The Triglyceride-Glucose Index Is Associated with Longitudinal Cognitive Decline in a Middle-Aged to Elderly Population: A Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Assessment of the TyG Index
2.4. Outcome Evaluation
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
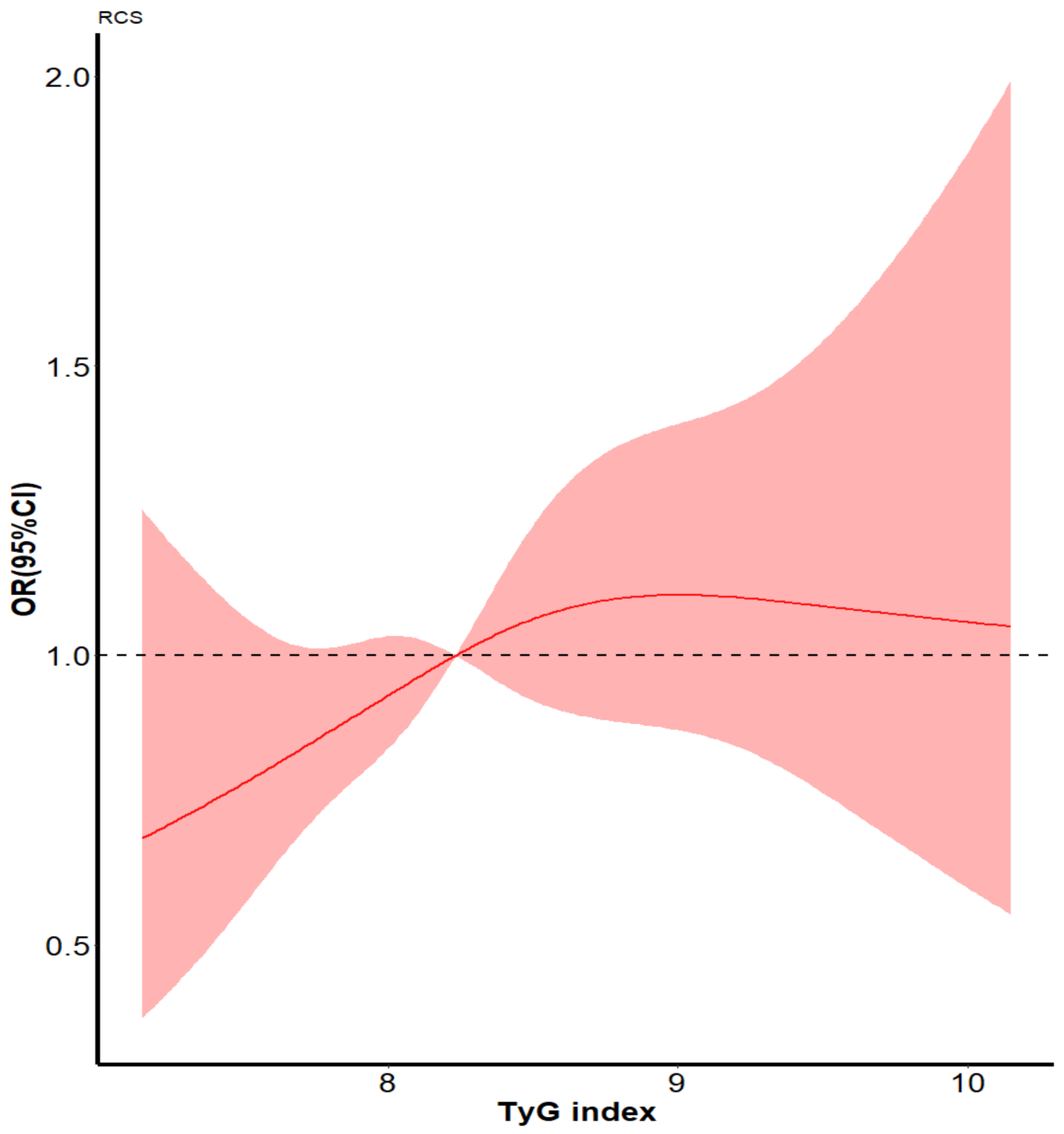
3.2. Effect of the Tyg Index on Cognitive Decline
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Zhu, M.; Jiao, H.; Song, Y.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ren, Y.; Wang, Y.; Hou, T.; Dong, Y.; Han, X.; Yin, L.; Zhang, Q.; Feng, J.; Wang, L.; et al. Mild cognitive impairment among rural-dwelling older adults in China: A community-based study. Alzheimer’s Dement. 2022, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Collaborators GBDD. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, R.; Liu, G.G. Cognitive impairment and mortality among the oldest-old Chinese. Int. J. Geriatr. Psychiatry 2016, 31, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Perna, L.; Wahl, H.W.; Mons, U.; Saum, K.U.; Holleczek, B.; Brenner, H. Cognitive impairment, all-cause and cause-specific mortality among non-demented older adults. Age Ageing 2015, 44, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Batty, G.D.; Deary, I.J.; Zaninotto, P. Association of cognitive function with causespecific mortality in middle and older age: Follow-up of participants in the English longitudinal study of ageing. Am. J. Epidemiol. 2016, 183, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, B.J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 3G–10G. [Google Scholar] [CrossRef]
- Novak, V.; Milberg, W.; Hao, Y.; Munshi, M.; Novak, P.; Galica, A.; Manor, B.; Roberson, P.; Craft, S.; Abduljalil, A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 2014, 37, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Mejia Arango, S.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Hooshmand, B.; Rusanen, M.; Ngandu, T.; Leiviskä, J.; Sindi, S.; von Arnim, C.A.; Falkai, P.; Soininen, H.; Tuomilehto, J.; Kivipelto, M. Serum insulin and cognitive performance in older adults: A longitudinal study. Am. J. Med. 2019, 132, 367–373. [Google Scholar] [CrossRef]
- Neergaard, J.S.; Dragsbæk, K.; Christiansen, C.; Nielsen, H.B.; Brix, S.; Karsdal, M.A.; Henriksen, K. Metabolic syndrome, insulin resistance, and cognitive dysfunction: Does your metabolic profile affect your brain? Diabetes 2017, 66, 1957–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutski, M.; Weinstein, G.; Goldbourt, U.; Tanne, D. Insulin Resistance and Future Cognitive Performance and Cognitive Decline in Elderly Patients with Cardiovascular Disease. J. Alzheimer’s Dis. 2017, 57, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, E.; Solis-Herrera, C.; Trautmann, M.; Malloy, J.; Triplitt, C. Assessment of pancreaticβ-cell function: Review of methods and clinical applications. Curr. Diabetes Rev. 2014, 10, 2–42. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Zhang, T.Y.; Cheng, Y.J.; Ma, Y.; Xu, Y.K.; Yang, J.Q.; Zhou, Y.J. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: Results from an observational cohort study in China. Cardiovasc. Diabetol. 2020, 19, 108. [Google Scholar] [CrossRef]
- Park, S.E.; Park, C.Y.; Sweeney, G. Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit. Rev. Clin. Lab. Sci. 2015, 52, 180–190. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Lv, X.; Lai, X.; Xu, L.; Feng, J.; Song, Y.; Wang, S.; Zhan, S. Sex-specific associations between lipids and cognitive decline in the middle-aged and elderly: A cohort study of Chinese adults. Alzheimer’s Res. Ther. 2020, 12, 164. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, F.; Yang, J.; Peng, H.; Li, Y.; Li, B.; Wang, S. Revealing a Novel Landscape of the Association Between Blood Lipid Levels and Alzheimer’s Disease: A Meta-Analysis of a Case-Control Study. Front. Aging Neurosci. 2020, 11, 370. [Google Scholar] [CrossRef]
- Ma, C.; Yin, Z.; Zhu, P.; Luo, J.; Shi, X.; Gao, X. Blood cholesterol in late-life and cognitive decline: A longitudinal study of the Chinese elderly. Mol. Neurodegener. 2017, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Power, M.C.; Rawlings, A.; Sharrett, A.R.; Bandeen-Roche, K.; Coresh, J.; Ballantyne, C.M.; Pokharel, Y.; Michos, E.D.; Penman, A.; Alonso, A.; et al. Association of midlife lipids with 20-year cognitive change: A cohort study. Alzheimer’s Dement. 2018, 14, 167–177. [Google Scholar] [CrossRef]
- Mielke, M.M.; Xue, Q.L.; Zhou, J.; Chaves, P.H.; Fried, L.P.; Carlson, M.C. Baseline serum cholesterol is selectively associated with motor speed and not rates of cognitive decline: The Women’s Health and Aging Study II. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wei, S.; Zhou, R.; Shang, S.; Dang, L.; Gao, L.; Chen, C.; Huo, K.; Wang, J.; Wang, J.; et al. The Relationships Between Lipid Accumulation Product Levels and Cognitive Decline Over 4 Years in a Rural Area of Xi’an, China. Front. Aging Neurosci. 2021, 13, 761886. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Yu, G.; Zhou, X.; Zhou, W.; Zhu, L.; Wang, T.; Huang, X.; Bao, H.; Cheng, X. Association Between Lipid Accumulation Product and Cognitive Function in Hypertensive Patients With Normal Weight: Insight From the China H-type Hypertension Registry Study. Front. Neurol. 2022, 12, 732757. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, Q.; Zhao, J.; Wu, T.; Ji, L.; Huang, G.; Ma, F. Relationship between plasma lipids and mild cognitive impairment in the elderly Chinese: A case-control study. Lipids Health Dis. 2016, 15, 146. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, V.; Frazier, D.T.; Bettcher, B.M.; Jastrzab, L.; Chao, L.; Reed, B.; Mungas, D.; Weiner, M.; DeCarli, C.; Chui, H.; et al. Triglycerides are negatively correlated with cognitive function in nondemented aging adults. Neuropsychology 2017, 31, 682–688. [Google Scholar] [CrossRef]
- Dimache, A.M.; Șalaru, D.L.; Sascău, R.; Stătescu, C. The Role of High Triglycerides Level in Predicting Cognitive Impairment: A Review of Current Evidence. Nutrients 2021, 13, 2118. [Google Scholar] [CrossRef]
- Nägga, K.; Gustavsson, A.M.; Stomrud, E.; Lindqvist, D.; van Westen, D.; Blennow, K.; Zetterberg, H.; Melander, O.; Hansson, O. Increased midlife triglycerides predict brain β-amyloid and tau pathology 20 years later. Neurology 2018, 90, e73–e81. [Google Scholar] [CrossRef] [Green Version]
- Banks, W.A.; Farr, S.A.; Salameh, T.S.; Niehoff, M.L.; Rhea, E.M.; Morley, J.E.; Hanson, A.J.; Hansen, K.M.; Craft, S. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int. J. Obes. 2018, 42, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Peloso, G.M.; Beiser, A.S.; Destefano, A.L.; Seshadri, S. Genetic Interaction with Plasma Lipids on Alzheimer’s Disease in the Framingham Heart Study. J. Alzheimer’s Dis. 2018, 66, 1275–1282. [Google Scholar] [CrossRef]
- Zimering, M.B.; Knight, J.; Ge, L.; Bahn, G.; VADT Investigators. Predictors of Cognitive Decline in Older Adult Type 2 Diabetes from the Veterans Affairs Diabetes Trial. Front. Endocrinol. 2016, 7, 123. [Google Scholar] [CrossRef]
- Shao, T.N.; Yin, G.Z.; Yin, X.L.; Wu, J.Q.; Du, X.D.; Zhu, H.L.; Liu, J.H.; Wang, X.Q.; Xu, D.W.; Tang, W.J.; et al. Elevated triglyceride levels are associated with cognitive impairments among patients with major depressive disorder. Compr. Psychiatry 2017, 75, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sobia, F.; Niazi, N.; Manzoor, S.; Fazal, N.; Ahmad, F. Metabolic clustering of risk factors: Evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Kengne, A.; Katsiki, N.; Mikhailidis, D.; Banach, M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J. Diabetes Complicat. 2018, 32, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Vasques, A.C.J.; Novaes, F.S.; de Oliveira, M.D.S.; Souza, J.R.M.; Yamanaka, A.; Pareja, J.C.; Tambascia, M.A.; Saad, M.J.A.; Geloneze, B. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011, 93, e98–e100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzegar, N.; Tohidi, M.; Hasheminia, M.; Azizi, F.; Hadaegh, F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran Lipid and Glucose Study. Cardiovasc. Diabetol. 2020, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Han, K.; Park, C. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: A population-based study. BMC Med. 2020, 18, 361. [Google Scholar] [CrossRef]
- Su, W.; Chen, S.; Huang, Y.; Huang, J.; Wu, P.; Hsu, W.; Lee, M. Comparison of the effects of fasting glucose, hemoglobin A, and triglyceride-glucose index on cardiovascular events in type 2 diabetes mellitus. Nutrients 2019, 11, 2838. [Google Scholar] [CrossRef] [Green Version]
- Teng, Z.; Feng, J.; Dong, Y.; Xu, J.; Jiang, X.; Chen, H.; Qi, Q.; Li, R.; Chen, W.; Lv, P. Triglyceride glucose index is associated with cerebral small vessel disease burden and cognitive impairment in elderly patients with type 2 diabetes mellitus. Front. Endocrinol. 2022, 13, 970122. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Z.; Wang, X.; Zhang, J.; Zhang, D.; Li, S. The chain mediating role of C-reactive protein and triglyceride-glucose index between lung function and cognitive function in a systemic low-grade inflammation state. J. Psychiatr. Res. 2022, 155, 380–386. [Google Scholar] [CrossRef]
- Hong, S.; Han, K.; Park, C.Y. The insulin resistance by triglyceride glucose index and risk for dementia: Population-based study. Alzheimer’s Res. Ther. 2021, 13, 9. [Google Scholar] [CrossRef]
- Song, D.Y.; Wang, X.W.; Wang, S.; Ge, S.Q.; Ding, G.Y.; Chen, X.Y.; Chen, Y.R.; Liu, H.M.; Xie, X.M.; Xing, W.J.; et al. Jidong cognitive impairment cohort study: Objectives, design, and baseline screening. Neural Regen. Res. 2020, 15, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jia, J.; Yang, Z. Mini-Mental State Examination in Elderly Chinese: A Population-Based Normative Study. J. Alzheimer’s Dis. 2016, 53, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, W.; Ma, Y.; Chen, H.; Zeng, Y.; Yu, X.; Hofman, A.; Wang, H. Cognitive decline and mortality among community-dwelling Chinese older people. BMC Med. 2019, 17, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.; Zheng, R.; Li, J.; Cao, Q.; Hou, T.; Zhao, Z.; Xu, M.; Chen, Y.; Lu, J.; Wang, T.; et al. Individual and Combined Associations of Glucose Metabolic Components With Cognitive Function Modified by Obesity. Front. Endocrinol. 2021, 12, 769120. [Google Scholar] [CrossRef] [PubMed]
- Pekkala, T.; Hall, A.; Mangialasche, F.; Kemppainen, N.; Mecocci, P.; Ngandu, T.; Rinne, J.O.; Soininen, H.; Tuomilehto, J.; Kivipelto, M.; et al. Association of Peripheral Insulin Resistance and Other Markers of Type 2 Diabetes Mellitus with Brain Amyloid Deposition in Healthy Individuals at Risk of Dementia. J. Alzheimer’s Dis. 2020, 76, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Cezaretto, A.; de Almeida-Pititto, B.; Alencar, G.P.; Suemoto, C.K.; Bensenor, I.; Lotufo, P.A.; Ferreira, S.R. Utility of combined inflammatory biomarkers for the identification of cognitive dysfunction in non-diabetic participants of the ELSA-Brasil. Psychoneuroendocrinology 2019, 103, 61–66. [Google Scholar] [CrossRef]
- Teixeira, M.M.; de Azeredo Passos, V.M.; Barreto, S.M.; Schmidt, M.I.; Duncan, B.B.; Beleigoli, A.M.; Fonseca, M.D.J.M.; Vidigal, P.G.; Figueiredo, R.C.; Colosimo, E.; et al. Markers of adiposity, insulin resistance, prediabetes and cognitive function at baseline of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Diabetes Res. Clin. Pract. 2020, 170, 108499. [Google Scholar] [CrossRef]
- Gómez-Martínez, C.; Babio, N.; Júlvez, J.; Becerra-Tomás, N.; Martínez-González, M.Á.; Corella, D.; Castañer, O.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Glycemic Dysregulations Are Associated with Worsening Cognitive Function in Older Participants at High Risk of Cardiovascular Disease: Two-Year Follow-up in the PREDIMED-Plus Study. Front. Endocrinol. 2021, 12, 754347. [Google Scholar] [CrossRef]
- Rönnemaa, E.; Zethelius, B.; Sundelöf, J.; Sundström, J.; Degerman-Gunnarsson, M.; Lannfelt, L.; Berne, C.; Kilander, L. Glucose metabolism and the risk of Alzheimer’s disease and dementia: A population-based 12 year follow-up study in 71-year-old men. Diabetologia 2009, 52, 1504–1510. [Google Scholar] [CrossRef] [Green Version]
- Tesauro, M.; Canale, M.P.; Rodia, G.; Di Daniele, N.; Lauro, D.; Scuteri, A.; Cardillo, C. Metabolic syndrome, chronic kidney, and cardiovascular diseases: Role of adipokines. Cardiol. Res. Pract. 2011, 2011, 653182. [Google Scholar] [CrossRef]
- Janus, A.; Szahidewicz-Krupska, E.; Mazur, G.; Doroszko, A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediat. Inflamm. 2016, 2016, 3634948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoscheidt, S.M.; Kellawan, J.M.; Berman, S.E.; Rivera-Rivera, L.A.; Krause, R.A.; Oh, J.M.; Beeri, M.S.; Rowley, H.A.; Wieben, O.; Carlsson, C.M.; et al. Insulin resistance is associated with lower arterial blood flow and reduced cortical perfusion in cognitively asymptomatic middle-aged adults. J. Cereb. Blood Flow Metab. 2017, 37, 2249–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feraco, A.; Marzolla, V.; Scuteri, A.; Armani, A.; Caprio, M. Mineralocorticoid Receptors in Metabolic Syndrome: From Physiology to Disease. Trends Endocrinol. Metab. 2020, 31, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Gorini, S.; Marzolla, V.; Mammi, C.; Armani, A.; Caprio, M. Mineralocorticoid Receptor and Aldosterone-Related Biomarkers of End-Organ Damage in Cardiometabolic Disease. Biomolecules 2018, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Scuteri, A.; Benetos, A.; Sierra, C.; Coca, A.; Chicherio, C.; Frisoni, G.B.; Gasecki, D.; Hering, D.; Lovic, D.; Manios, E.; et al. Routine assessment of cognitive function in older patients with hypertension seen by primary care physicians: Why and how-a decision-making support from the working group on ‘hypertension and the brain’ of the European Society of Hypertension and from the European Geriatric Medicine Society. J. Hypertens. 2021, 39, 90–100. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Laurent, S.; Cunha, P.G.; Olsen, M.H.; Rietzschel, E.; Franco, O.H.; Ryliškytė, L.; Strazhesko, I.; Vlachopoulos, C.; Chen, C.H.; et al. Characteristics of healthy vascular ageing in pooled population-based cohort studies: The global Metabolic syndrome and Artery Research Consortium. J. Hypertens. 2018, 36, 2340–2349. [Google Scholar] [CrossRef]
- Lamballais, S.; Sajjad, A.; Leening, M.J.G.; Gaillard, R.; Franco, O.H.; Mattace-Raso, F.U.S.; Jaddoe, V.W.V.; Roza, S.J.; Tiemeier, H.; Ikram, M.A. Association of Blood Pressure and Arterial Stiffness with Cognition in 2 Population-Based Child and Adult Cohorts. J. Am. Heart Assoc. 2018, 7, e009847. [Google Scholar] [CrossRef] [Green Version]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Grillo, C.A.; Woodruff, J.L.; Macht, V.A.; Reagan, L.P. Insulin resistance and hippocampal dysfunction: Disentangling peripheral and brain causes from consequences. Exp. Neurol. 2019, 318, 71–77. [Google Scholar] [CrossRef]
- Ekblad, L.L.; Johansson, J.; Helin, S.; Viitanen, M.; Laine, H.; Puukka, P.; Jula, A.; Rinne, J.O. Midlife insulin resistance, APOE genotype, and late-life brain amyloid accumulation. Neurology 2018, 90, e1150–e1157. [Google Scholar] [CrossRef]
- Laws, S.M.; Gaskin, S.; Woodfield, A.; Srikanth, V.; Bruce, D.; Fraser, P.E.; Porter, T.; Newsholme, P.; Wijesekara, N.; Burnham, S.; et al. Insulin resistance is associated with reductions in specific cognitive domains and increases in CSF tau in cognitively normal adults. Sci. Rep. 2017, 7, 9766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willette, A.A.; Bendlin, B.B.; Starks, E.J.; Birdsill, A.C.; Johnson, S.C.; Christian, B.T.; Okonkwo, O.C.; La Rue, A.; Hermann, B.P.; Koscik, R.L.; et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer’s disease. JAMA Neurol. 2015, 72, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Hoscheidt, S.M.; Starks, E.J.; Oh, J.M.; Zetterberg, H.; Blennow, K.; Krause, R.A.; Gleason, C.E.; Puglielli, L.; Atwood, C.S.; Carlsson, C.M.; et al. Insulin Resistance is Associated with Increased Levels of Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease and Reduced Memory Function in At-Risk Healthy Middle-Aged Adults. J. Alzheimer’s Dis. 2016, 52, 1373–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willette, A.A.; Xu, G.; Johnson, S.C.; Birdsill, A.C.; Jonaitis, E.M.; Sager, M.A.; Hermann, B.P.; La Rue, A.; Asthana, S.; Bendlin, B.B. Insulin Resistance, Brain Atrophy, and Cognitive Performance in Late Middle–Aged Adults. Diabetes Care 2013, 36, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, A.; Pugazhenthi, S. Targeting Insulin Resistance to Treat Cognitive Dysfunction. Mol. Neurobiol. 2021, 58, 2672–2691. [Google Scholar] [CrossRef]
- Spinelli, M.; Fusco, S.; Grassi, C. Brain Insulin Resistance and Hippocampal Plasticity: Mechanisms and Biomarkers of Cognitive Decline. Front. Neurosci. 2019, 13, 788. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Vilaret, K.M.; Cantero, J.L.; Fernandez-Alvarez, M.; Calero, M.; Calero, O.; Lindín, M.; Zurrón, M.; Díaz, F.; Atienza, M. Impaired glucose metabolism reduces the neuroprotective action of adipocytokines in cognitively normal older adults with insulin resistance. Aging 2021, 13, 23936–23952. [Google Scholar] [CrossRef]
- Rönnbäck, C.; Hansson, E. The Importance and Control of Low-Grade Inflammation Due to Damage of Cellular Barrier Systems That May Lead to Systemic Inflammation. Front. Neurol. 2019, 10, 533. [Google Scholar] [CrossRef] [Green Version]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Ekblad, L.L.; Rinne, J.O.; Puukka, P.J.; Laine, H.K.; Ahtiluoto, S.E.; Sulkava, R.O.; Viitanen, M.H.; Jula, A.M. Insulin resistance is associated with poorer verbal fluency performance in women. Diabetologia 2015, 58, 2545–2553. [Google Scholar] [CrossRef]
- Schuur, M.; Henneman, P.; van Swieten, J.C.; Zillikens, M.C.; de Koning, I.; Janssens, A.C.; Witteman, J.C.; Aulchenko, Y.S.; Frants, R.R.; Oostra, B.A.; et al. Insulin-resistance and metabolic syndrome are related to executive function in women in a large family-based study. Eur. J. Epidemiol. 2010, 25, 561–568. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, L.K.; Laughlin, G.A.; Barrett-Connor, E.; Bergstrom, J.; Kritz-Silverstein, D.; Der-Martirosian, C.; von Mühlen, D. Metabolic syndrome and 16-year cognitive decline in community-dwelling older adults. Ann. Epidemiol. 2012, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total | TyG Index | p Value | |||
|---|---|---|---|---|---|---|
| Q1 (<7.87) | Q2 (7.87–8.25) | Q3 (8.25–8.68) | Q4 (≥8.68) | |||
| N, (%) | 1774 | 437 | 450 | 444 | 443 | |
| Age, year, (mean ± SD) | 53.48 ± 8.47 | 51.91 ± 8.58 | 53.59 ± 8.57 | 54.48 ± 8.47 | 53.92 ± 8.09 | <0.001 |
| Sex, n (%) | <0.001 | |||||
| Male | 851(47.97) | 148(33.87) | 198(44.00) | 250(56.31) | 255(57.56) | |
| Female | 923(52.03) | 289(66.13) | 252(56.00) | 194(43.69) | 188(42.44) | |
| Educational level, n (%) | 0.601 | |||||
| Illiterate | 65(3.66) | 12(2.75) | 17(3.78) | 22(4.95) | 14(3.16) | |
| Primary | 95(5.36) | 21(4.81) | 28(6.22) | 22(4.95) | 24(5.42) | |
| Junior or above | 1614(90.98) | 404(92.45) | 405(90.00) | 400(90.09) | 405(91.42) | |
| Body mass index, kg/m2, (mean ± SD) | 25.02 ± 5.39 | 23.55 ± 9.22 | 24.52 ± 3.03 | 25.57 ± 2.99 | 26.43 ± 3.18 | <0.001 |
| Current smoking, n (%) | 394(22.21) | 66(15.10) | 81(18.00) | 114(25.68) | 133(30.02) | <0.001 |
| Current drinking, n (%) | 553(31.78) | 101(23.54) | 121(27.38) | 151(34.55) | 180(41.67) | <0.001 |
| Regular physical activity, n (%) | 883(60.90) | 210(57.69) | 222(61.16) | 239(66.02) | 212(58.73) | 0.489 |
| Medical history, n (%) | ||||||
| Hypertension | 632(35.63) | 87(19.91) | 135(30.00) | 179(40.32) | 231(52.14) | <0.001 |
| Diabetes mellitus | 226(12.74) | 10(2.29) | 25(5.56) | 56(12.61) | 135(30.47) | <0.001 |
| Dyslipidemia | 1012(57.05) | 60(13.73) | 132(29.33) | 382(86.04) | 438(98.87) | <0.001 |
| Laboratory test, (mean ± SD) | ||||||
| TyG index | 8.31 ± 0.62 | 7.60 ± 0.21 | 8.06 ± 0.11 | 8.45 ± 0.12 | 9.14 ± 0.44 | <0.001 |
| FBG, mg/dL | 6.21 ± 1.42 | 5.67 ± 0.49 | 5.90 ± 0.72 | 6.17 ± 0.96 | 7.08 ± 2.29 | <0.001 |
| LDL, mg/dL | 3.42 ± 0.82 | 3.01 ± 0.63 | 3.38 ± 0.79 | 3.61 ± 0.82 | 3.66 ± 0.86 | <0.001 |
| HDL, mg/dL | 1.27 ± 0.27 | 1.43 ± 0.27 | 1.32 ± 0.25 | 1.21 ± 0.24 | 1.12 ± 0.23 | <0.001 |
| TC, mg/dL | 5.15 ± 0.98 | 4.71 ± 0.78 | 5.09 ± 0.91 | 5.29 ± 0.95 | 5.51 ± 1.06 | <0.001 |
| TG, mg/dL | 2.00 ± 1.53 | 0.91 ± 0.17 | 1.36 ± 0.19 | 1.94 ± 0.31 | 3.79 ± 2.09 | <0.001 |
| Overall | Q1 | Q2 | Q3 | Q4 | p Value | |
|---|---|---|---|---|---|---|
| MMSE in 2015 (mean ± SD) | 28.45 ± 1.58 | 28.51 ± 1.49 | 28.43 ± 1.62 | 28.39 ± 1.56 | 28.46 ± 1.64 | 0.742 |
| MMSE in 2019 (mean ± SD) | 27.38 ± 3.06 | 27.80 ± 2.79 | 27.48 ± 2.99 | 27.03 ± 3.30 | 27.24 ± 3.12 | 0.002 |
| MMSE decline from 2015 to 2019 (mean ± SD) | 1.06 ± 3.08 | 0.70 ± 2.83 | 0.95 ± 2.95 | 1.35 ± 3.18 | 1.21 ± 3.30 | 0.009 |
| Cognitive decline incidence N (%) | 820 (46.22) | 180 (41.19) | 198 (44.00) | 218 (49.10) | 224 (50.56) | 0.017 |
| Outcome | TyG Levels | p for Trend | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Cognitive decline from 2015 to 2019 | |||||
| Events, N(%) | 180 (41.19) | 198 (44.00) | 218 (49.10) | 224 (50.56) | |
| Unadjusted | 1 | 1.12 (0.86–1.46) | 1.38 (1.06–1.80) | 1.46 (1.12–1.91) | 0.002 |
| Model 1 | 1 | 1.07 (0.82–1.40) | 1.30 (0.99–1.70) | 1.40 (1.07–1.84) | 0.006 |
| Model 2 | 1 | 1.17 (0.85–1.62) | 1.31 (0.93–1.83) | 1.51 (1.06–2.14) | 0.020 |
| Outcome | Model | C-Statistic | NRI | IDI | |||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | p Value | Estimate (95% CI) | p Value | Estimate (95% CI) | p Value | ||
| Cognitive decline from 2015 to 2019 | Conventional model | 0.64 (0.61–0.67) | 0.414 | Ref. | 0.004 | Ref. | 0.030 |
| Conventional model +TyG | 0.65 (0.62–0.68) | 0.16 (0.05–0.27) | 0.004 (0.003–0.01) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Deng, X.; Zhang, Y. The Triglyceride-Glucose Index Is Associated with Longitudinal Cognitive Decline in a Middle-Aged to Elderly Population: A Cohort Study. J. Clin. Med. 2022, 11, 7153. https://doi.org/10.3390/jcm11237153
Li S, Deng X, Zhang Y. The Triglyceride-Glucose Index Is Associated with Longitudinal Cognitive Decline in a Middle-Aged to Elderly Population: A Cohort Study. Journal of Clinical Medicine. 2022; 11(23):7153. https://doi.org/10.3390/jcm11237153
Chicago/Turabian StyleLi, Siqi, Xuan Deng, and Yumei Zhang. 2022. "The Triglyceride-Glucose Index Is Associated with Longitudinal Cognitive Decline in a Middle-Aged to Elderly Population: A Cohort Study" Journal of Clinical Medicine 11, no. 23: 7153. https://doi.org/10.3390/jcm11237153





