Simultaneous Quantification of Anisotropic Microcirculation and Microstructure in Peripheral Nerve
Abstract
:1. Introduction
2. Materials and Methods
2.1. MRI
2.2. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noble, J.; Munro, C.A.; Prasad, V.S.; Midha, R. Analysis of Upper and Lower Extremity Peripheral Nerve Injuries in a Population of Patients with Multiple Injuries. J. Trauma 1998, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Lad, S.P.; Nathan, J.K.; Schubert, R.D.; Boakye, M. Trends in Median, Ulnar, Radial, and Brachioplexus Nerve Injuries in the United States. Neurosurgery 2010, 66, 953–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundborg, G. A 25-Year Perspective of Peripheral Nerve Surgery: Evolving Neuroscientific Concepts and Clinical Significance. J. Hand Surg. Am. 2000, 25, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Tetzlaff, W. Regeneration-Associated Genes Decline in Chronically Injured Rat Sciatic Motoneurons. Eur. J. Neurosci. 2015, 42, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.G.; Spinner, R.J.; Kline, D.G.; Kliot, M. Advances in the Neurological and Neurosurgical Management of Peripheral Nerve Trauma. J. Neurol. Neurosurg. Psychiatry 2016, 87, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Lundborg, G. Structure and Function of the Intraneural Microvessels as Related to Trauma, Edema Formation, and Nerve Function. J. Bone Jt. Surg. Am. 1975, 57, 938–948. [Google Scholar] [CrossRef]
- Lundborg, G. The Intrinsic Vascularization of Human Peripheral Nerves: Structural and Functional Aspects. J. Hand Surg. Am. 1979, 4, 34–41. [Google Scholar] [CrossRef]
- Boyer, R.B.; Kelm, N.D.; Riley, D.C.; Sexton, K.W.; Pollins, A.C.; Shack, R.B.; Dortch, R.D.; Nanney, L.B.; Does, M.D.; Thayer, W.P. 4.7-T Diffusion Tensor Imaging of Acute Traumatic Peripheral Nerve Injury. Neurosurg. Focus 2015, 39, E9. [Google Scholar] [CrossRef] [Green Version]
- Simon, N.G.; Kliot, M. Diffusion Weighted MRI and Tractography for Evaluating Peripheral Nerve Degeneration and Regeneration. Neural. Regen. Res. 2014, 9, 2122–2124. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Hong, G.; Sun, C.; Wu, X.; Peng, M.J.; Zeng, G. In Vivo DTI Longitudinal Measurements of Acute Sciatic Nerve Traction Injury and the Association with Pathological and Functional Changes. Eur. J. Radiol. 2013, 82, e707–e714. [Google Scholar] [CrossRef]
- Morisaki, S.; Kawai, Y.; Umeda, M.; Nishi, M.; Oda, R.; Fujiwara, H.; Yamada, K.; Higuchi, T.; Tanaka, C.; Kawata, M.; et al. In Vivo Assessment of Peripheral Nerve Regeneration by Diffusion Tensor Imaging. J. Magn. Reson. Imaging 2011, 33, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Fujiwara, H.; Oda, R.; Mikami, Y.; Ikeda, T.; Nagae, M.; Shirai, T.; Morisaki, S.; Ikoma, K.; Masugi-Tokita, M.; et al. In Vivo Evaluation of Rabbit Sciatic Nerve Regeneration with Diffusion Tensor Imaging (DTI): Correlations with Histology and Behavior. Magn. Reson. Imaging 2015, 33, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bonekamp, D.; Degaonkar, M.; Barker, P.B. Quantitative Cerebral Blood Flow in Dynamic Susceptibility Contrast MRI Using Total Cerebral Flow from Phase Contrast Magnetic Resonance Angiography. Magn. Reson. Med. 2011, 66, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Yu, S.; Alger, J.R.; Zuo, Z.; Chen, J.; Wang, R.; An, J.; Wang, B.; Zhao, J.; Xue, R.; et al. Multi-Delay Arterial Spin Labeling Perfusion MRI in Moyamoya Disease—Comparison with CT Perfusion Imaging. Eur. Radiol. 2014, 24, 1135–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, E.; Sugrue, L.; Wintermark, M. Understanding the Neurophysiology and Quantification of Brain Perfusion. Top. Magn. Reson. Imaging 2017, 26, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kroll, H.; Zaharchuk, G.; Christen, T.; Heit, J.J.; Iv, M. Resting-State BOLD MRI for Perfusion and Ischemia. Top. Magn. Reson. Imaging 2017, 26, 91–96. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR Imaging of Intravoxel Incoherent Motions: Application to Diffusion and Perfusion in Neurologic Disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, O.M.; Gomez, A.D.; Merchant, S.; Heidinger, M.; Poelzing, S.; Hsu, E.W. Orientation Dependence of Microcirculation-Induced Diffusion Signal in Anisotropic Tissues. Magn. Reson. Med. 2016, 76, 1252–1262. [Google Scholar] [CrossRef] [Green Version]
- Koay, C.G.; Chang, L.-C.; Carew, J.D.; Pierpaoli, C.; Basser, P.J. A Unifying Theoretical and Algorithmic Framework for Least Squares Methods of Estimation in Diffusion Tensor Imaging. J. Magn. Reson. 2006, 182, 115–125. [Google Scholar] [CrossRef]
- Barmpoutis, A.; Vemuri, B.C. Unified Framework for Estimating Diffusion Tensors of Any Order with Symmetric Positive-Definite Constraints. In Proceedings of the 2010 7th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, ISBI 2010—Proceedings, Rotterdam, The Netherlands, 14–17 April 2010; pp. 1385–1388. [Google Scholar]
- Mozumder, M.; Beltrachini, L.; Collier, Q.; Pozo, J.M.; Frangi, A.F. Simultaneous Magnetic Resonance Diffusion and Pseudo-Diffusion Tensor Imaging. Magn. Reson. Med. 2018, 79, 2367–2378. [Google Scholar] [CrossRef] [Green Version]
- Federau, C.; Meuli, R.; O’Brien, K.; Maeder, P.; Hagmann, P. Perfusion Measurement in Brain Gliomas with Intravoxel Incoherent Motion MRI. Am. J. Neuroradiol. 2014, 35, 256–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevallier, O.; Zhou, N.; Cercueil, J.-P.; He, J.; Loffroy, R.; Wáng, Y.X.J. Comparison of Tri-Exponential Decay vs. Bi-Exponential Decay and Full Fitting vs. Segmented Fitting for Modeling Liver Intravoxel Incoherent Motion Diffusion MRI. bioRxiv 2018, 32, 429977. [Google Scholar] [CrossRef] [Green Version]
- Bisdas, S.; Koh, T.S.; Roder, C.; Braun, C.; Schittenhelm, J.; Ernemann, U.; Klose, U. Intravoxel Incoherent Motion Diffusion-Weighted MR Imaging of Gliomas: Feasibility of the Method and Initial Results. Neuroradiology 2013, 55, 1189–1196. [Google Scholar] [CrossRef]
- Chow, A.M.; Gao, D.S.; Fan, S.J.; Qiao, Z.; Lee, F.Y.; Yang, J.; Man, K.; Wu, E.X. Liver Fibrosis: An Intravoxel Incoherent Motion (IVIM) Study. J. Magn. Reson. Imaging 2012, 36, 159–167. [Google Scholar] [CrossRef]
- Luciani, A.; Vignaud, A.; Cavet, M.; Van Nhieu, J.; Mallat, A.; Ruel, L.; Laurent, A.; Deux, J.; Brugieres, P.; Rahmouni, A. Liver Cirrhosis: Intravoxel Incoherent Motion MR Imaging—Pilot Study. Radiology 2008, 249, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Callot, V.; Bennett, E.; Decking, U.K.M.; Balaban, R.S.; Wen, H. In Vivo Study of Microcirculation in Canine Myocardium Using the IVIM Method. Magn. Reson. Med. 2003, 50, 531–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Baalen, S.; Leemans, A.; Dik, P.; Lilien, M.R.; Ten Haken, B.; Froeling, M. Intravoxel Incoherent Motion Modeling in the Kidneys: Comparison of Mono-, Bi-, and Triexponential Fit. J. Magn. Reson. Imaging 2017, 46, 228–239. [Google Scholar] [CrossRef]
- Notohamiprodjo, M.; Chandarana, H.; Mikheev, A.; Rusinek, H.; Grinstead, J.; Feiweier, T.; Raya, J.G.; Lee, V.S.; Sigmund, E.E. Combined Intravoxel Incoherent Motion and Diffusion Tensor Imaging of Renal Diffusion and Flow Anisotropy. Magn. Reson. Med. 2015, 73, 1526–1532. [Google Scholar] [CrossRef]
- Cattin, A.-L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.A.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [Green Version]
- Caillaud, M.; Richard, L.; Vallat, J.-M.; Desmoulière, A.; Billet, F. Peripheral Nerve Regeneration and Intraneural Revascularization. Neural Regen. Res. 2019, 14, 24–33. [Google Scholar] [CrossRef]
- Campbell, W.W. Evaluation and Management of Peripheral Nerve Injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pridmore, M.D.; Glassman, G.E.; Pollins, A.C.; Manzanera Esteve, I.V.; Drolet, B.C.; Weikert, D.R.; Does, M.D.; Perdikis, G.; Thayer, W.P.; Dortch, R.D. Initial findings in traumatic peripheral nerve injury and repair with diffusion tensor imaging. Ann. Clin. Transl. Neurol. 2021, 8, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Esteve, I.V.M.; Farinas, A.F.; Pollins, A.C.; Nussenbaum, M.E.; Cardwell, N.L.; Kahn, H.; Does, M.D.; Dortch, R.D.; Thayer, W.P. Noninvasive diffusion MRI to determine the severity of peripheral nerve injury. Magn. Reson. Imaging 2021, 83, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, S.; Warner, W.S.; Merchant, S.S.; Hsu, E.W.; Agoston, D.V.; Mahan, M.A. Incorporating Blood Flow in Nerve Injury and Regeneration Assessment. Front. Surg. 2022, 9, 862478. [Google Scholar] [CrossRef]
- Dewey, M.; Siebes, M.; Kachelrieß, M.; Kofoed, K.F.; Maurovich-Horvat, P.; Nikolaou, K.; Bai, W.; Kofler, A.; Manka, R.; Kozerke, S.; et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia. Nat. Rev. Cardiol. 2020, 17, 427–450. [Google Scholar] [CrossRef] [Green Version]
- Kholmovski, E.G.; Morris, A.K.; Chelu, M.G. Cardiac MRI and Fibrosis Quantification. Card. Electrophysiol. Clin. 2019, 11, 537–549. [Google Scholar] [CrossRef]
- Aramendía-Vidaurreta, V.; Echeverría-Chasco, R.; Vidorreta, M.; Bastarrika, G.; Fernández-Seara, M.A. Quantification of Myocardial Perfusion with Vasodilation Using Arterial Spin Labeling at 1.5T. J. Magn. Reson. Imaging JMRI 2021, 53, 777–788. [Google Scholar] [CrossRef]
- Kärger, J. NMR Self-Diffusion Studies in Heterogeneous Systems. Adv. Colloid Interface Sci. 1985, 23, 129–148. [Google Scholar] [CrossRef]
- Niendorf, T.; Dijkhuizen, R.M.; Norris, D.G.; van Lookeren Campagne, M.; Nicolay, K. Biexponential Diffusion Attenuation in Various States of Brain Tissue: Implications for Diffusion-Weighted Imaging. Magn. Reson. Med. 1996, 36, 847–857. [Google Scholar] [CrossRef]
- Farrell, J.A.D.; Landman, B.A.; Jones, C.K.; Smith, S.A.; Prince, J.L.; van Zijl, P.C.M.; Mori, S. Effects of Signal-to-Noise Ratio on the Accuracy and Reproducibility of Diffusion Tensor Imaging-Derived Fractional Anisotropy, Mean Diffusivity, and Principal Eigenvector Measurements at 1.5 T. J. Magn. Reson. Imaging 2007, 26, 756–767. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.; Wang, Z.J.; Morriss, M.C.; Rollins, N.K. Minimum SNR and Acquisition for Bias-Free Estimation of Fractional Anisotropy in Diffusion Tensor Imaging—A Comparison of Two Analytical Techniques and Field Strengths. Magn. Reson. Imaging 2012, 30, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
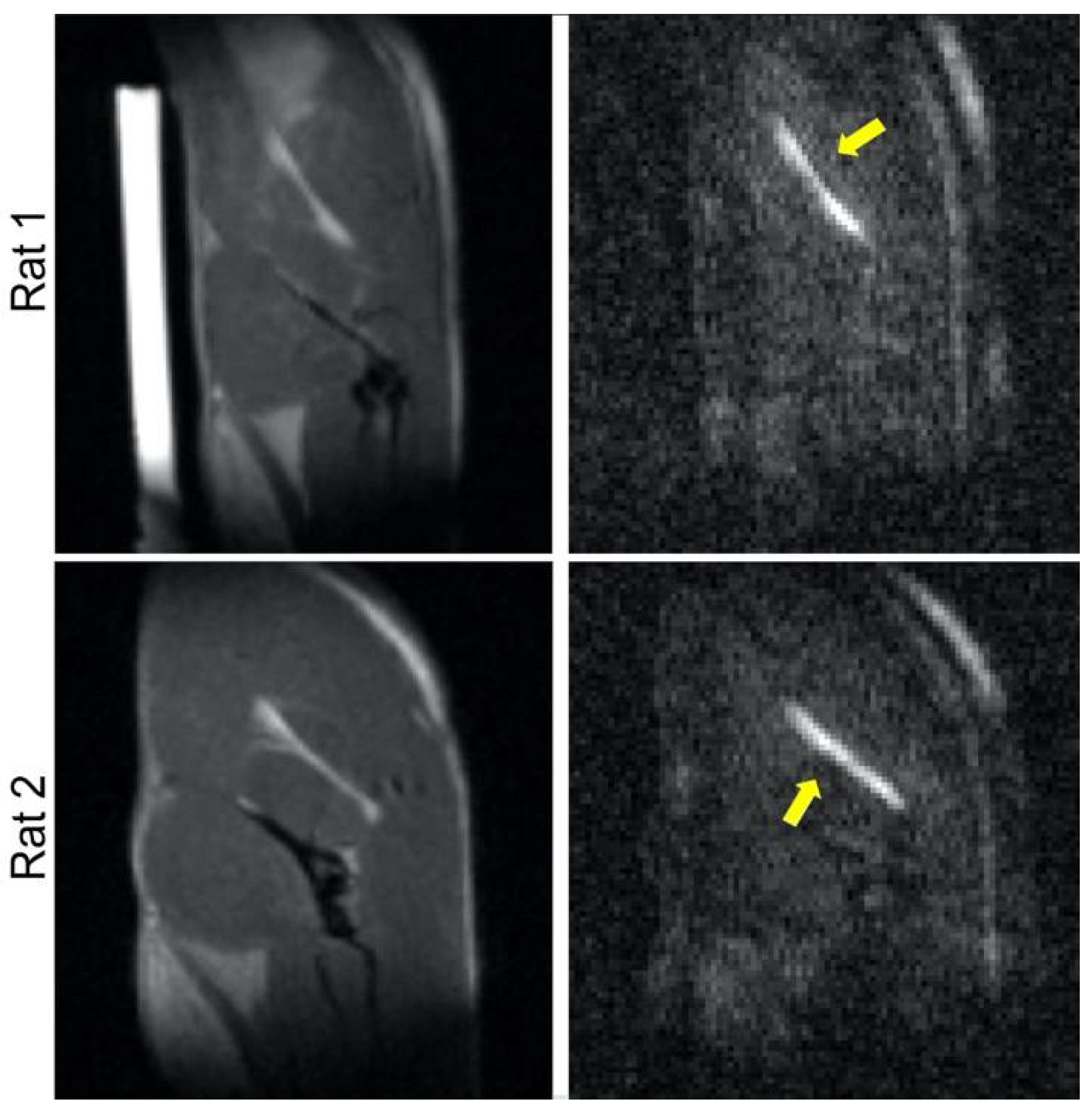
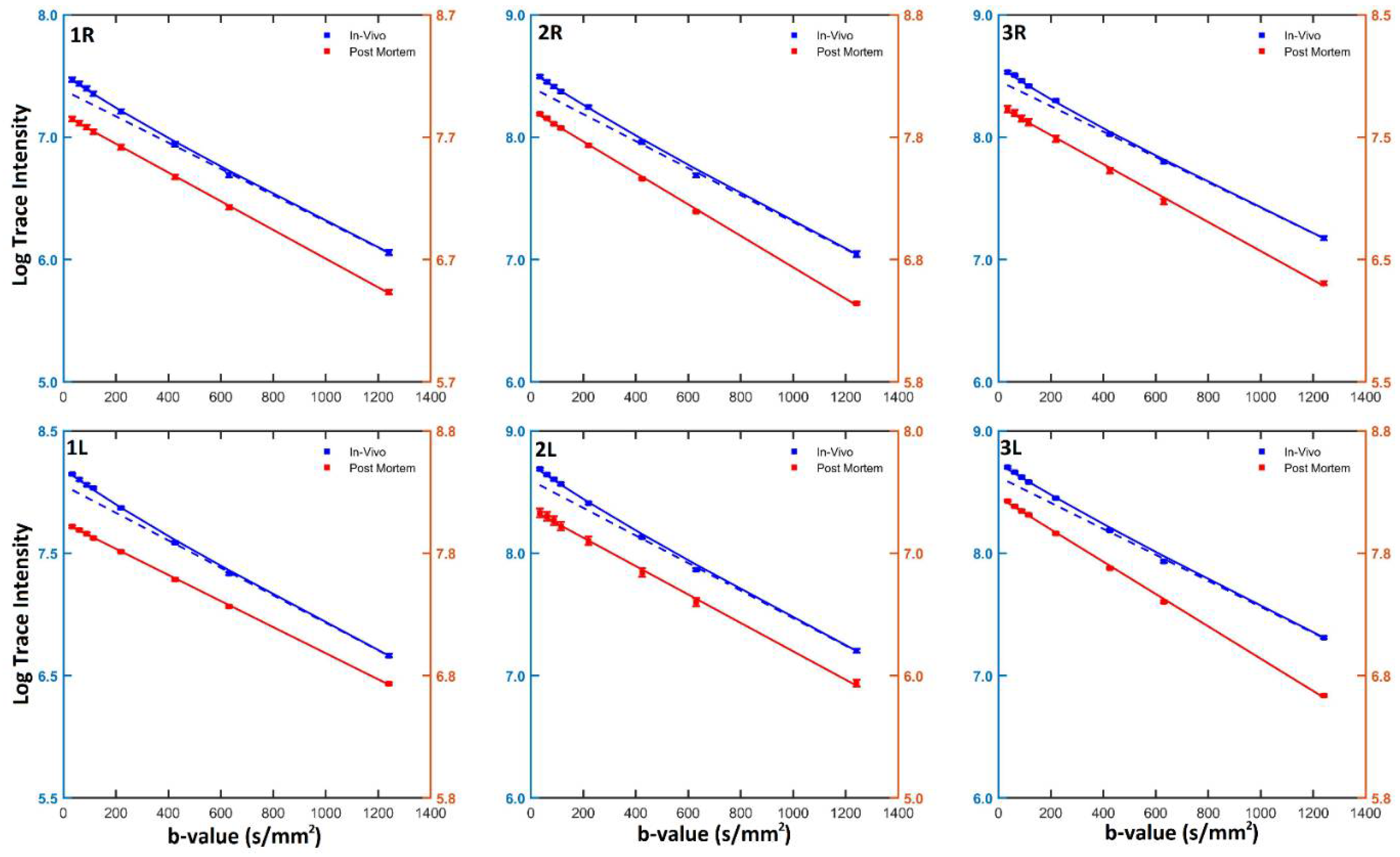
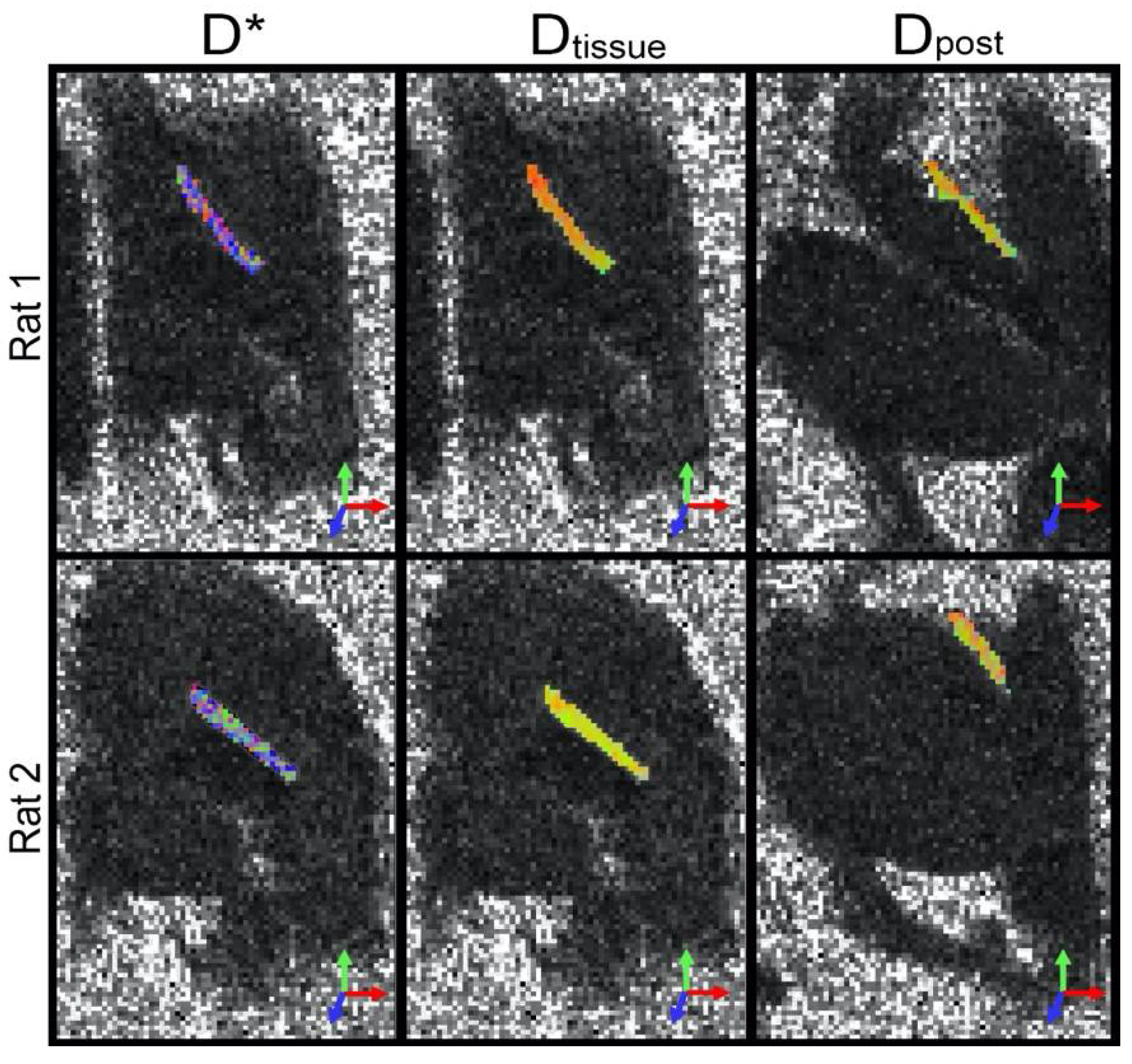
| Rat | Limb | Fast Component | Slow Component | Vascular Fraction | Postmortem | |||
|---|---|---|---|---|---|---|---|---|
| MD* (mm2/s) | FA* | MDtissue (mm2/s) | FAtissue | f | MDpost (mm2/s) | FApost | ||
| 1 | Right | 2.38 | 0.50 | 1.08 | 0.55 | 0.15 | 0.76 | 0.41 |
| Left | 2.35 | 0.49 | 1.02 | 0.48 | 0.16 | 0.84 | 0.31 | |
| 2 | Right | 2.36 | 0.51 | 1.07 | 0.60 | 0.16 | 1.04 | 0.42 |
| Left | 2.24 | 0.51 | 1.01 | 0.50 | 0.18 | 1.01 | 0.37 | |
| 3 | Right | 2.46 | 0.48 | 1.02 | 0.56 | 0.15 | 1.05 | 0.46 |
| Left | 2.13 | 0.51 | 1.06 | 0.49 | 0.12 | 1.04 | 0.35 | |
| Mean | 2.32 | 0.50 | 1.04 | 0.53 | 0.15 | 0.96 | 0.38 | |
| ±sem | 0.04 | 0.01 | 0.01 | 0.02 | 0.01 | 0.05 | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merchant, S.; Yeoh, S.; Mahan, M.A.; Hsu, E.W. Simultaneous Quantification of Anisotropic Microcirculation and Microstructure in Peripheral Nerve. J. Clin. Med. 2022, 11, 3036. https://doi.org/10.3390/jcm11113036
Merchant S, Yeoh S, Mahan MA, Hsu EW. Simultaneous Quantification of Anisotropic Microcirculation and Microstructure in Peripheral Nerve. Journal of Clinical Medicine. 2022; 11(11):3036. https://doi.org/10.3390/jcm11113036
Chicago/Turabian StyleMerchant, Samer, Stewart Yeoh, Mark A. Mahan, and Edward W. Hsu. 2022. "Simultaneous Quantification of Anisotropic Microcirculation and Microstructure in Peripheral Nerve" Journal of Clinical Medicine 11, no. 11: 3036. https://doi.org/10.3390/jcm11113036
APA StyleMerchant, S., Yeoh, S., Mahan, M. A., & Hsu, E. W. (2022). Simultaneous Quantification of Anisotropic Microcirculation and Microstructure in Peripheral Nerve. Journal of Clinical Medicine, 11(11), 3036. https://doi.org/10.3390/jcm11113036






