Pretreatment Serum Levels of IL-1 Receptor Antagonist and IL-4 Are Predictors of Overall Survival in Multiple Myeloma Patients Treated with Bortezomib
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Cytokine Analysis
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
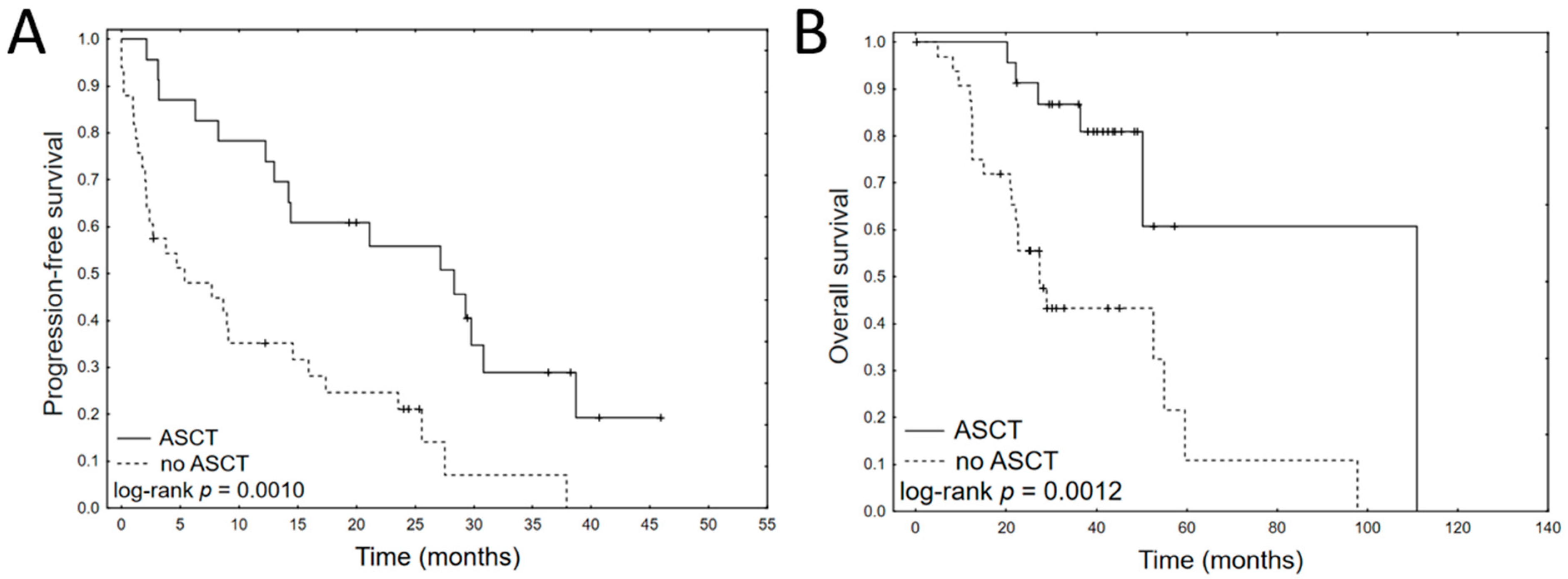
3.2. Prognostic Impact of Clinical Variables
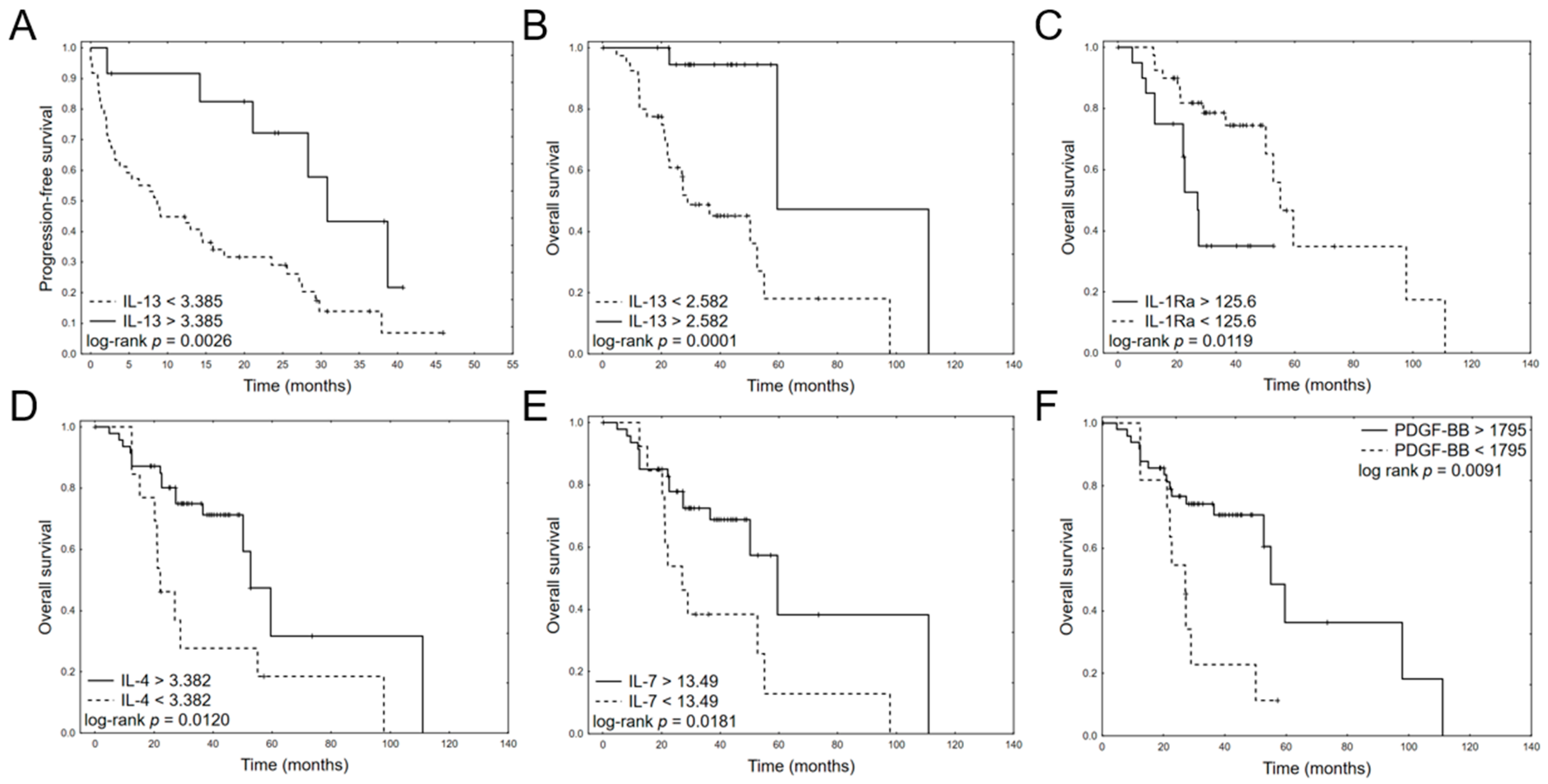
3.3. Prognostic Impact of Cytokine Levels
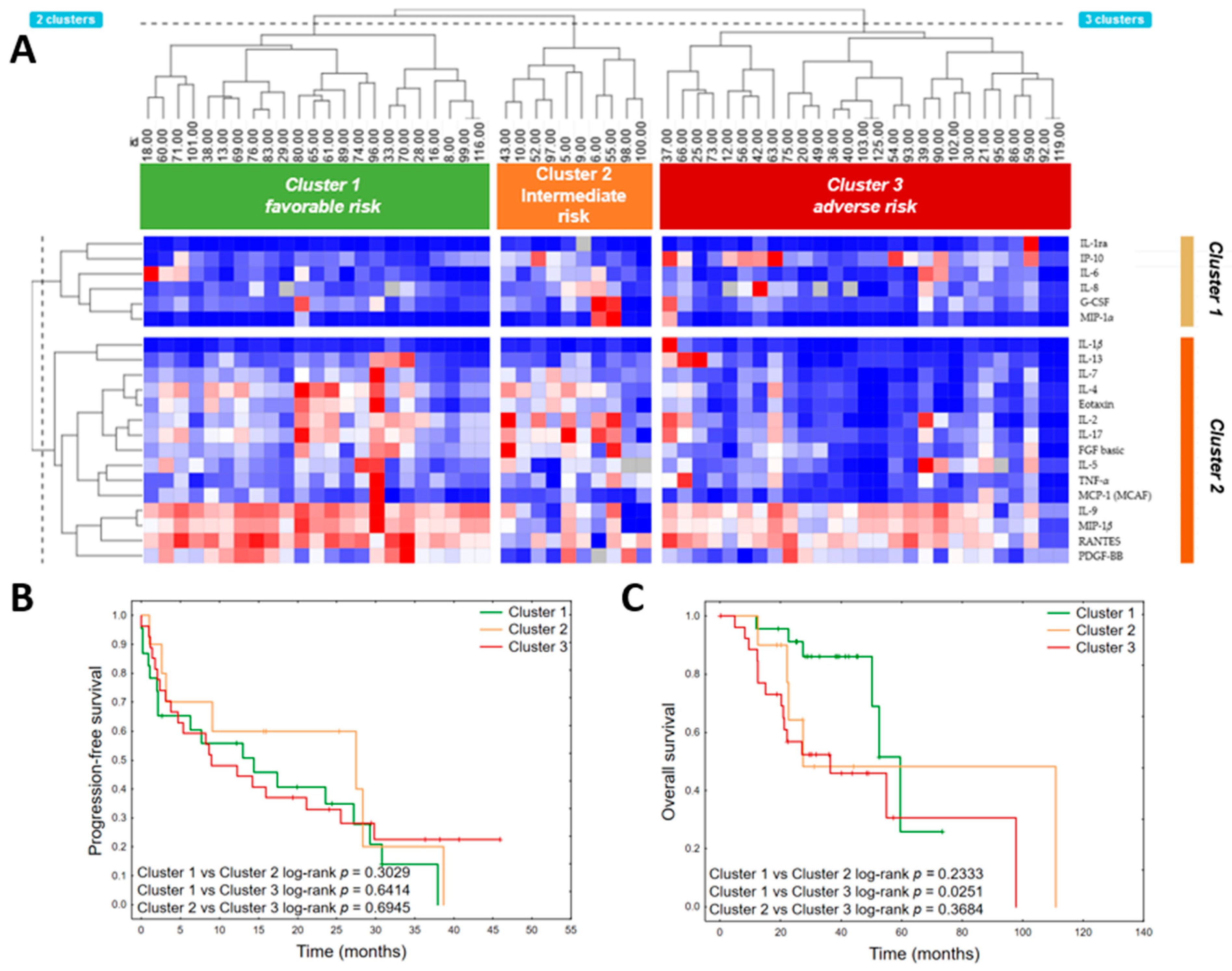
3.4. Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations:
| ASCT | autologous stem cell transplantation |
| BM | bone marrow |
| BM-MSC | bone marrow-derived mesenchymal stem cells |
| CR | complete response |
| CRAB | calcium-elevated, renal failure, anemia, and bone lesions |
| CXCL1 | C-X-C motif chemokine ligand |
| GM-CSF | granulocyte, monocyte colony-stimulating factor |
| IFN | interferon |
| IL-1-Ra | IL-1 receptor antagonist |
| IMiDs | immunomodulatory drugs |
| IMWG | International Myeloma Working Group |
| LCD | light chain disease |
| LDH | lactate dehydrogenase |
| MIP | macrophage inflammatory protein |
| MM | multiple myeloma |
| MSC | mesenchymal stem cells |
| OS | overall survival |
| PC | plasma cells |
| PCM | plasma cell myeloma |
| PD | progressive disease |
| PDGF | platelet-derived growth factor |
| PFS | progression-free survival |
| PI | proteasome inhibitor |
| POEMS | polyradiculoneuropathy, organomegaly, endocrinopathy, M-spike, skin changes |
| PR | partial resposne |
| RANTES | regulated upon activation, normal T cell expressed, and presumably secreted |
| SD | stable disease |
| TGF-β | transforming growth factor β |
| TNF | tumor necrosis factor |
| VCD | bortezomib, cyclophosphamide, dexamethasone |
| VEGF | vascular endothelial growth factor |
| VGPR | very good partial response |
| VMP | bortezomib, melphalan, and prednisone |
| VTD | bortezomib, thalidomide, dexamethasone |
References
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Pawlyn, C.; Yong, K.L. Multiple Myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Alexander, D.D.; Mink, P.J.; Adami, H.-O.; Cole, P.; Mandel, J.S.; Oken, M.M.; Trichopoulos, D. Multiple Myeloma: A Review of the Epidemiologic Literature. Int. J. Cancer 2007, 120, 40–61. [Google Scholar] [CrossRef]
- Hemminki, K.; Försti, A.; Houlston, R.; Sud, A. Epidemiology, Genetics and Treatment of Multiple Myeloma and Precursor Diseases. Int. J. Cancer 2021, 149, 1980–1996. [Google Scholar] [CrossRef]
- Robak, P.; Robak, T. Novel drugs for multiple myeloma. Top. Anti-Cancer Res. 2019, 8, 1–43. [Google Scholar]
- Robak, P.; Robak, T. Bortezomib for the Treatment of Hematologic Malignancies: 15 Years Later. Drugs R. D. 2019, 19, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Hayden, P.J.; Will, A.; Wheatley, K.; Coyne, I. Bortezomib for the Treatment of Multiple Myeloma. Cochrane Database Syst. Rev. 2016, 4, CD010816. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug Resistance in Multiple Myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef]
- Zheng, M.M.; Zhang, Z.; Bemis, K.; Belch, A.R.; Pilarski, L.M.; Shively, J.E.; Kirshner, J. The Systemic Cytokine Environment Is Permanently Altered in Multiple Myeloma. PLoS ONE 2013, 8, e58504. [Google Scholar] [CrossRef]
- Robak, P.; Wȩgłowska, E.; Dróżdż, I.; Mikulski, D.; Jarych, D.; Ferlińska, M.; Wawrzyniak, E.; Misiewicz, M.; Smolewski, P.; Fendler, W.; et al. Cytokine and Chemokine Profile in Patients with Multiple Myeloma Treated with Bortezomib. Mediators Inflamm. 2020, 2020, 1835836. [Google Scholar] [CrossRef]
- Jasrotia, S.; Gupta, R.; Sharma, A.; Halder, A.; Kumar, L. Cytokine Profile in Multiple Myeloma. Cytokine 2020, 136, 155271. [Google Scholar] [CrossRef] [PubMed]
- Bębnowska, D.; Hrynkiewicz, R.; Grywalska, E.; Pasiarski, M.; Sosnowska-Pasiarska, B.; Smarz-Widelska, I.; Góźdź, S.; Roliński, J.; Niedźwiedzka-Rystwej, P. Immunological Prognostic Factors in Multiple Myeloma. Int. J. Mol. Sci. 2021, 22, 3587. [Google Scholar] [CrossRef]
- Kyle, R.A.; Rajkumar, S.V. Criteria for Diagnosis, Staging, Risk Stratification and Response Assessment of Multiple Myeloma. Leukemia 2009, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Updated Diagnostic Criteria and Staging System for Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. B. 2016, 36, e418–e423. [Google Scholar] [CrossRef] [PubMed]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Győrffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A Comprehensive and Straightforward Web Application Enabling Rapid Biomarker Cutoff Optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, X.; Zhang, Y.; Bao, C.; Zhou, Z.; Jin, J. Cytokine Profiles in Patients with Newly Diagnosed Multiple Myeloma: Survival Is Associated with IL-6 and IL-17A Levels. Cytokine 2021, 138, 155358. [Google Scholar] [CrossRef]
- Stocchi, V.; Wang, T.; Randelli, E.; Mazzini, M.; Gerdol, M.; Pallavicini, A.; Secombes, C.J.; Scapigliati, G.; Buonocore, F. Evolution of Th2 Responses: Characterization of IL-4/13 in Sea Bass (Dicentrarchus labrax L.) and Studies of Expression and Biological Activity. Sci. Rep. 2017, 7, 2240. [Google Scholar] [CrossRef]
- Rifas, L.; Su-Li, C. IL-13 Regulates Vascular Cell Adhesion Molecule-1 Expression in Human Osteoblasts. J. Cell. Biochem. 2003, 89, 213–219. [Google Scholar] [CrossRef]
- Di Lullo, G.; Marcatti, M.; Heltai, S.; Brunetto, E.; Tresoldi, C.; Bondanza, A.; Bonini, C.; Ponzoni, M.; Tonon, G.; Ciceri, F.; et al. Th22 Cells Increase in Poor Prognosis Multiple Myeloma and Promote Tumor Cell Growth and Survival. Oncoimmunology 2015, 4, e1005460. [Google Scholar] [CrossRef]
- Bochner, B.S.; Klunk, D.A.; Sterbinsky, S.A.; Coffman, R.L.; Schleimer, R.P. IL-13 Selectively Induces Vascular Cell Adhesion Molecule-1 Expression in Human Endothelial Cells. J. Immunol. 1995, 154, 799–803. [Google Scholar]
- Doucet, C.; Brouty-Boyé, D.; Pottin-Clemenceau, C.; Jasmin, C.; Canonica, G.W.; Azzarone, B. IL-4 and IL-13 Specifically Increase Adhesion Molecule and Inflammatory Cytokine Expression in Human Lung Fibroblasts. Int. Immunol. 1998, 10, 1421–1433. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Alaeddine, M.; Prat, M.; Poinsot, V.; Gouazé-Andersson, V.; Authier, H.; Meunier, E.; Lefèvre, L.; Alric, C.; Dardenne, C.; Bernad, J.; et al. IL13-Mediated Dectin-1 and Mannose Receptor Overexpression Promotes Macrophage Antitumor Activities through Recognition of Sialylated Tumor Cells. Cancer Immunol. Res. 2019, 7, 321–334. [Google Scholar] [CrossRef]
- Özyörük, D.; Yavuz, G.; Dinçaslan, H.; Cabı-Unal, E.; Taçyıldız, N.; Karataş, D.; Doğu, F.; İkincioğulları, A. Serum IL-13 Levels at Diagnosis and Remission in Children with Malignant Lymphoma. Turk. J. Pediatr. 2016, 58, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Dimopoulos, M.-A. Myeloma Bone Disease: Pathophysiology and Management. Ann. Oncol. 2005, 16, 1223–1231. [Google Scholar] [CrossRef]
- Kyrstsonis, M.-C.; Dedou, S.G.; Bax, E.C.; Stamate Lou, M.; Maniatis, A. Serum Interleukin-6 (IL-6) and Interleukin-4 (IL-4) in Patients with Multiple Myeloma (MM). Br. J. Haematol. 1996, 92, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Andreeff, M.; Gruss, H.J.; Brach, M.A.; Lubbert, M.; Mertelsmann, R. Interleukin-4 Inhibits Growth of Multiple Myelomas by Suppressing Interleukin-6 Expression. Blood 1991, 78, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
- Garat, C.; Arend, W.P. Intracellular IL-1Ra Type 1 Inhibits IL-1-Induced IL-6 and IL-8 Production in Caco-2 Intestinal Epithelial Cells through Inhibition of P38 Mitogen-Activated Protein Kinase and NF-ΚB Pathways. Cytokine 2003, 23, 31–40. [Google Scholar] [CrossRef]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 Pathways in Inflammation and Human Diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Bosseboeuf, A.; Allain-Maillet, S.; Mennesson, N.; Tallet, A.; Rossi, C.; Garderet, L.; Caillot, D.; Moreau, P.; Piver, E.; Girodon, F.; et al. Pro-Inflammatory State in Monoclonal Gammopathy of Undetermined Significance and in Multiple Myeloma Is Characterized by Low Sialylation of Pathogen-Specific and Other Monoclonal Immunoglobulins. Front. Immunol. 2017, 8, 1347. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Luetkens, T.; Kobold, S.; Hildebrandt, Y.; Gordic, M.; Lajmi, N.; Meyer, S.; Bartels, K.; Zander, A.R.; Bokemeyer, C.; et al. The Cytokine/Chemokine Pattern in the Bone Marrow Environment of Multiple Myeloma Patients. Exp. Hematol. 2010, 38, 860–867. [Google Scholar] [CrossRef]
- Keyzner, A.; D’Souza, A.; Lacy, M.; Gertz, M.; Hayman, S.; Buadi, F.; Kumar, S.; Dingli, D.; Engebretson, A.; Tong, C.; et al. Low Levels of Interleukin-1 Receptor Antagonist (IL-1RA) Predict Engraftment Syndrome after Autologous Stem Cell Transplantation in POEMS Syndrome and Other Plasma Cell Neoplasms. Biol. Blood Marrow Transplant. 2013, 19, 1395–1398. [Google Scholar] [CrossRef][Green Version]
- Donovan, K.A.; Moon-Tasson, L.L.; Lust, J.A. Interplay Between IL-1, IL-6 and IL-17 in IL-1 Receptor Antagonist (IL-1Ra) Treated Multiple Myeloma Patients. Blood 2012, 120, 1874. [Google Scholar] [CrossRef]
- Lust, J.A.; Lacy, M.Q.; Zeldenrust, S.R.; Witzig, T.E.; Moon-Tasson, L.L.; Dinarello, C.A.; Donovan, K.A. Reduction in C-Reactive Protein Indicates Successful Targeting of the IL-1/IL-6 Axis Resulting in Improved Survival in Early Stage Multiple Myeloma. Am. J. Hematol. 2016, 91, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Nierste, B.A.; Glackin, C.A.; Kirshner, J. Dkk-1 and IL-7 in Plasma of Patients with Multiple Myeloma Prevent Differentiation of Mesenchymal Stem Cells into Osteoblasts. Am. J. Blood Res. 2014, 4, 73–85. [Google Scholar]
- Greco, C.; D’Agnano, I.; Vitelli, G.; Vona, R.; Marino, M.; Mottolese, M.; Zuppi, C.; Capoluongo, E.; Ameglio, F. C-Myc Deregulation Is Involved in Melphalan Resistance of Multiple Myeloma: Role of PDGF-BB. Int. J. Immunopathol. Pharmacol. 2006, 19, 205873920601900100. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhao, F.; Zhang, B.; Zhang, Y.; Cai, L.; Qiao, B.; Hu, Y.; Sun, C. Prognostic Nomogram Incorporating Cytokines for Overall Survival in Patients with Newly Diagnosed Multiple Myeloma. Int. Immunopharmacol. 2021, 99, 108016. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Chi, P.-D.; Wang, W.; Chen, X.-Q.; Geng, Q.-R.; Xia, Z.-J.; Lu, Y. High Level of Interleukin-10 in Serum Predicts Poor Prognosis in Multiple Myeloma. Br. J. Cancer 2016, 114, 463–468. [Google Scholar] [CrossRef]
- Aggarwal, M.; Agrawal, N.; Yadav, N.; Verma, P.; Ahmed, R.; Mehta, P.; Kapoor, J.; Bhurani, D. Autologous Stem Cell Transplantation in First Remission Is Associated with Better Progression-Free Survival in Multiple Myeloma. Ann. Hematol. 2018, 97, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Harousseau, J.L.; Stoppa, A.M.; Sotto, J.J.; Fuzibet, J.G.; Rossi, J.F.; Casassus, P.; Maisonneuve, H.; Facon, T.; Ifrah, N.; et al. A Prospective, Randomized Trial of Autologous Bone Marrow Transplantation and Chemotherapy in Multiple Myeloma. Intergroupe Français Du Myélome. N. Engl. J. Med. 1996, 335, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Szabo, A.; Chhabra, S.; Hamadani, M.; D’Souza, A.; Usmani, S.Z.; Sieracki, R.; Gyawali, B.; Jackson, J.L.; Asimakopoulos, F.; et al. Autologous Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Novel Agent Induction: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-H.; Kwon, S.Y.; Min, J.-J.; Bom, H.-S.; Ahn, S.-Y.; Jung, S.-Y.; Lee, S.-S.; Park, M.-R.; Yang, D.-H.; Ahn, J.-S.; et al. (18)F-FDG PET/CT Is Useful for Determining Survival Outcomes of Patients with Multiple Myeloma Classified as Stage II and III with the Revised International Staging System. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Narita, K.; Kobayashi, H.; Kitadate, A.; Takeuchi, M.; O’uchi, T.; Matsue, K. Medullary Abnormalities in Appendicular Skeletons Detected With (18)F-FDG PET/CT Predict an Unfavorable Prognosis in Newly Diagnosed Multiple Myeloma Patients with High-Risk Factors. AJR. Am. J. Roentgenol. 2019, 213, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.-I.; Yoon, D.H.; Ryu, J.-S. Prognostic Value of 18 F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Newly Diagnosed Multiple Myeloma: A Systematic Review and Meta-analysis. Eur. Radiol. 2021, 31, 152–162. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total |
|---|---|
| Number of patients | 61 (100) |
| Gender | M: 32 (52.5) |
| F: 29 (47.5) | |
| Age at diagnosis | 61.9 ± 11.3 |
| mean + SD (range) | (38.3–83.7) |
| Bortezomib regimen: | |
| VCD | 49 (80.3) |
| VMP | 5 (8.2) |
| VTD | 4 (6.6) |
| Other | 3 (4.9) |
| Paraprotein | |
| IgG | 33 (54) |
| IgA | 14 (23) |
| LCD | 14 (23) |
| Bone disease at diagnosis | 36 (59) |
| Calcium > 2.75 mmol/L at diagnosis | 12 (19.7) |
| HB < 10 g/dL at diagnosis | 21 (34.4) |
| Creatinine > 2 mg/dL at diagnosis | 10 (16.4) |
| International Staging System (ISS) | I-17 (27.9) |
| II-13 (21.3) | |
| III-29 (47.5) | |
| Beta2-microglobulin increased (>3mg/L) | 40 (65.6) |
| LDH > 240U/L | 7 (11.5) |
| Response to induction therapy | |
| CR | 24 (39.3) |
| VGPR | 13 (21.3) |
| PR | 14 (23.0) |
| SD | 7 (11.5) |
| PD | 3 (4.9) |
| ASCT | 23 (37.3) |
| Cytogenetics * | N = 33 |
| t(11;14) | 1 (3) |
| t(4;14) | 5 (15.2) |
| t(14;16) | 0 |
| t(14;20) | 0 |
| del(17p) | 3 (9.1) |
| amp(1q) | 18 (54.5) |
| del(13q) | 8 (24.2) |
| PFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient | p | HR | 95% CI | Coefficient | p | HR | 95% CI | ||
| Lower | Upper | Lower | Upper | |||||||
| ISS III | −0.13 | 0.680 | 0.88 | 0.48 | 1.60 | 0.78 | 0.060 | 2.18 | 0.97 | 4.90 |
| ASCT | −1.11 | 0.001 | 0.33 | 0.17 | 0.64 | −1.46 | 0.004 | 0.23 | 0.09 | 0.62 |
| HB < 10 g/dL at diagnosis | 0.12 | 0.720 | 1.13 | 0.59 | 2.17 | 0.12 | 0.778 | 1.13 | 0.49 | 2.58 |
| Calcium > 2.75 mmol/l at diagnosis | 0.46 | 0.213 | 1.58 | 0.77 | 3.23 | −0.40 | 0.464 | 0.67 | 0.23 | 1.95 |
| Creatinine > 2 mg/dL at diagnosis | −0.48 | 0.323 | 0.62 | 0.24 | 1.60 | 0.07 | 0.893 | 1.08 | 0.36 | 3.18 |
| Bone disease | 0.64 | 0.054 | 1.89 | 0.99 | 3.61 | 0.61 | 0.156 | 1.85 | 0.79 | 4.30 |
| Age > 70 | 0.34 | 0.323 | 1.41 | 0.71 | 2.80 | 0.63 | 0.118 | 1.87 | 0.85 | 4.10 |
| PFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Coefficient | p | HR | 95% CI | Coefficient | p | HR | 95% CI | ||
| Lower | Upper | Lower | Upper | |||||||
| Eotaxin | 0.0022 | 0.9558 | 1.0022 | 0.9279 | 1.0824 | −0.0954 | 0.0620 | 0.9090 | 0.8224 | 1.0048 |
| FGF basic | −0.0825 | 0.6462 | 0.9208 | 0.6475 | 1.3096 | −0.2964 | 0.2059 | 0.7435 | 0.4697 | 1.1769 |
| G-CSF | −0.0009 | 0.8658 | 0.9991 | 0.9882 | 1.0101 | 0.0002 | 0.9755 | 1.0002 | 0.9852 | 1.0155 |
| GM-CSF | −1.7105 | 0.0694 | 0.1808 | 0.0285 | 1.1456 | −4.9713 | 0.0643 | 0.0069 | 0.00004 | 1.3441 |
| IFN-γ | −0.0230 | 0.8837 | 0.9773 | 0.7184 | 1.3296 | −0.1371 | 0.5175 | 0.8719 | 0.5756 | 1.3206 |
| IL-10 | −0.0005 | 0.9984 | 0.9995 | 0.6270 | 1.5934 | 0.0049 | 0.0531 | 1.0049 | 0.9999 | 1.0099 |
| IL−12 (p70) | 0.6213 | 0.3417 | 1.8613 | 0.5172 | 6.6991 | 0.3990 | 0.7549 | 1.4903 | 0.1217 | 18.2445 |
| IL-13 | −1.9675 | 0.0185 | 0.1398 | 0.0272 | 0.7189 | −4.4187 | 0.0087 | 0.0121 | 0.0004 | 0.3271 |
| IL-15 | 0.0138 | 0.5837 | 1.0139 | 0.9652 | 1.0650 | −0.0110 | 0.7869 | 0.9890 | 0.9131 | 1.0713 |
| IL-17 | −0.0975 | 0.5954 | 0.9071 | 0.6329 | 1.3001 | −0.4021 | 0.1243 | 0.6689 | 0.4006 | 1.1170 |
| IL-1ra | 0.0012 | 0.8111 | 1.0012 | 0.9917 | 1.0107 | 0.0514 | 0.0029 | 1.0527 | 1.0177 | 1.0889 |
| IL-1β | −1.6824 | 0.1799 | 0.1859 | 0.0159 | 2.1737 | −1.3066 | 0.5299 | 0.2707 | 0.0046 | 15.9668 |
| IL-2 | −0.3222 | 0.6034 | 0.7246 | 0.2149 | 2.4429 | −1.2680 | 0.1562 | 0.2814 | 0.0488 | 1.6234 |
| IL-4 | −0.1722 | 0.7859 | 0.8418 | 0.2430 | 2.9165 | −1.6615 | 0.0364 | 0.1899 | 0.0401 | 0.9000 |
| IL-5 | 0.1369 | 0.2194 | 1.1467 | 0.9217 | 1.4268 | −0.0654 | 0.6848 | 0.9367 | 0.6829 | 1.2847 |
| IL-6 | 0.0328 | 0.9321 | 1.0334 | 0.4860 | 2.1970 | −0.2548 | 0.6893 | 0.7751 | 0.2223 | 2.7027 |
| IL-7 | 0.0450 | 0.7981 | 1.0460 | 0.7411 | 1.4762 | −0.4600 | 0.0497 | 0.6313 | 0.3988 | 0.9993 |
| IL-8 | 0.1698 | 0.3739 | 1.1850 | 0.8151 | 1.7229 | 0.0972 | 0.7127 | 1.1021 | 0.6570 | 1.8487 |
| IL-9 | 0.0217 | 0.1296 | 1.0219 | 0.9937 | 1.0509 | −0.0149 | 0.3157 | 0.9852 | 0.9570 | 1.0143 |
| IP-10 | −0.0005 | 0.9984 | 0.9995 | 0.6270 | 1.5934 | 0.0049 | 0.0531 | 1.0049 | 0.9999 | 1.0099 |
| MCP-1 | 0.0856 | 0.4049 | 1.0894 | 0.8906 | 1.3326 | −0.2965 | 0.0992 | 0.7435 | 0.5226 | 1.0575 |
| MIP-1α | −0.1895 | 0.3722 | 0.8273 | 0.5457 | 1.2545 | 0.0639 | 0.7731 | 1.0660 | 0.6903 | 1.6461 |
| MIP-1β | 0.1054 | 0.0990 | 1.1112 | 0.9804 | 1.2595 | −0.0463 | 0.4618 | 0.9548 | 0.8441 | 1.0800 |
| PDGF-BB | −0.0012 | 0.2900 | 0.9988 | 0.9965 | 1.0011 | −0.0037 | 0.0316 | 0.9963 | 0.9930 | 0.9997 |
| RANTES | −0.0007 | 0.1152 | 0.9993 | 0.9984 | 1.0002 | −0.0010 | 0.0572 | 0.9990 | 0.9981 | 1.0000 |
| TNF-α | −0.1612 | 0.3417 | 0.8511 | 0.6105 | 1.1866 | −0.3491 | 0.1528 | 0.7053 | 0.4371 | 1.1382 |
| VEGF | 0.0022 | 0.7425 | 1.0022 | 0.9890 | 1.0157 | −0.0395 | 0.1177 | 0.9613 | 0.9149 | 1.0100 |
| Variable | Coefficient | p | HR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| PFS | |||||
| IL-13 | −1.958 | 0.0302 | 0.1411 | 0.0240 | 0.8291 |
| ASCT | −0.494 | 0.0065 | 0.3722 | 0.1826 | 0.7585 |
| OS | |||||
| IL-1Ra | 0.017 | 0.0091 | 1.017 | 1.004 | 1.030 |
| IL-4 | −1.828 | 0.0147 | 0.161 | 0.037 | 0.698 |
| ASCT | −0.975 | 0.0007 | 0.142 | 0.046 | 0.438 |
| bone disease | 0.671 | 0.0059 | 3.826 | 1.471 | 9.949 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulski, D.; Robak, P.; Perdas, E.; Węgłowska, E.; Łosiewicz, A.; Dróżdż, I.; Jarych, D.; Misiewicz, M.; Szemraj, J.; Fendler, W.; et al. Pretreatment Serum Levels of IL-1 Receptor Antagonist and IL-4 Are Predictors of Overall Survival in Multiple Myeloma Patients Treated with Bortezomib. J. Clin. Med. 2022, 11, 112. https://doi.org/10.3390/jcm11010112
Mikulski D, Robak P, Perdas E, Węgłowska E, Łosiewicz A, Dróżdż I, Jarych D, Misiewicz M, Szemraj J, Fendler W, et al. Pretreatment Serum Levels of IL-1 Receptor Antagonist and IL-4 Are Predictors of Overall Survival in Multiple Myeloma Patients Treated with Bortezomib. Journal of Clinical Medicine. 2022; 11(1):112. https://doi.org/10.3390/jcm11010112
Chicago/Turabian StyleMikulski, Damian, Paweł Robak, Ewelina Perdas, Edyta Węgłowska, Aleksandra Łosiewicz, Izabela Dróżdż, Dariusz Jarych, Małgorzata Misiewicz, Janusz Szemraj, Wojciech Fendler, and et al. 2022. "Pretreatment Serum Levels of IL-1 Receptor Antagonist and IL-4 Are Predictors of Overall Survival in Multiple Myeloma Patients Treated with Bortezomib" Journal of Clinical Medicine 11, no. 1: 112. https://doi.org/10.3390/jcm11010112
APA StyleMikulski, D., Robak, P., Perdas, E., Węgłowska, E., Łosiewicz, A., Dróżdż, I., Jarych, D., Misiewicz, M., Szemraj, J., Fendler, W., & Robak, T. (2022). Pretreatment Serum Levels of IL-1 Receptor Antagonist and IL-4 Are Predictors of Overall Survival in Multiple Myeloma Patients Treated with Bortezomib. Journal of Clinical Medicine, 11(1), 112. https://doi.org/10.3390/jcm11010112







