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/data/series/sr_02/Sr02_113.pdf (accessed on 6 November 2022).
- Fitzgerald, B.P.; Hawley, C.E.; Harrold, C.Q.; Garrett, J.S.; Polson, A.M.; Rams, T.E. Reproducibility of Manual Periodontal Probing Following a Comprehensive Standardization and Calibration Training Program. J. Oral Biol. (Northborough) 2022, 8, 63. [Google Scholar] [CrossRef]
- Farook, F.F.; Alodwene, H.; Alharbi, R.; Alyami, M.; Alshahrani, A.; Almohammadi, D.; Alnasyan, B.; Aboelmaaty, W. Reliability Assessment between Clinical Attachment Loss and Alveolar Bone Level in Dental Radiographs. Clin. Exp. Dent. Res. 2020, 6, 596–601. [Google Scholar] [CrossRef]
- Saravi, B.E.; Putz, M.; Patzelt, S.; Alkalak, A.; Uelkuemen, S.; Boeker, M. Marginal Bone Loss around Oral Implants Supporting Fixed versus Removable Prostheses: A Systematic Review. Int. J. Implant Dent. 2020, 6, 20. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Luo, J.; Dong, X.; Jiang, X. The Association between Periodontitis and Lung Function: Results from the National Health and Nutrition Examination Survey 2009 to 2012. J. Periodontol. 2022, 93, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Saravi, B.; Hassel, F.; Ülkümen, S.; Zink, A.; Shavlokhova, V.; Couillard-Despres, S.; Boeker, M.; Obid, P.; Lang, G.M. Artificial Intelligence-Driven Prediction Modeling and Decision Making in Spine Surgery Using Hybrid Machine Learning Models. J. Pers. Med. 2022, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Fu, E.; Li, C.-H.; Huang, R.-Y.; Chiu, H.-C.; Cheng, W.-C.; Chen, W.-L. Association between Periodontitis and Pulmonary Function Based on the Third National Health and Nutrition Examination Survey (NHANES III). J. Clin. Periodontol. 2020, 47, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Hobbins, S.; Chapple, I.L.; Sapey, E.; Stockley, R.A. Is Periodontitis a Comorbidity of COPD or Can Associations Be Explained by Shared Risk Factors/Behaviors? Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1339–1349. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.H.; Hwang, H.-J.; Kim, S.-H.; Kim, T.H. Associations between Periodontitis and Chronic Obstructive Pulmonary Disease: The 2010 to 2012 Korean National Health and Nutrition Examination Survey. J. Periodontol. 2016, 87, 864–871. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Leake, J.L. Systematic Review of the Association between Respiratory Diseases and Oral Health. J. Periodontol. 2006, 77, 1465–1482. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, E.; Kormi, I.; Basoglu, O.K.; Gurgun, A.; Kaval, B.; Sorsa, T.; Buduneli, N. Periodontal Health and Serum, Saliva Matrix Metalloproteinases in Patients with Mild Chronic Obstructive Pulmonary Disease. J. Periodontal Res. 2013, 48, 269–275. [Google Scholar] [CrossRef]
- Baldomero, A.K.; Siddiqui, M.; Lo, C.-Y.; Petersen, A.; Pragman, A.A.; Connett, J.E.; Kunisaki, K.M.; Wendt, C.H. The Relationship between Oral Health and COPD Exacerbations. COPD 2019, 14, 881–892. [Google Scholar] [CrossRef] [Green Version]
- Sundh, J.; Tanash, H.; Arian, R.; Neves-Guimaraes, A.; Broberg, K.; Lindved, G.; Kern, T.; Zych, K.; Nielsen, H.B.; Halling, A.; et al. Advanced Dental Cleaning Is Associated with Reduced Risk of COPD Exacerbations—A Randomized Controlled Trial. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 3203–3215. [Google Scholar] [CrossRef]
- Murphy, T.F.; Sethi, S. Bacterial Infection in Chronic Obstructive Pulmonary Disease. Am. Rev. Respir. Dis. 1992, 146, 1067–1083. [Google Scholar] [CrossRef] [Green Version]
- Whittemore, A.S.; Perlin, S.A.; DiCiccio, Y. Chronic Obstructive Pulmonary Disease in Lifelong Nonsmokers: Results from NHANES. Am. J. Public Health 1995, 85, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.A.; Weiss, P.J. Capnocytophagal Pneumonia in a Healthy Man. West. J. Med. 1994, 160, 79–80. [Google Scholar] [PubMed]
- Goolam Mahomed, A.; Feldman, C.; Smith, C.; Promnitz, D.A.; Kaka, S. Does Primary Streptococcus Viridans Pneumonia Exist? S. Afr. Med. J. 1992, 82, 432–434. [Google Scholar] [PubMed]
- Morris, J.F.; Sewell, D.L. Necrotizing Pneumonia Caused by Mixed Infection with Actinobacillus Actinomycetemcomitans and Actinomyces Israelii: Case Report and Review. Clin. Infect. Dis. 1994, 18, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Demmer, R.T.; Trinquart, L.; Zuk, A.; Fu, B.C.; Blomkvist, J.; Michalowicz, B.S.; Ravaud, P.; Desvarieux, M. The Influence of Anti-Infective Periodontal Treatment on C-Reactive Protein: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e77441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demmer, R.T.; Breskin, A.; Rosenbaum, M.; Zuk, A.; LeDuc, C.; Leibel, R.; Paster, B.; Desvarieux, M.; Jacobs Jr, D.R.; Papapanou, P.N. The Subgingival Microbiome, Systemic Inflammation and Insulin Resistance: The Oral Infections, Glucose Intolerance and Insulin Resistance Study. J. Clin. Periodontol. 2017, 44, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teeuw, W.J.; Slot, D.E.; Susanto, H.; Gerdes, V.E.A.; Abbas, F.; D’Aiuto, F.; Kastelein, J.J.P.; Loos, B.G. Treatment of Periodontitis Improves the Atherosclerotic Profile: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2014, 41, 70–79. [Google Scholar] [CrossRef]
- Kataoka, S.; Kimura, M.; Yamaguchi, T.; Egashira, K.; Yamamoto, Y.; Koike, Y.; Ogawa, Y.; Fujiharu, C.; Namai, T.; Taguchi, K.; et al. A Cross-Sectional Study of Relationships between Periodontal Disease and General Health: The Hitachi Oral Healthcare Survey. BMC Oral Health 2021, 21, 644. [Google Scholar] [CrossRef]
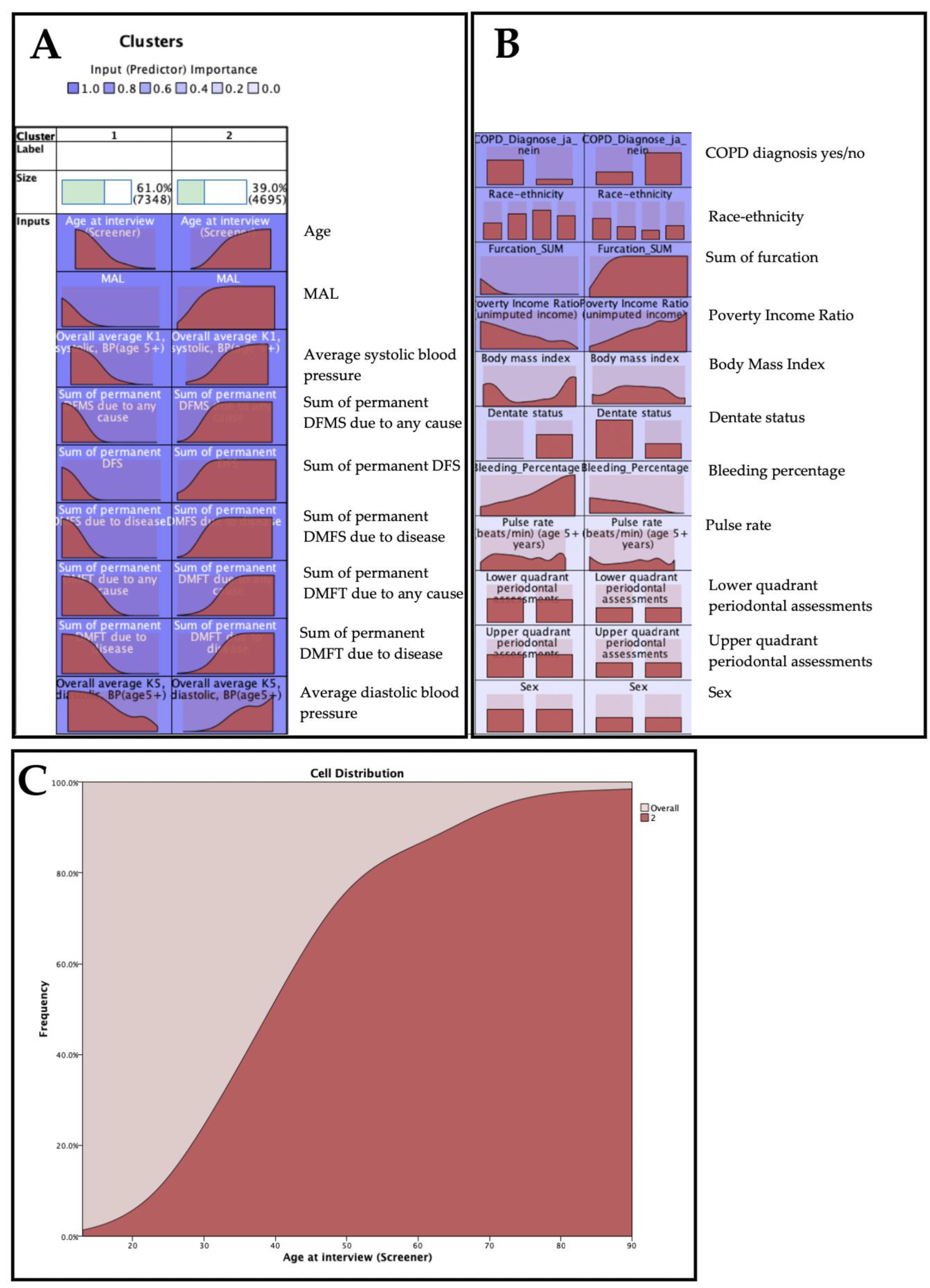
| Low-Risk Cluster | High-Risk Cluster | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Count | Row N % | Mean | Standard Deviation | Count | Row N % | Mean | Standard Deviation | ||
| COPD diagnosis | no | 7187 | 65.5% | 3780 | 34.5% | ||||
| yes | 161 | 15.0% | 915 | 85.0% | |||||
| Race/ethnicity | Non-Hispanic white | 1946 | 44.0% | 2477 | 56.0% | ||||
| Non-Hispanic black | 2295 | 66.4% | 1163 | 33.6% | |||||
| Mexican-American | 2786 | 76.3% | 867 | 23.7% | |||||
| Other | 321 | 63.1% | 188 | 36.9% | |||||
| Sex | Male | 3525 | 61.1% | 2242 | 38.9% | ||||
| Female | 3823 | 60.9% | 2453 | 39.1% | |||||
| Upper-quadrant periodontal assessments | 1 | 3694 | 60.7% | 2389 | 39.3% | ||||
| 2 | 3654 | 61.3% | 2306 | 38.7% | |||||
| Lower-quadrant periodontal assessments | 3 | 3659 | 61.5% | 2291 | 38.5% | ||||
| 4 | 3689 | 60.5% | 2404 | 39.5% | |||||
| Dentate status | Completely edentulous | 0 | 0.0% | 0 | 0.0% | ||||
| Edentulous in one arch | 0 | 0.0% | 177 | 100.0% | |||||
| Teeth present | 7348 | 61.9% | 4518 | 38.1% | |||||
| Age at interview (Screener) | 28 | 11 | 53 | 16 | |||||
| Poverty income ratio (unimputed income) | 3 | 2 | 2 | 2 | |||||
| Pulse rate (beats/min) (age 5+ years) | 74 | 12 | 75 | 12 | |||||
| Overall average K1, systolic, BP (age 5+) | 114 | 12 | 129 | 19 | |||||
| Overall average K5, diastolic, BP (age 5+) | 69 | 11 | 76 | 10 | |||||
| Body mass index | 25.6 | 5.9 | 27.5 | 5.7 | |||||
| Sum of permanent DMFS due to disease | 12 | 11 | 56 | 23 | |||||
| Sum of permanent DFMS due to any cause | 13 | 11 | 58 | 23 | |||||
| Sum of permanent DFS | 8 | 7 | 30 | 19 | |||||
| Sum of permanent DMFT due to disease | 5 | 4 | 16 | 5 | |||||
| Sum of permanent DMFT due to any cause | 5 | 4 | 17 | 5 | |||||
| Bleeding_Percentage | 10.43 | 14.37 | 7.88 | 11.26 | |||||
| Furcation_SUM | 0.05 | 0.30 | 0.57 | 1.40 | |||||
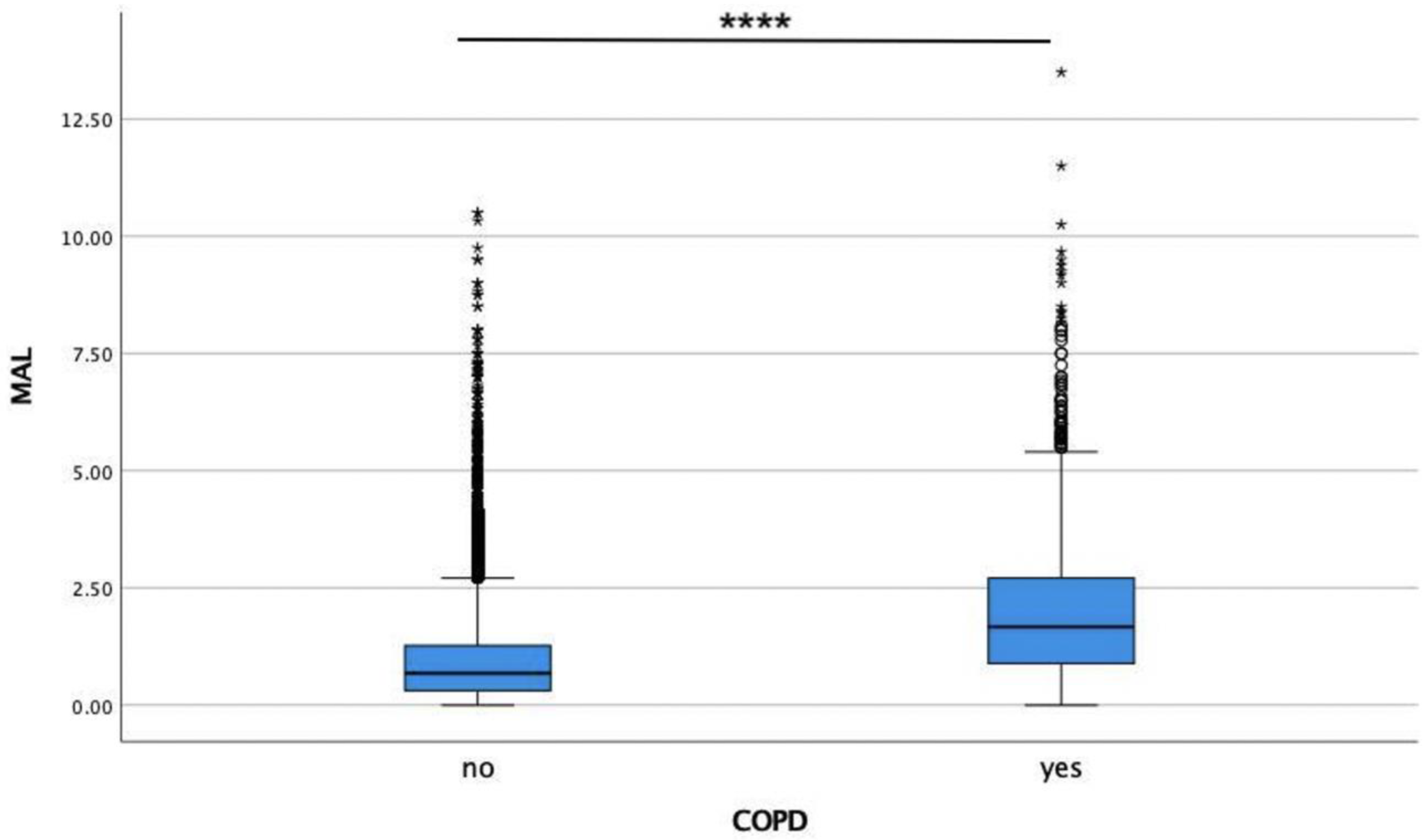
| MAL | 0.58 | 0.53 | 1.51 | 1.16 | |||||
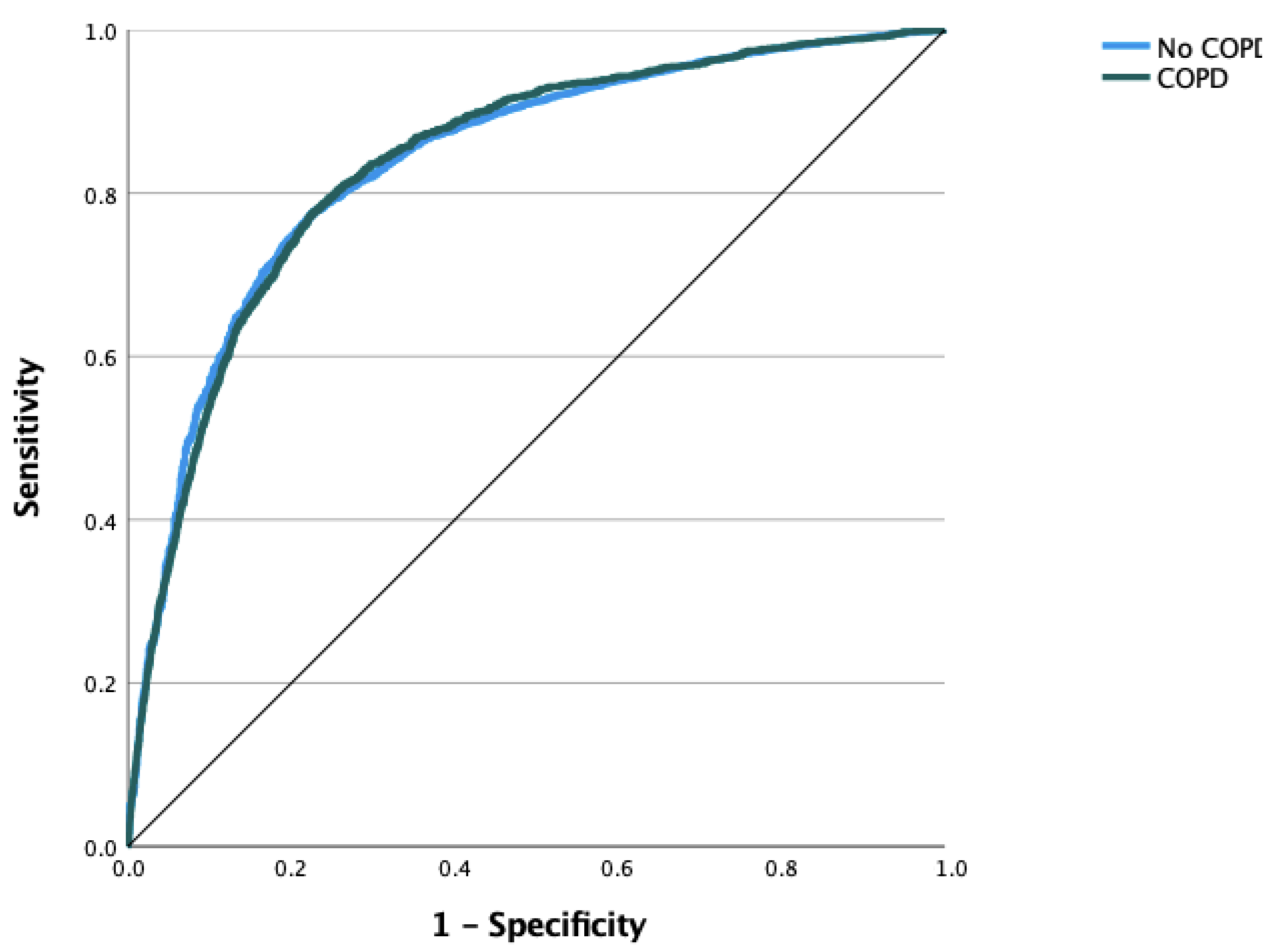
| Algorithm | Accuracy | AUC |
|---|---|---|
| Logistic regression | 0.884 | 0.835 |
| Random forest classifier | 0.883 | 0.819 |
| SGD classifier | 0.879 | 0.804 |
| K-nearest neighbors | 0.872 | 0.723 |
| Decision tree classifier | 0.823 | 0.608 |
| GaussianNB | 0.803 | 0.807 |
| SVM | 0.884 | 0.685 |
| Custom CNN | 0.887 | 0.953 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollmer, A.; Vollmer, M.; Lang, G.; Straub, A.; Shavlokhova, V.; Kübler, A.; Gubik, S.; Brands, R.; Hartmann, S.; Saravi, B. Associations between Periodontitis and COPD: An Artificial Intelligence-Based Analysis of NHANES III. J. Clin. Med. 2022, 11, 7210. https://doi.org/10.3390/jcm11237210
Vollmer A, Vollmer M, Lang G, Straub A, Shavlokhova V, Kübler A, Gubik S, Brands R, Hartmann S, Saravi B. Associations between Periodontitis and COPD: An Artificial Intelligence-Based Analysis of NHANES III. Journal of Clinical Medicine. 2022; 11(23):7210. https://doi.org/10.3390/jcm11237210
Chicago/Turabian StyleVollmer, Andreas, Michael Vollmer, Gernot Lang, Anton Straub, Veronika Shavlokhova, Alexander Kübler, Sebastian Gubik, Roman Brands, Stefan Hartmann, and Babak Saravi. 2022. "Associations between Periodontitis and COPD: An Artificial Intelligence-Based Analysis of NHANES III" Journal of Clinical Medicine 11, no. 23: 7210. https://doi.org/10.3390/jcm11237210







