Promising Tools to Facilitate the Implementation of TDM of Biologics in Clinical Practice
Abstract
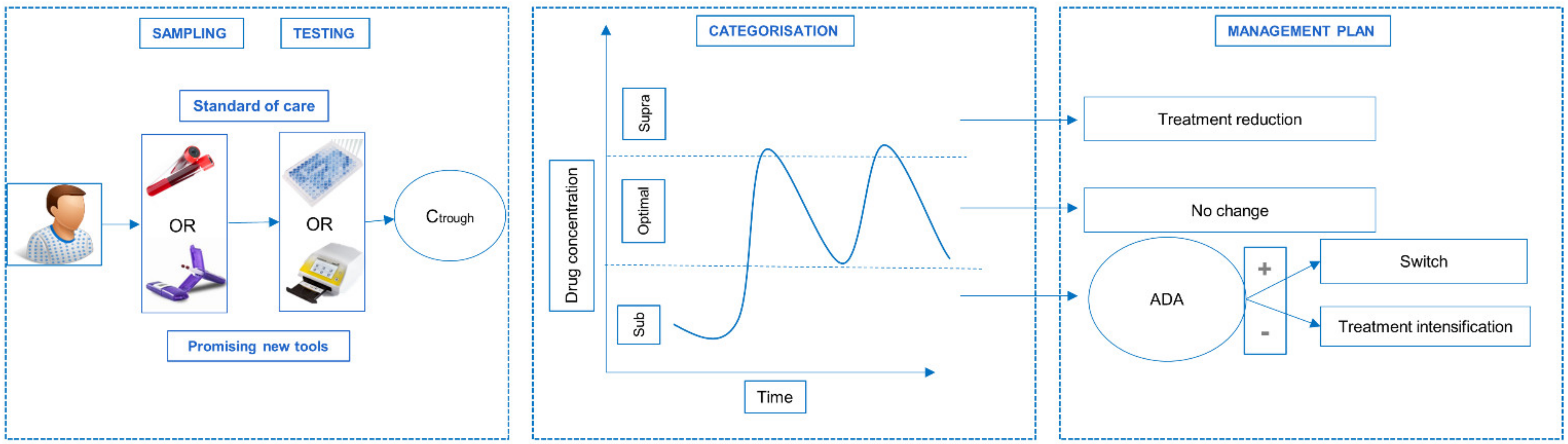
:1. Introduction
2. Materials and Methods
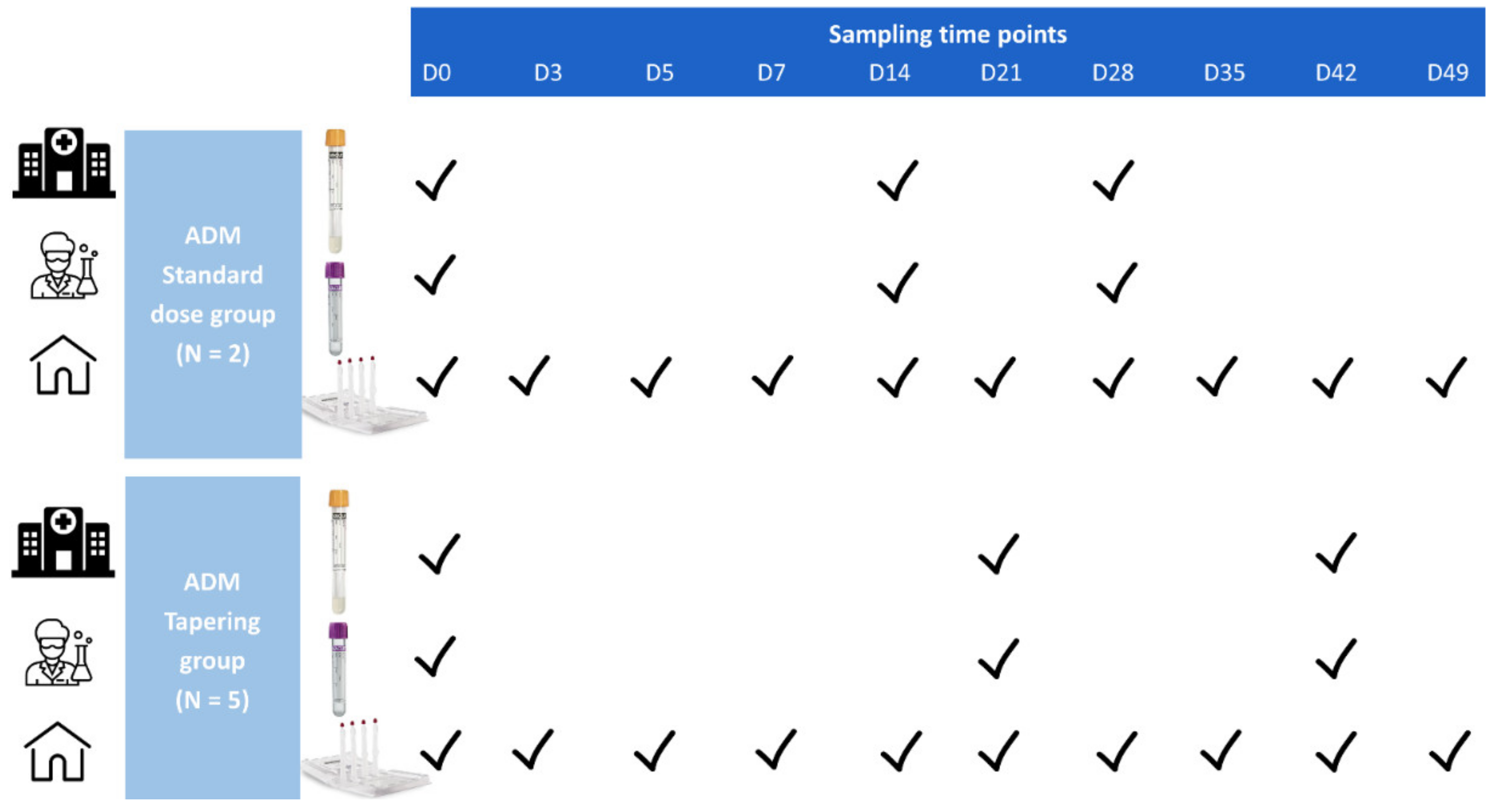
2.1. Study Design and Data Collection
2.2. Sample Collection, Transportation, Preparation and Storage
2.3. Extraction Efficiency of Adalimumab in Volumetric Absorptive Microsamples
2.4. Quantification of Adalimumab Concentrations
2.4.1. Rapid Testing with Lateral Flow Technique
2.4.2. Traditional Detection with ELISA
2.5. Statistical Analysis
3. Results
3.1. Demographics of Study Cohort
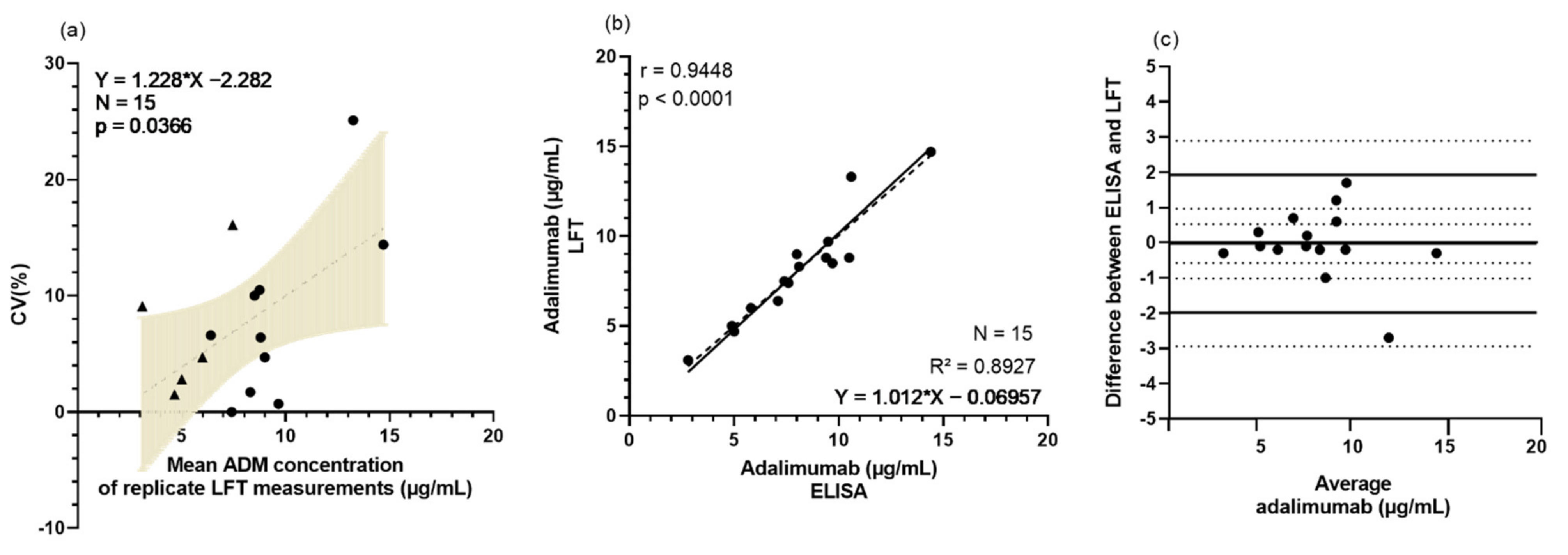
3.2. Rapid Testing of Adalimumab Is Feasible and Valuable in Clinical Setting
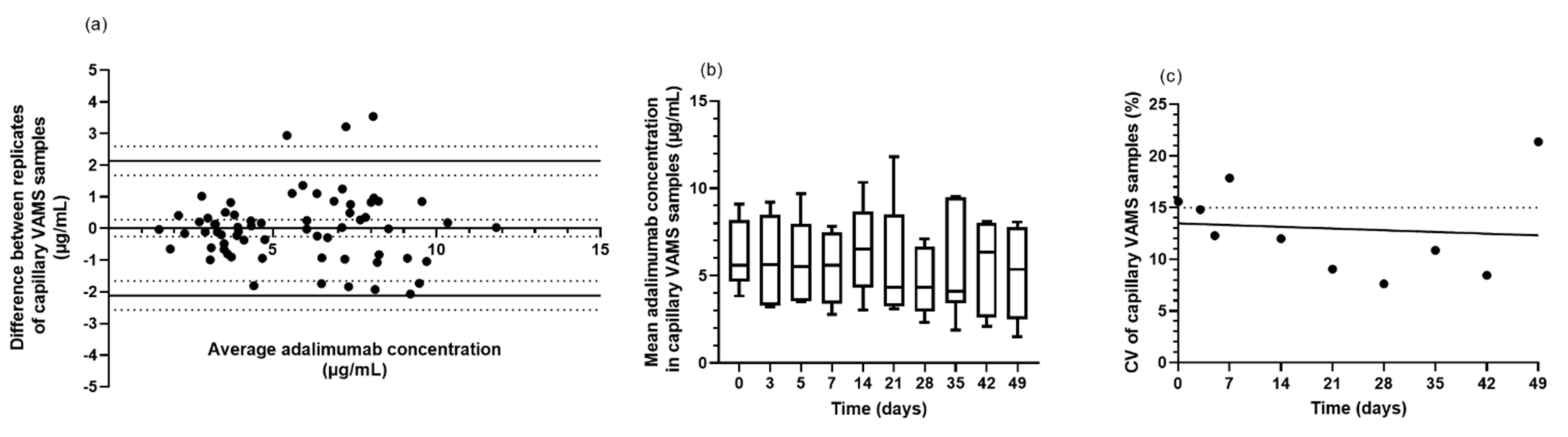
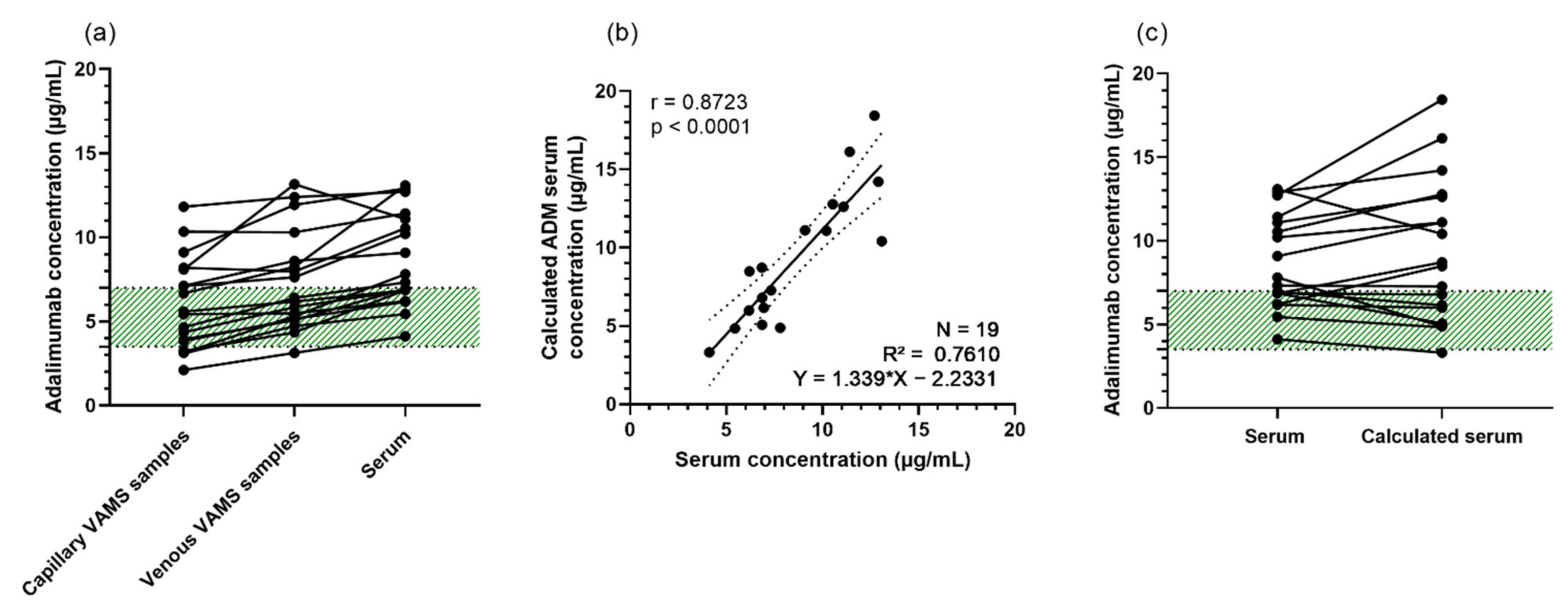
3.3. VAMS Is Suitable for Extraction of Adalimumab
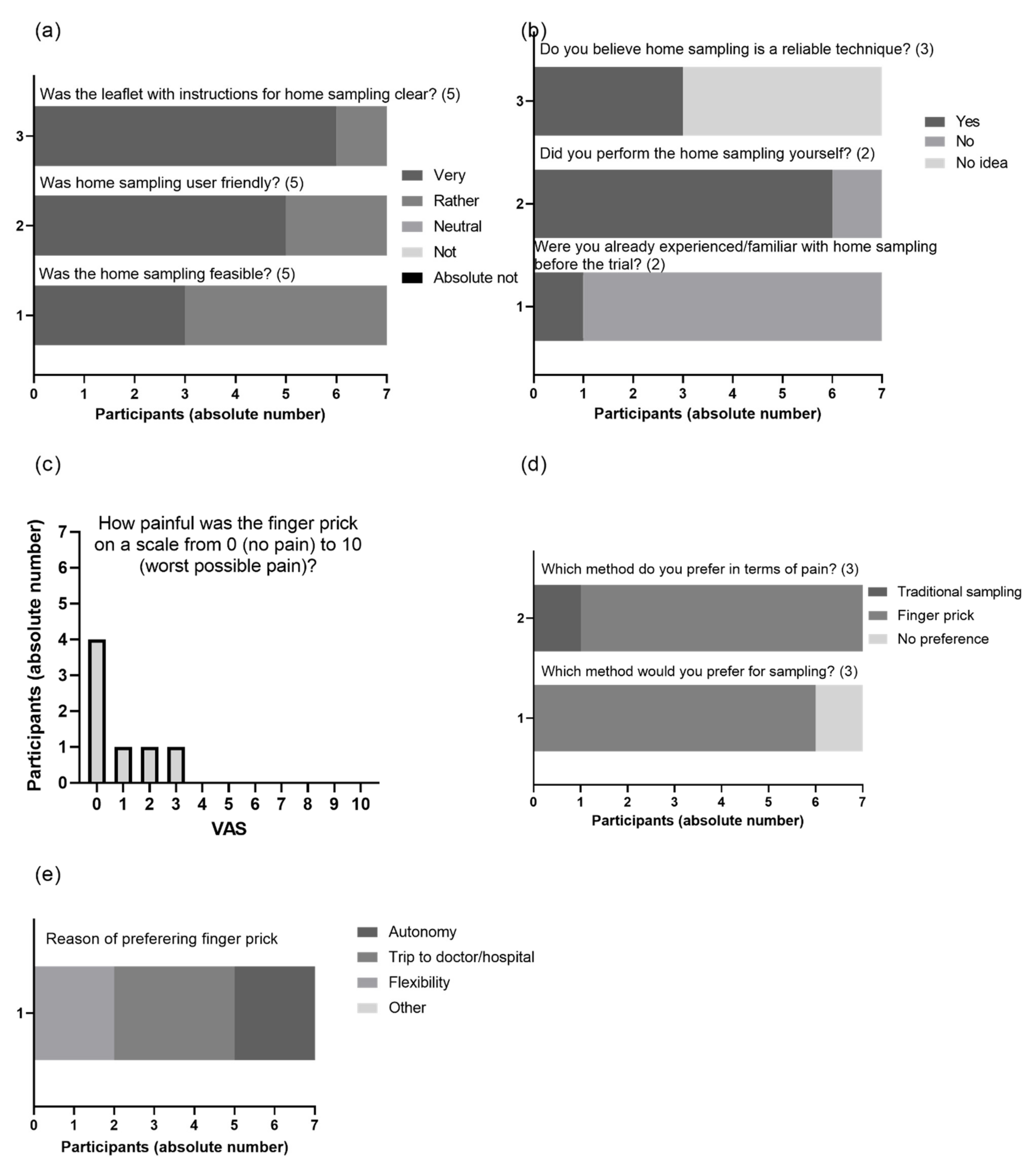
3.4. Feasibility of Home Sampling by Non-Experienced Patients Using VAMS
3.5. Patient Experience with VAMS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brownstone, N.D.; Hong, J.; Mosca, M.; Hadeler, E.; Liao, W.; Bhutani, T.; Koo, J. Biologic treatments of psoriasis: An update for the clinician. Biol. Targets Ther. 2021, 15, 39–51. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Schmitt, J.; Rosumeck, S.; Thomaschewski, G.; Sporbeck, B.; Haufe, E.; Nast, A. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: Meta-analysis of randomized controlled trials. Br. J. Dermatol. 2014, 170, 274–303. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Rampakakis, E.; Movahedi, M.; Cesta, A.; Stutz, M.; Sampalis, J.S.; Nantel, F.; Maslova, K.; Bombardier, C. Toward Defining Primary and Secondary Nonresponse in Rheumatoid Arthritis Patients Treated with Anti-TNF: Results from the BioTRAC and OBRI Registries. J. Rheumatol. 2020, 47, 510–527. [Google Scholar] [CrossRef]
- Bracke, S.; Lambert, J. Viewpoint on handling anti-TNF failure in psoriasis. Arch. Dermatol. Res. 2013, 305, 945–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Brassinne, M.; Ghislain, P.-D.; Lambert, J.L.W.; Lambert, J.; Segaert, S.; Willaert, F. Recommendations for managing a suboptimal response to biologics for moderate-to-severe psoriasis: A Belgian perspective. J. Dermatolog. Treat. 2016, 27, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Edson-Heredia, E.; Sterling, K.L.; Alatorre, C.I.; Cuyun Carter, G.; Paczkowski, R.; Zarotsky, V.; Maeda-Chubachi, T. Heterogeneity of Response to Biologic Treatment: Perspective for Psoriasis. J. Investig. Dermatol. 2014, 134, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menting, S.P.; Coussens, E.; Pouw, M.F.; van den Reek, J.M.P.A.P.A.; Temmerman, L.; Boonen, H.; De Jong, E.M.G.J.G.J.; Spuls, P.I.; Lambert, J. Developing a Therapeutic Range of Adalimumab Serum Concentrations in Management of Psoriasis. JAMA Dermatology 2015, 151, 616. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, N.; Tsakok, T.; Dand, N.; Bloem, K.; Duckworth, M.; Baudry, D.; Pushpa-Rajah, A.; Griffiths, C.E.E.M.; Reynolds, N.J.; Barker, J.; et al. Defining the Therapeutic Range for Adalimumab and Predicting Response in Psoriasis: A Multicenter Prospective Observational Cohort Study. J. Invest. Dermatol. 2019, 139, 115–123. [Google Scholar] [CrossRef]
- Papamichael, K.; Cheifetz, A.S. Therapeutic Drug Monitoring in IBD: The New Standard-of-Care for Anti-TNF Therapy. Am. J. Gastroenterol. 2017, 112, 673–676. [Google Scholar] [CrossRef]
- Juncadella, A.; Papamichael, K.; Vaughn, B.P.; Cheifetz, A.S. Maintenance Adalimumab Concentrations Are Associated with Biochemical, Endoscopic, and Histologic Remission in Inflammatory Bowel Disease. Dig. Dis. Sci. 2018, 63, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Vande Casteele, N.; Feagan, B.G.; Wolf, D.C.; Pop, A.; Yassine, M.; Horst, S.N.; Ritter, T.E.; Sandborn, W.J. Therapeutic Drug Monitoring of Tumor Necrosis Factor Antagonists in Crohn Disease: A Theoretical Construct to Apply Pharmacokinetics and Guidelines to Clinical Practice. Inflamm. Bowel Dis. 2021, 27, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Grine, L.; Soenen, R. Dose Tapering Study of Adalimumab in Psoriasis—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT04028713 (accessed on 24 January 2022).
- Harahap, Y.; Diptasaadya, R.; Purwanto, D.J. Volumetric absorptive microsampling as a sampling alternative in clinical trials and therapeutic drug monitoring during the covid-19 pandemic: A review. Drug Des. Dev. Ther. 2020, 14, 5757–5771. [Google Scholar] [CrossRef] [PubMed]
- Kneepkens, E.L.; Pouw, M.F.; Wolbink, G.J.; Schaap, T.; Nurmohamed, M.T.; de Vries, A.; Rispens, T.; Bloem, K. Dried blood spots from finger prick facilitate therapeutic drug monitoring of adalimumab and anti-adalimumab in patients with inflammatory diseases. Br. J. Clin. Pharmacol. 2017, 83, 2474–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mingas, P.D.; Zdovc, J.; Grabnar, I.; Vovk, T. The Evolving Role of Microsampling in Therapeutic Drug Monitoring of Monoclonal Antibodies in Inflammatory Diseases. Molecules 2021, 26, 1787. [Google Scholar] [CrossRef]
- Bloem, K.; Schaap, T.; Boshuizen, R.; Kneepkens, E.L.; Wolbink, G.J.; de Vries, A.; Rispens, T. Capillary blood microsampling to determine serum biopharmaceutical concentration: Mitra ® microsampler vs dried blood spot. Bioanalysis 2018, 10, 815–823. [Google Scholar] [CrossRef]
- Delahaye, L.; Veenhof, H.; Koch, B.C.P.; Alffenaar, J.-W.C.; Linden, R.; Stove, C. Alternative Sampling Devices to Collect Dried Blood Microsamples. Ther. Drug Monit. 2021, 43, 310–321. [Google Scholar] [CrossRef]
- Martial, L.C.; Aarnoutse, R.E.; Schreuder, M.F.; Henriet, S.S.; Brüggemann, R.J.M.; Joore, M.A. Cost Evaluation of Dried Blood Spot Home Sampling as Compared to Conventional Sampling for Therapeutic Drug Monitoring in Children. PLoS ONE 2016, 11, e0167433. [Google Scholar] [CrossRef]
- De Kesel, P.M.M.; Sadones, N.; Capiau, S.; Lambert, W.E.; Stove, C.P. Hemato-critical issues in quantitative analysis of dried blood spots: Challenges and solutions. Bioanalysis 2013, 5, 2023–2041. [Google Scholar] [CrossRef]
- Velghe, S.; Delahaye, L.; Stove, C.P. Is the hematocrit still an issue in quantitative dried blood spot analysis? J. Pharm. Biomed. Anal. 2019, 163, 188–196. [Google Scholar] [CrossRef]
- Spooner, N.; Denniff, P.; Michielsen, L.; De Vries, R.; Ji, Q.C.; Arnold, M.E.; Woods, K.; Woolf, E.J.; Xu, Y.; Boutet, V.; et al. A device for dried blood microsampling in quantitative bioanalysis: Overcoming the issues associated blood hematocrit. Bioanalysis 2015, 7, 653–659. [Google Scholar] [CrossRef]
- Verougstraete, N.; Lapauw, B.; Van Aken, S.; Delanghe, J.; Stove, C.; Stove, V. Volumetric absorptive microsampling at home as an alternative tool for the monitoring of HbA1c in diabetes patients. Clin. Chem. Lab. Med. 2017, 55, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, I.; Gruson, D.; Kabamba, B.; Dahma, H.; Van den Wijngaert, S.; Reza, S.; Carbone, V.; Vandenberg, O.; Gulbis, B.; Wolff, F.; et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020, 128, 104413. [Google Scholar] [CrossRef]
- Verstockt, B.; Moors, G.; Bian, S.; Van Stappen, T.; Van Assche, G.; Vermeire, S.; Gils, A.; Ferrante, M. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: The usefulness of rapid testing. Aliment. Pharmacol. Ther. 2018, 48, 731–739. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Uytfanghe, K.; Heughebaert, L.; Stove, C.P. Self-sampling at home using volumetric absorptive microsampling: Coupling analytical evaluation to volunteers’ perception in the context of a large scale study. Clin. Chem. Lab. Med. 2021, 59, e185–e187. [Google Scholar] [CrossRef]
- Castillo, C. GN3043_RIDA QUICK_ADM Monitoring_2018-06-28_EN. Available online: https://clinical.r-biopharm.com/wp-content/uploads/2018/08/gn3043_rida-quick_adm-monitoring_2018-06-28_en.pdf (accessed on 24 January 2022).
- Bian, S.; Van Stappen, T.; Baert, F.; Compernolle, G.; Brouwers, E.; Tops, S.; de Vries, A.; Rispens, T.; Lammertyn, J.; Vermeire, S.; et al. Generation and characterization of a unique panel of anti-adalimumab specific antibodies and their application in therapeutic drug monitoring assays. J. Pharm. Biomed. Anal. 2016, 125, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Mbughuni, M.M.; Stevens, M.A.; Langman, L.J.; Kudva, Y.C.; Sanchez, W.; Dean, P.G.; Jannetto, P.J. Volumetric Microsampling of Capillary Blood Spot vs Whole Blood Sampling for Therapeutic Drug Monitoring of Tacrolimus and Cyclosporin A: Accuracy and Patient Satisfaction. J. Appl. Lab. Med. 2020, 5, 516–530. [Google Scholar] [CrossRef]
- Morgan, P.E. Microsampling Devices for Routine Therapeutic Drug Monitoring-Are We There Yet? Ther. Drug Monit. 2021, 43, 322–334. [Google Scholar] [CrossRef]
- Li, H.; Myzithras, M.; Bolella, E.; Leonard, A.; Ahlberg, J. Whole blood stability evaluation of monoclonal antibody therapeutics using volumetric absorptive microsampling. Bioanalysis 2021, 13, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.; Afonso, J.; Lago, P.; Arroja, B.; Vieira, A.I.; Dias, C.C.; Magro, F. Accuracy of the new rapid test for monitoring adalimumab levels. Therap. Adv. Gastroenterol. 2019, 12, 1756284819828238. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soenen, R.; Stove, C.; Capobianco, A.; De Schutter, H.; Dobbelaere, M.; Mahjor, T.; Follens, M.; Lambert, J.; Grine, L. Promising Tools to Facilitate the Implementation of TDM of Biologics in Clinical Practice. J. Clin. Med. 2022, 11, 3011. https://doi.org/10.3390/jcm11113011
Soenen R, Stove C, Capobianco A, De Schutter H, Dobbelaere M, Mahjor T, Follens M, Lambert J, Grine L. Promising Tools to Facilitate the Implementation of TDM of Biologics in Clinical Practice. Journal of Clinical Medicine. 2022; 11(11):3011. https://doi.org/10.3390/jcm11113011
Chicago/Turabian StyleSoenen, Rani, Christophe Stove, Alessio Capobianco, Hanne De Schutter, Marie Dobbelaere, Tahmina Mahjor, Merel Follens, Jo Lambert, and Lynda Grine. 2022. "Promising Tools to Facilitate the Implementation of TDM of Biologics in Clinical Practice" Journal of Clinical Medicine 11, no. 11: 3011. https://doi.org/10.3390/jcm11113011





