Infection Probability Index: Implementation of an Automated Chronic Wound Infection Marker
Abstract
1. Introduction
2. Materials and Methods
2.1. Infection Probability Index
- The wound base forms the center of the wound.
- The anatomical wound margin is visible to the naked eye, surrounding the wound base.
- The thermal wound margin comprising the wound area within the thermal image.
Wound Types
2.2. Automation of the Infection Probability Index
2.2.1. Data Acquisition
2.2.2. Camera Calibration
2.2.3. Preprocessing
| Algorithm 1: Preprocessing. |
|
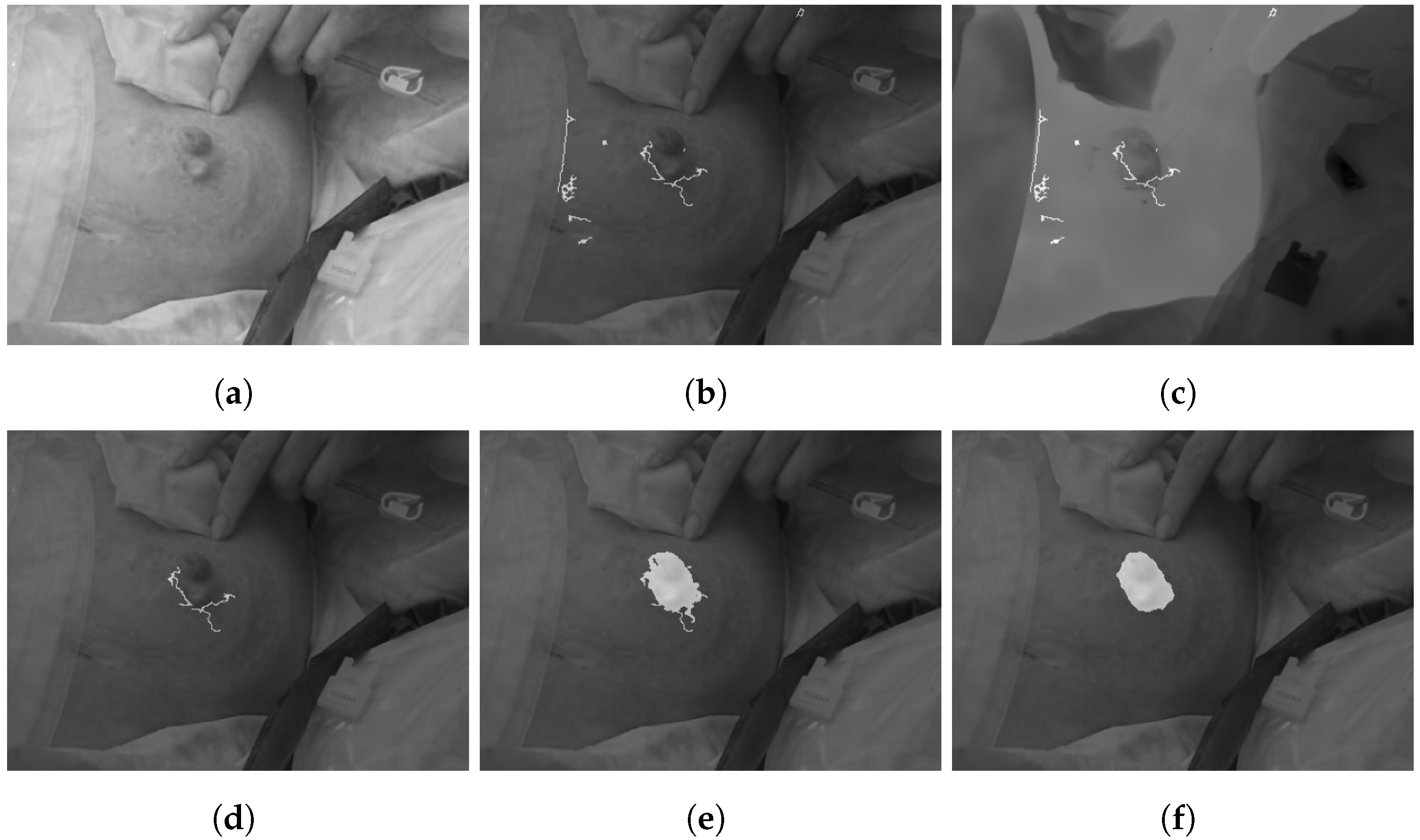
2.2.4. Wound Segmentation
| Algorithm 2: Wound segmentation. |
|
2.3. Comparison of the Infection Probability Index to a Clinical Parameter
Statistical Analysis
3. Results
3.1. Infection Probability Index
- Cold spots (punctual within wound base);
- Temperature difference (wound to intact skin);
- Temperature distribution (within the wound base);
- Thermal wound margin.
3.1.1. Cold Spots
3.1.2. Temperature Difference
3.1.3. Temperature Distribution
3.1.4. Wound Margin
3.2. Automation of the Infection Probability Index
3.2.1. Cold Spots
3.2.2. Temperature Difference
3.2.3. Temperature Distribution
3.2.4. Wound Margin
3.3. Comparison of the Infection Probability Index to a Clinical Parameter
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRP | C-reactive protein |
| IPI | Infection Probability Index |
| NFC | Near field communication |
| SSI | Surgical site infection |
References
- Sun, H.; Pulakat, L.; Anderson, D.W. Challenges and New Therapeutic Approaches in the Management of Chronic Wounds. Curr. Drug Targets 2020, 21, 1264–1275. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review: The burden of chronic wounds. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Heyer, K.; Herberger, K.; Protz, K.; Glaeske, G.; Augustin, M. Epidemiology of chronic wounds in Germany: Analysis of statutory health insurance data: Epidemiology of chronic wounds in Germany. Wound Repair Regen. 2016, 24, 434–442. [Google Scholar] [CrossRef]
- Raeder, K.; Jachan, D.E.; Müller-Werdan, U.; Lahmann, N.A. Prevalence and risk factors of chronic wounds in nursing homes in Germany: A Cross-Sectional Study. Int. Wound J. 2020, 17, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Stülpnagel, C.C.; Silva, N.; Augustin, M.; Montfrans, C.; Fife, C.; Fagerdahl, A.; Gamus, A.; Klein, T.M.; Blome, C.; Sommer, R. Assessing the quality of life of people with chronic wounds by using the cross-culturally valid and revised Wound-QoL questionnaire. Wound Repair Regen. 2021, 29, 452–459. [Google Scholar] [CrossRef]
- Gomes, A.; Teixeira, C.; Ferraz, R.; Prudêncio, C.; Gomes, P. Wound-Healing Peptides for Treatment of Chronic Diabetic Foot Ulcers and Other Infected Skin Injuries. Molecules 2017, 22, 1743. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Sommer, K.; Sander, A.L.; Albig, M.; Weber, R.; Henrich, D.; Frank, J.; Marzi, I.; Jakob, H. Delayed Wound Repair in Sepsis Is Associated with Reduced Local Pro-Inflammatory Cytokine Expression. PLoS ONE 2013, 8, e73992. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Punchard, N.A.; Whelan, C.J.; Adcock, I. The Journal of Inflammation. J. Inflamm. 2004, 1, 1. [Google Scholar] [CrossRef][Green Version]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on Wound Antisepsis: Update 2018. Skin Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Pang, M.; Zhu, M.; Lei, X.; Xu, P.; Cheng, B. Microbiome Imbalances: An Overlooked Potential Mechanism in Chronic Nonhealing Wounds. Int. J. Low. Extrem. Wounds 2019, 18, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Ashley, B.; Koh, A. Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Front. Bioeng. Biotechnol. 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Fierheller, M.; Sibbald, R.G. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv. Ski. Wound Care 2010, 23, 369–379, quiz 380–381. [Google Scholar] [CrossRef]
- Liu, C.; van der Heijden, F.; Klein, M.E.; van Baal, J.G.; Bus, S.A.; van Netten, J.J. Infrared dermal thermography on diabetic feet soles to predict ulcerations: A case study. In Advanced Biomedical and Clinical Diagnostic Systems XI; International Society for Optics and Photonics: Bellingham, WA, USA, 2013; p. 85720N. [Google Scholar] [CrossRef]
- Bryant, R.A.; Nix, D.P. (Eds.) Acute & Chronic Wounds: Current Management Concepts, 4th ed.; Elsevier/Mosby: St. Louis, MO, USA, 2012. [Google Scholar]
- Florczak, B.; Scheurich, A.; Croghan, J.; Sheridan, P.; Kurtz, D.; McGill, W.; McClain, B. An observational study to assess an electronic point-of-care wound documentation and reporting system regarding user satisfaction and potential for improved care. Ostomy/Wound Manag. 2012, 58, 46–51. [Google Scholar]
- Dong, W.; Nie, L.J.; Wu, M.J.; Xie, T.; Liu, Y.K.; Tang, J.J.; Dong, J.Y.; Qing, C.; Lu, S.L. WoundCareLog APP—A new application to record wound diagnosis and healing. Chin. J. Traumatol. = Zhonghua Chuang Shang Za Zhi 2019, 22, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; McSwiggan, J.; Parker, J.; Halas, G.A.; Friesen, M. An mHealth App for Decision-Making Support in Wound Dressing Selection (WounDS): Protocol for a User-Centered Feasibility Study. JMIR Res. Protoc. 2018, 7, e108. [Google Scholar] [CrossRef]
- Jacob, C.; Sanchez-Vazquez, A.; Ivory, C. Factors Impacting Clinicians’ Adoption of a Clinical Photo Documentation App and its Implications for Clinical Workflows and Quality of Care: Qualitative Case Study (Preprint). JMIR mHealth uHealth 2020, preprint. [Google Scholar] [CrossRef]
- Do Khac, A.; Jourdan, C.; Fazilleau, S.; Palayer, C.; Laffont, I.; Dupeyron, A.; Verdun, S.; Gelis, A. mHealth App for Pressure Ulcer Wound Assessment in Patients with Spinal Cord Injury: Clinical Validation Study. JMIR mHealth uHealth 2021, 9, e26443. [Google Scholar] [CrossRef]
- Pires, I.M.; Garcia, N. Wound Area Assessment using Mobile Application. In Proceedings of the International Conference on Biomedical Electronics and Devices, Lisbon, Portugal, 12–15 January 2015; pp. 271–282. [Google Scholar] [CrossRef]
- Mamone, V.; Fonzo, M.D.; Esposito, N.; Ferrari, M.; Ferrari, V. Monitoring Wound Healing with Contactless Measurements and Augmented Reality. IEEE J. Transl. Eng. Health Med. 2020, 8, 2700412. [Google Scholar] [CrossRef]
- Hani, A.F.M.; Eltegani, N.M.; Hussein, S.H.; Jamil, A.; Gill, P. Assessment of Ulcer Wounds Size Using 3D Skin Surface Imaging. In Visual Informatics: Bridging Research and Practice; Hutchison, D., Kanade, T., Kittler, J., Kleinberg, J.M., Mattern, F., Mitchell, J.C., Naor, M., Nierstrasz, O., Pandu Rangan, C., Steffen, B., Eds.; Series Title: Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5857, pp. 243–253. [Google Scholar] [CrossRef]
- Körber, A.; Rietkötter, J.; Grabbe, S.; Dissemond, J. Three-dimensional documentation of wound healing: First results of a new objective method for measurement. JDDG 2006, 4, 848–854. [Google Scholar] [CrossRef]
- Yee, A.; Harmon, J.; Yi, S. Quantitative Monitoring Wound Healing Status Through Three-dimensional Imaging on Mobile Platforms. J. Am. Coll. Clin. Wound Spec. 2016, 8, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Huang, W.; Tian, H.; Hu, J.; Cheng, Y.; Peng, Y. A New Smart Mobile System for Chronic Wound Care Management. IEEE Access 2018, 6, 52355–52365. [Google Scholar] [CrossRef]
- Wang, S.C.; Anderson, J.A.E.; Evans, R.; Woo, K.; Beland, B.; Sasseville, D.; Moreau, L. Point-of-care wound visioning technology: Reproducibility and accuracy of a wound measurement app. PLoS ONE 2017, 12, e0183139. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Yoon, J.H.; Yoo, S.S.; Choi, B.G.; Yoo, H.J. A Batteryless Chronic Wound Monitoring System with NFC. In Proceedings of the IEEE Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability (ECBIOS), Okinawa, Japan, 31 May–3 June 2019; pp. 31–34. [Google Scholar] [CrossRef]
- Sattar, H.; Bajwa, I.S.; Amin, R.U.; Sarwar, N.; Jamil, N.; Malik, M.G.A.; Mahmood, A.; Shafi, U. An IoT-Based Intelligent Wound Monitoring System. IEEE Access 2019, 7, 144500–144515. [Google Scholar] [CrossRef]
- Hsu, J.T.; Chen, Y.W.; Ho, T.W.; Tai, H.C.; Wu, J.M.; Sun, H.Y.; Hung, C.S.; Zeng, Y.C.; Kuo, S.Y.; Lai, F. Chronic wound assessment and infection detection method. BMC Med. Inform. Decis. Mak. 2019, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Paoli, A.; Razionale, A.V. Assessment of Chronic Wounds by Three-Dimensional Optical Imaging Based on Integrating Geometrical, Chromatic, and Thermal Data. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Dini, V.; Salvo, P.; Janowska, A.; Di Francesco, F.; Barbini, A.; Romanelli, M. Correlation Between Wound Temperature Obtained with an Infrared Camera and Clinical Wound Bed Score in Venous Leg Ulcers. Wounds Compend. Clin. Res. Pract. 2015, 27, 274–278. [Google Scholar]
- Chanmugam, A.; Langemo, D.; Thomason, K.; Haan, J.; Altenburger, E.A.; Tippett, A.; Henderson, L.; Zortman, T.A. Relative Temperature Maximum in Wound Infection and Inflammation as Compared with a Control Subject Using Long-Wave Infrared Thermography. Adv. Ski. Wound Care 2017, 30, 406–414. [Google Scholar] [CrossRef]
- Kraft, C.N.; Krüger, T.; Westhoff, J.; Lüring, C.; Weber, O.; Wirtz, D.C.; Pennekamp, P.H. CRP and leukocyte-count after lumbar spine surgery: Fusion vs. nucleotomy. Acta Orthop. 2011, 82, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- László, I.; Trásy, D.; Molnár, Z.; Fazakas, J. Sepsis: From Pathophysiology to Individualized Patient Care. J. Immunol. Res. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Li, S.; Mohamedi, A.H.; Senkowsky, J.; Nair, A.; Tang, L. Imaging in Chronic Wound Diagnostics. Adv. Wound Care 2020, 9, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Saghaleini, S.H.; Dehghan, K.; Shadvar, K.; Sanaie, S.; Mahmoodpoor, A.; Ostadi, Z. Pressure Ulcer and Nutrition. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2018, 22, 283–289. [Google Scholar] [CrossRef]
- Boyko, T.V.; Longaker, M.T.; Yang, G.P. Review of the Current Management of Pressure Ulcers. Adv. Wound Care 2018, 7, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.R.H.; Sharma, S. Decubitus Ulcer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Amir, Y.; Lohrmann, C.; Halfens, R.J.; Schols, J.M. Pressure ulcers in four Indonesian hospitals: Prevalence, patient characteristics, ulcer characteristics, prevention and treatment: Pressure ulcers in four Indonesian hospitals. Int. Wound J. 2017, 14, 184–193. [Google Scholar] [CrossRef]
- Prevention and Treatment of Pressure Ulcers/Injuries: Quick Reference Guide. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. PUAP/NPIAP/PPPIA. 2019. Available online: https://www.internationalguideline.com/ (accessed on 28 November 2021).
- Mervis, J.S.; Phillips, T.J. Pressure ulcers: Prevention and management. J. Am. Acad. Dermatol. 2019, 81, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.A.; Klotz, R.; Harnoss, J.C.; Heger, P.; Ritter, A.S.; Doerr-Harim, C.; Knebel, P.; Schneider, M.; Büchler, M.W.; Diener, M.K.; et al. Standard of Care and Outcomes of Primary Laparotomy Versus Laparotomy in Patients with Prior Open Abdominal Surgery (ReLap Study; DRKS00013001). J. Gastrointest. Surg. 2021, 25, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Azim, A. Emergency Laparotomies: Causes, Pathophysiology, and Outcomes. Indian J. Crit. Care Med. 2020, 24, S183–S189. [Google Scholar] [CrossRef]
- Abebe, K.; Bekele, M.; Tsehaye, A.; Lemmu, B.; Abebe, E. Laparotomy for Abdominal Injury Indication & Outcome of patients at a Teaching Hospital in Addis Ababa, Ethiopia. Ethiop. J. Health Sci. 2019, 29, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Sahebally, S.M.; McKevitt, K.; Stephens, I.; Fitzpatrick, F.; Deasy, J.; Burke, J.P.; McNamara, D. Negative Pressure Wound Therapy for Closed Laparotomy Incisions in General and Colorectal Surgery: A Systematic Review and Meta-analysis. JAMA Surg. 2018, 153, e183467. [Google Scholar] [CrossRef]
- Vilz, T.O.; v. Websky, M.; Kalff, J.C.; Stoffels, B. Intestinale Stomata. Der Chir. 2020, 91, 269–280. [Google Scholar] [CrossRef]
- Tsujinaka, S.; Tan, K.Y.; Miyakura, Y.; Fukano, R.; Oshima, M.; Konishi, F.; Rikiyama, T. Current Management of Intestinal Stomas and Their Complications. J. Anus Rectum Colon 2020, 4, 25–33. [Google Scholar] [CrossRef]
- Grubišić, I.; Gjenero, L.; Lipić, T.; Sović, I.; Skala, T. Active 3D scanning based 3D thermography system and medical applications. In Proceedings of the 34th International Convention MIPRO, Opatija, Croatia, 23–27 May 2011; pp. 269–273. [Google Scholar]
- Beauvisage, A.; Aouf, N. Low cost and low power multispectral thermal-visible calibration. In Proceedings of the IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Divya, P.; Anusudha, K. Segmentation of Defected Regions in Leaves using K- Means and OTSU’s Method. In Proceedings of the 4th International Conference on Electrical Energy Systems (ICEES), Chennai, India, 7–9 February 2018; pp. 111–115. [Google Scholar] [CrossRef]
- Johnson, T.R.; Gómez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef]
- Chen, H.; Ding, H.; He, X.; Zhuang, H. Color image segmentation based on seeded region growing with Canny edge detection. In Proceedings of the 12th International Conference on Signal Processing (ICSP), Hangzhou, China, 19–23 October 2014; pp. 683–686. [Google Scholar] [CrossRef]
- Narkhede, P.R.; Gokhale, A.V. Color image segmentation using edge detection and seeded region growing approach for CIELab and HSV color spaces. In Proceedings of the International Conference on Industrial Instrumentation and Control (ICIC), Pune, India, 28–30 May 2015; pp. 1214–1218. [Google Scholar] [CrossRef]
- Yu, Y.-W.; Wang, J.-H. Image segmentation based on region growing and edge detection. In Proceedings of the 1999 IEEE International Conference on Systems, Man, and Cybernetics (Cat. No.99CH37028), Tokyo, Japan, 12–15 October 1999; Volume 6, pp. 798–803. [Google Scholar] [CrossRef]
- Susan, S.; Verma, O.P.; Swarup, J. Object Segmentation by an Automatic Edge Constrained Region Growing Technique. In Proceedings of the Fourth International Conference on Computational Intelligence and Communication Networks, Mathura, Uttar Pradesh, India, 3–5 November 2012; pp. 378–381. [Google Scholar] [CrossRef]
- Shimbashi, T.; Kokubo, Y.; Shirota, N. Region segmentation using edge based circle growing. In Proceedings of the International Conference on Image Processing, Washington, DC, USA, 23–26 October 1995; Volume 3, pp. 65–68. [Google Scholar] [CrossRef]
- Rashid, M.H.O.; Mamun, M.A.; Hossain, M.A.; Uddin, M.P. Brain Tumor Detection Using Anisotropic Filtering, SVM Classifier and Morphological Operation from MR Images. In Proceedings of the International Conference on Computer, Communication, Chemical, Material and Electronic Engineering (IC4ME2), Rajshahi, Bangladesh, 8–9 February 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Childs, C.; Wright, N.; Willmott, J.; Davies, M.; Kilner, K.; Ousey, K.; Soltani, H.; Madhuvrata, P.; Stephenson, J. The surgical wound in infrared: Thermographic profiles and early stage test-accuracy to predict surgical site infection in obese women during the first 30 days after caesarean section. Antimicrob. Resist. Infect. Control 2019, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, G.; Tait, R.; Howell, K.; Hopgood, A.; Woo, P.; Harper, J. Automated Overlay of Infrared and Visual Medical Images. In User Centered Design for Medical Visualization; IGI Global: Hershey, PA, USA, 2008; p. 10. [Google Scholar]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5. [Google Scholar] [CrossRef]


| Parameter | Name: Description | Weight |
|---|---|---|
| Cold spots | None: No cold spot within the wound base or nearby the wound | 0 |
| Particular: 3 or less cold spots within wound base or nearby | 2 | |
| Distinct: More than 3 cold spots within wound base or nearby | 4 | |
| Temperature difference | Difference between wound base and intact skin between and | 0 |
| Difference between wound base and intact skin < | 1 | |
| Difference between wound base and intact skin > | 2 | |
| Temperature distribution | Homogeneous: > of temperature fluctuations below | 0 |
| Inhomogeneous: > of temperature fluctuations above | 1 | |
| Concentrated: 3 or less regions of mean temperature above | 1 | |
| Wound Margin | Discontinuing: Thermal margin crosses the anatomical margin | 1 |
| IPI (Infection probability index): | ∑ | |
| Wounds | Images | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients | Laparotomy | Pressure Injury | Stoma | Laparotomy | Pressure Injury | Stoma | Total | CRP |
| ID1 | 1 | - | 1 | 17 | - | 10 | 27 | 70.98 |
| ID2 | - | - | 1 | - | - | 3 | 3 | 51.58 |
| ID3 | 1 | - | 1 | 4 | - | 2 | 6 | 55.88 |
| ID4 | - | - | 1 | - | - | 3 | 3 | 17.13 |
| ID5 | 1 | 1 | 1 | 8 | 3 | 4 | 15 | 147.87 |
| ID6 | - | 1 | - | - | 2 | - | 2 | 11.1 |
| ID7 | - | - | 1 | - | - | 4 | 4 | 100.95 |
| total | 3 | 2 | 6 | 29 | 5 | 26 | 60 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schollemann, F.; Kunczik, J.; Dohmeier, H.; Pereira, C.B.; Follmann, A.; Czaplik, M. Infection Probability Index: Implementation of an Automated Chronic Wound Infection Marker. J. Clin. Med. 2022, 11, 169. https://doi.org/10.3390/jcm11010169
Schollemann F, Kunczik J, Dohmeier H, Pereira CB, Follmann A, Czaplik M. Infection Probability Index: Implementation of an Automated Chronic Wound Infection Marker. Journal of Clinical Medicine. 2022; 11(1):169. https://doi.org/10.3390/jcm11010169
Chicago/Turabian StyleSchollemann, Franziska, Janosch Kunczik, Henriette Dohmeier, Carina Barbosa Pereira, Andreas Follmann, and Michael Czaplik. 2022. "Infection Probability Index: Implementation of an Automated Chronic Wound Infection Marker" Journal of Clinical Medicine 11, no. 1: 169. https://doi.org/10.3390/jcm11010169
APA StyleSchollemann, F., Kunczik, J., Dohmeier, H., Pereira, C. B., Follmann, A., & Czaplik, M. (2022). Infection Probability Index: Implementation of an Automated Chronic Wound Infection Marker. Journal of Clinical Medicine, 11(1), 169. https://doi.org/10.3390/jcm11010169








