High Right Ventricular Afterload during Exercise in Patients with Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. Acute and Chronic RV Responses to Exercise
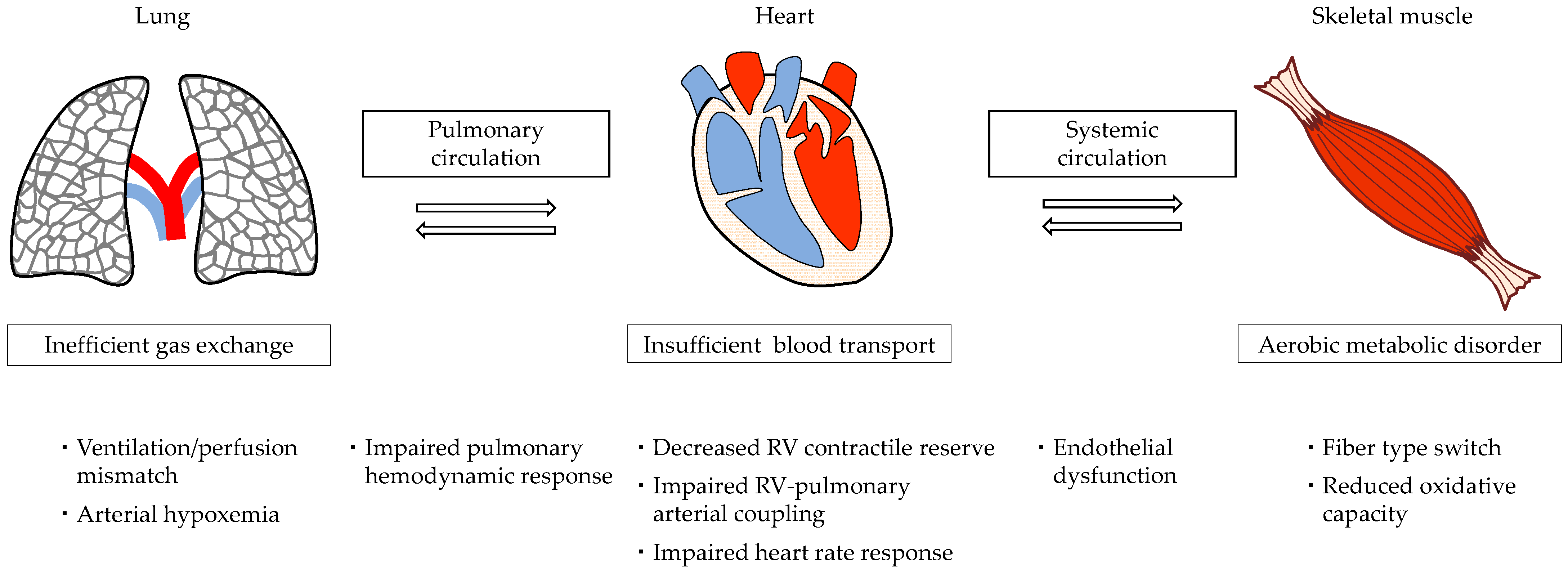
3. Pathophysiology of Exercise Intolerance in Patients with PAH
4. Pulmonary Hemodynamic Response to Exercise in Normal Subjects
5. Pulmonary Hemodynamic Response to Exercise in Patients with PAH
6. The Efficacy and Safety of Exercise Training in Patients with PAH
7. Exercise Training Program Based on Risk Stratification to Enhance Safety and Efficacy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk-Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Naeije, R.; Manes, A. The right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. 2014, 23, 476–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.Q.; Abbate, A.; Bogaard, H.J. Role of cardiac inflammation in right ventricular failure. Cardiovasc. Res. 2017, 113, 1441–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barst, R.J.; Rubin, L.J.; Long, W.A.; McGoon, M.D.; Rich, S.; Badesch, D.B.; Groves, B.M.; Tapson, V.F.; Bourge, R.C.; Brundage, B.H.; et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N. Engl. J. Med. 1996, 334, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Monaco, T.J.; Davila, C.D. Safety, efficacy, and clinical utility of macitentan in the treatment of pulmonary arterial hypertension. Drug Des. Dev. Ther. 2016, 10, 1675–1682. [Google Scholar] [CrossRef] [Green Version]
- Sastry, B.K.S.; Narasimhan, C.; Reddy, N.K.; Raju, B.S. Clinical Efficacy of Sildenafil in Primary Pulmonary Hypertension: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study. J. Am. Coll. Cardiol. 2004, 43, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Claessen, G. Is exercise good for the right ventricle? Concepts for health and disease. Can. J. Cardiol. 2015, 31, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, M.; Ogawa, A.; Matsubara, H. Response to exercise in patients with pulmonary arterial hypertension treated with combination therapy. ERJ Open Res. 2021, 7, 00725–02020. [Google Scholar] [CrossRef] [PubMed]
- Grünig, E.; Eichstaedt, C.; Barberà, J.A.; Benjamin, N.; Blanco, I.; Bossone, E.; Cittadini, A.; Coghlan, G.; Corris, P.; D’Alto, M.; et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1800332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waller, L.; Krüger, K.; Conrad, K.; Weiss, A.; Alack, K. Effects of Different Types of Exercise Training on Pulmonary Arterial Hypertension: A Systematic Review. J. Clin. Med. 2020, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Garg, S.; Khunger, M.; Garg, S.; Kumbhani, D.J.; Chin, K.M.; Berry, J.D. Efficacy and Safety of Exercise Training in Chronic Pulmonary Hypertension: Systematic Review and Meta-Analysis. Circ. Heart Fail. 2015, 8, 1032–1043. [Google Scholar] [CrossRef]
- West, J.B.; Luks, A.M. Blood flow and metabolism: How the pulmonary circulation removes gas from the lung and alters some metabolites. In West’s Respiratory Physiology. The Essentials, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2016; pp. 41–62. [Google Scholar]
- Friedberg, M.K.; Redington, A.N. Right versus left ventricular failure: Differences, similarities, and interactions. Circulation 2014, 129, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Burns, A.T.; Mooney, D.J.; Inder, W.J.; Taylor, A.J.; Bogaert, J.; MacIsaac, A.I.; Heidbüchel, H.; Prior, D.L. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur. Heart J. 2012, 33, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- La Gerche, A.; Heidbüchel, H.; Burns, A.T.; Mooney, D.J.; Taylor, A.J.; Pfluger, H.B.; Inder, W.J.; MacIsaac, A.I.; Prior, D.L. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med. Sci. Sports Exerc. 2011, 43, 974–981. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Prior, D.L.; La Gerche, A. Ventricular arrhythmias associated with long-term endurance sports: What is the evidence? Br. J. Sports Med. 2012, 46, i44–i50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handoko, M.L.; de Man, F.S.; Happé, C.M.; Schalij, I.; Musters, R.J.P.; Westerhof, N.; Postmus, P.E.; Paulus, W.J.; van der Laarse, W.J.; Vonk-Noordegraaf, A. Opposite Effects of Training in Rats With Stable and Progressive Pulmonary Hypertension. Circulation 2009, 120, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, K. Breathig during exercise. N. Engl. J. Med. 1978, 298, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Morrell, N.W.; Archer, S.L.; Stenmark, K.R.; Maclean, M.R.; Lang, I.M.; Christman, B.W.; Weir, E.K.; Eickelberg, O.; Voelkel, N.F.; et al. Cellular and Molecular Pathobiology of Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2004, 43, 13S–24S. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.G.; Hansen, J.E.; Oudiz, R.J.; Wasserman, K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation 2001, 104, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spruijt, O.A.; de Man, F.S.; Groepenhoff, H.; Oosterveer, F.; Westerhof, N.; Vonk-Noordegraaf, A.; Bogaard, H.J. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2015, 191, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Champion, H.C.; Michelakis, E.D.; Hassoun, P.M. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit State of the art and clinical and research implications. Circulation 2009, 120, 992–1007. [Google Scholar] [CrossRef] [Green Version]
- Vonk Noordegraaf, A.; Chin, K.M.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.R.; Kawut, S.M.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Holverda, S.; Gan, C.T.-J.; Marcus, J.T.; Postmus, P.E.; Boonstra, A.; Vonk-Noordegraaf, A. Impaired Stroke Volume Response to Exercise in Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2006, 47, 1732–1733. [Google Scholar] [CrossRef] [Green Version]
- Provencher, S.; Chemla, D.; Hervé, P.; Sitbon, O.; Humbert, M.; Simonneau, G. Heart rate responses during the 6-minute walk test in pulmonary arterial hypertension. Eur. Respir. J. 2006, 27, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mainguy, V.; Maltais, F.; Saey, D.; Gagnon, P.; Martel, S.; Simon, M.; Provencher, S. Peripheral muscle dysfunction in idiopathic pulmonary arterial hypertension. Thorax 2010, 65, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Peled, N.; Bendayan, D.; Shitrit, D.; Fox, B.; Yehoshua, L.; Kramer, M.R. Peripheral endothelial dysfunction in patients with pulmonary arterial hypertension. Respir. Med. 2008, 102, 1791–1796. [Google Scholar] [CrossRef] [Green Version]
- Tolle, J.J.; Waxman, A.B.; Van Horn, T.L.; Pappagianopoulos, P.P.; Systrom, D.M. Exercise-Induced Pulmonary Arterial Hypertension. Circulation 2008, 118, 2183–2189. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.D.; Bossone, E.; Naeije, R.; Grünig, E.; Saggar, R.; Lancellotti, P.; Ghio, S.; Varga, J.; Rajagopalan, S.; Oudiz, R.; et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013, 128, 1470–1479. [Google Scholar] [CrossRef]
- Kovacs, G.; Berghold, A.; Scheidl, S.; Olschewski, H. Pulmonary arterial pressure during rest and exercise in healthy subjects: A systematic review. Eur. Respir. J. 2009, 34, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Naeije, R.; Vanderpool, R.; Dhakal, B.P.; Saggar, R.; Saggar, R.; Vachiery, J.-L.; Lewis, G.D. Exercise-induced Pulmonary Hypertension: Physiological Basis and Methodological Concerns. Am. J. Respir. Crit. Care Med. 2013, 187, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.; Herve, P.; Barbera, J.A.; Chaouat, A.; Chemla, D.; Condliffe, R.; Garcia, G.; Grünig, E.; Howard, L.; Humbert, M.; et al. An official European Respiratory Society statement: Pulmonary haemodynamics during exercise. Eur. Respir. J. 2017, 50, 1700578. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Chesler, N. Pulmonary circulation at exercise. Compr. Physiol. 2012, 2, 711–741. [Google Scholar] [CrossRef] [Green Version]
- Badesch, D.B.; Champion, H.C.; Gomez Sanchez, M.A.; Hoeper, M.M.; Loyd, J.E.; Manes, A.; McGoon, M.; Naeije, R.; Olschewski, H.; Oudiz, R.J.; et al. Diagnosis and Assessment of Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2009, 54, S55–S66. [Google Scholar] [CrossRef]
- Singh, I.; Rahaghi, F.N.; Naeije, R.; Oliveira, R.K.F.; Vanderpool, R.R.; Waxman, A.B.; Systrom, D.M. Dynamic right ventricular–pulmonary arterial uncoupling during maximum incremental exercise in exercise pulmonary hypertension and pulmonary arterial hypertension. Pulm. Circ. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weatherald, J.; Boucly, A.; Lau, E.; Godinas, L.; Savale, L.; Jaïs, X.; Montani, D.; Sitbon, O.; Simonneau, G.; Humbert, M.; et al. Are indexed values better for defining exercise pulmonary hypertension? Eur. Respir. J. 2017, 50, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herve, P.; Lau, E.M.; Sitbon, O.; Savale, L.; Montani, D.; Godinas, L.; Lador, F.; Jaïs, X.; Parent, F.; Günther, S.; et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur. Respir. J. 2015, 46, 728–737. [Google Scholar] [CrossRef] [Green Version]
- Portillo, K.; Torralba, Y.; Blanco, I.; Burgos, F.; Rodriguez-Roisin, R.; Rios, J.; Roca, J.; Barberà, J.A. Pulmonary hemodynamic profile in chronic obstructive pulmonary disease. Int. J. COPD 2015, 10, 1313–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janicki, J.S.; Weber, K.T.; Likoff, M.J.; Fishman, A.P. The pressure-flow response of the pulmonary circulation in patients with heart failure and pulmonary vascular disease. Circulation 1985, 72, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Blumberg, F.C.; Riegger, G.A.J.; Pfeifer, M. Hemodynamic Effects of Aerosolized Iloprost in Pulmonary Hypertension at Rest and During Exercise. Chest 2002, 121, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Castelain, V.; Chemla, D.; Humbert, M.; Sitbon, O.; Simonneau, G.; Lecarpentier, Y.; Hervé, P. Pulmonary Artery Pressure—Flow Relations after Prostacyclin in Primary Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2002, 165, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.; Hervé, P.; Sitbon, O.; Humbert, M.; Simonneau, G.; Chemla, D. Changes in exercise haemodynamics during treatment in pulmonary arterial hypertension. Eur. Respir. J. 2008, 32, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemla, D.; Castelain, V.; Hoette, S.; Creuzé, N.; Provencher, S.; Zhu, K.; Humbert, M.; Herve, P. Strong linear relationship between heart rate and mean pulmonary artery pressure in exercising patients with severe precapillary pulmonary hypertension. Am. J. Physiol. Hear. Circ. Physiol. 2013, 305, H769–H777. [Google Scholar] [CrossRef] [Green Version]
- Chaouat, A.; Sitbon, O.; Mercy, M.; Ponçot-Mongars, R.; Steeve Provencher, A.G.; Gomez, E.; Selton-Suty, C.; Malvestio, P.; Regent, D.; Paris, C.; et al. Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur. Respir. J. 2014, 44, 704–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasler, E.D.; Müller-Mottet, S.; Furian, M.; Saxer, S.; Huber, L.C.; Maggiorini, M.; Speich, R.; Bloch, K.E.; Ulrich, S. Pressure-Flow During Exercise Catheterization Predicts Survival in Pulmonary Hypertension. Chest 2016, 150, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ehlken, N.; Lichtblau, M.; Klose, H.; Weidenhammer, J.; Fischer, C.; Nechwatal, R.; Uiker, S.; Halank, M.; Olsson, K.; Seeger, W.; et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: A prospective, randomized, controlled trial. Eur. Heart J. 2016, 37, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Galiè, N.; Palazzini, M.; Manes, A. Pulmonary arterial hypertension: From the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur. Heart J. 2010, 31, 2080–2086. [Google Scholar] [CrossRef]
- Mereles, D.; Ehlken, N.; Kreuscher, S.; Ghofrani, S.; Hoeper, M.M.; Halank, M.; Meyer, F.J.; Karger, G.; Buss, J.; Juenger, J.; et al. Exercise and Respiratory Training Improve Exercise Capacity and Quality of Life in Patients With Severe Chronic Pulmonary Hypertension. Circulation 2006, 114, 1482–1489. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, A.A.; Chin, L.M.K.; Keyser, R.E.; Kennedy, M.; Nathan, S.D.; Woolstenhulme, J.G.; Connors, G.; Chan, L. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respir. Med. 2013, 107, 778–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, L.; Chin, L.M.K.; Kennedy, M.; Woolstenhulme, J.G.; Nathan, S.D.; Weinstein, A.A.; Connors, G.; Weir, N.A.; Drinkard, B.; Lamberti, J.; et al. Benefits of Intensive Treadmill Exercise Training on Cardiorespiratory Function and Quality of Life in Patients With Pulmonary Hypertension. Chest 2013, 143, 333–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, S.; Fink, C.; Risse, F.; Ehlken, N.; Fischer, C.; Ley-Zaporozhan, J.; Kauczor, H.-U.; Klose, H.; Gruenig, E. Magnetic resonance imaging to assess the effect of exercise training on pulmonary perfusion and blood flow in patients with pulmonary hypertension. Eur. Radiol. 2013, 23, 324–331. [Google Scholar] [CrossRef] [PubMed]
- González-Saiz, L.; Fiuza-Luces, C.; Sanchis-Gomar, F.; Santos-Lozano, A.; Quezada-Loaiza, C.A.; Flox-Camacho, A.; Munguía-Izquierdo, D.; Ara, I.; Santalla, A.; Morán, M.; et al. Benefits of skeletal-muscle exercise training in pulmonary arterial hypertension: The WHOLEi + 12 trial. Int. J. Cardiol. 2017, 231, 277–283. [Google Scholar] [CrossRef]
- Grünig, E.; Lichtblau, M.; Ehlken, N.; Ghofrani, H.A.; Reichenberger, F.; Staehler, G.; Halank, M.; Fischer, C.; Seyfarth, H.-J.; Klose, H.; et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur. Respir. J. 2012, 40, 84–92. [Google Scholar] [CrossRef] [Green Version]
- De Man, F.S.; Handoko, M.L.; Groepenhoff, H.; van ’t Hul, A.J.; Abbink, J.; Koppers, R.J.H.; Grotjohan, H.P.; Twisk, J.W.R.; Bogaard, H.-J.; Boonstra, A.; et al. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2009, 34, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American heart association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Gaine, S.P.; Howard, L.S.; Leuchte, H.H.; Mathier, M.A.; Mehta, S.; Palazzini, M.; Park, M.H.; Tapson, V.F.; Sitbon, O. Treatment goals of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D73–D81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
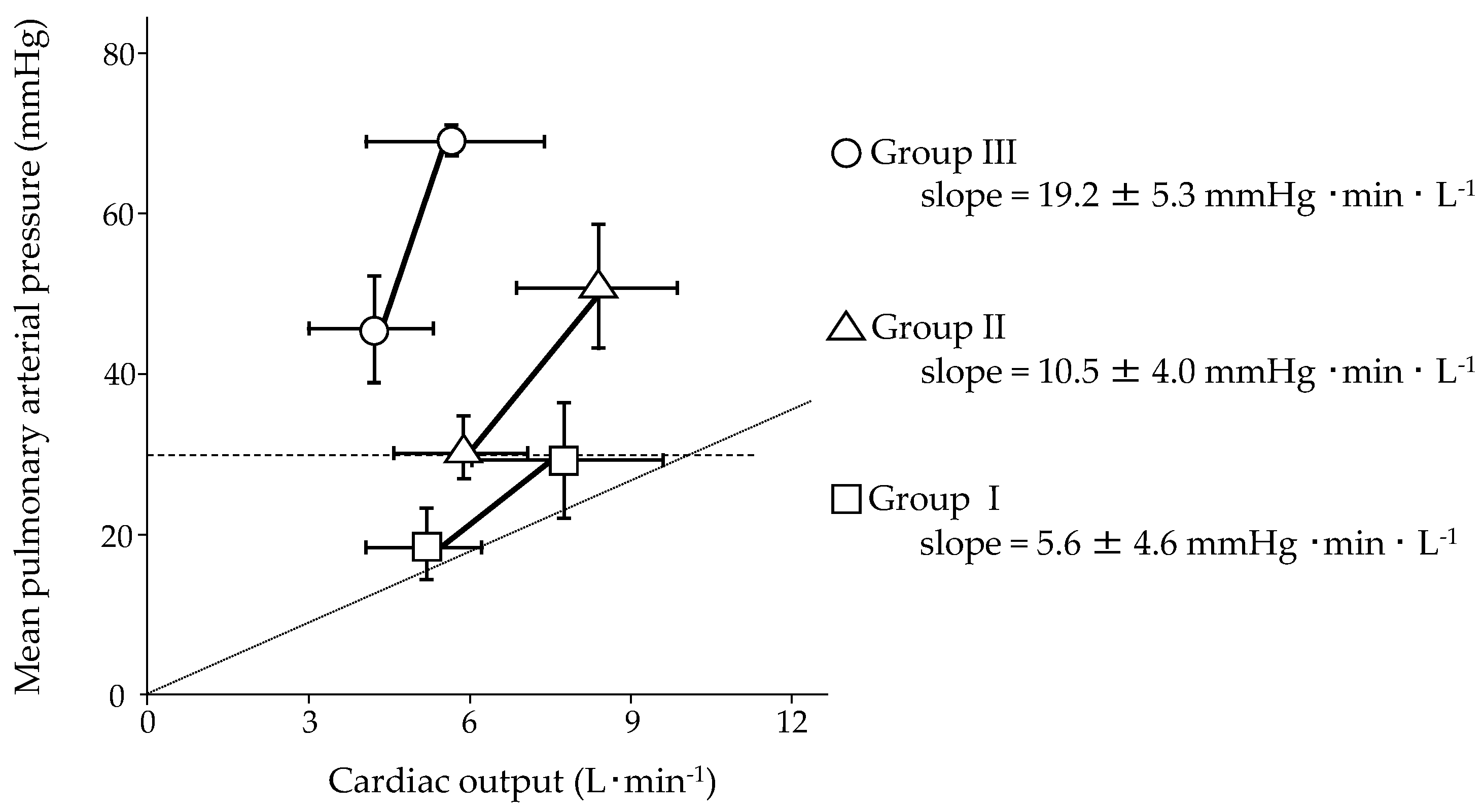
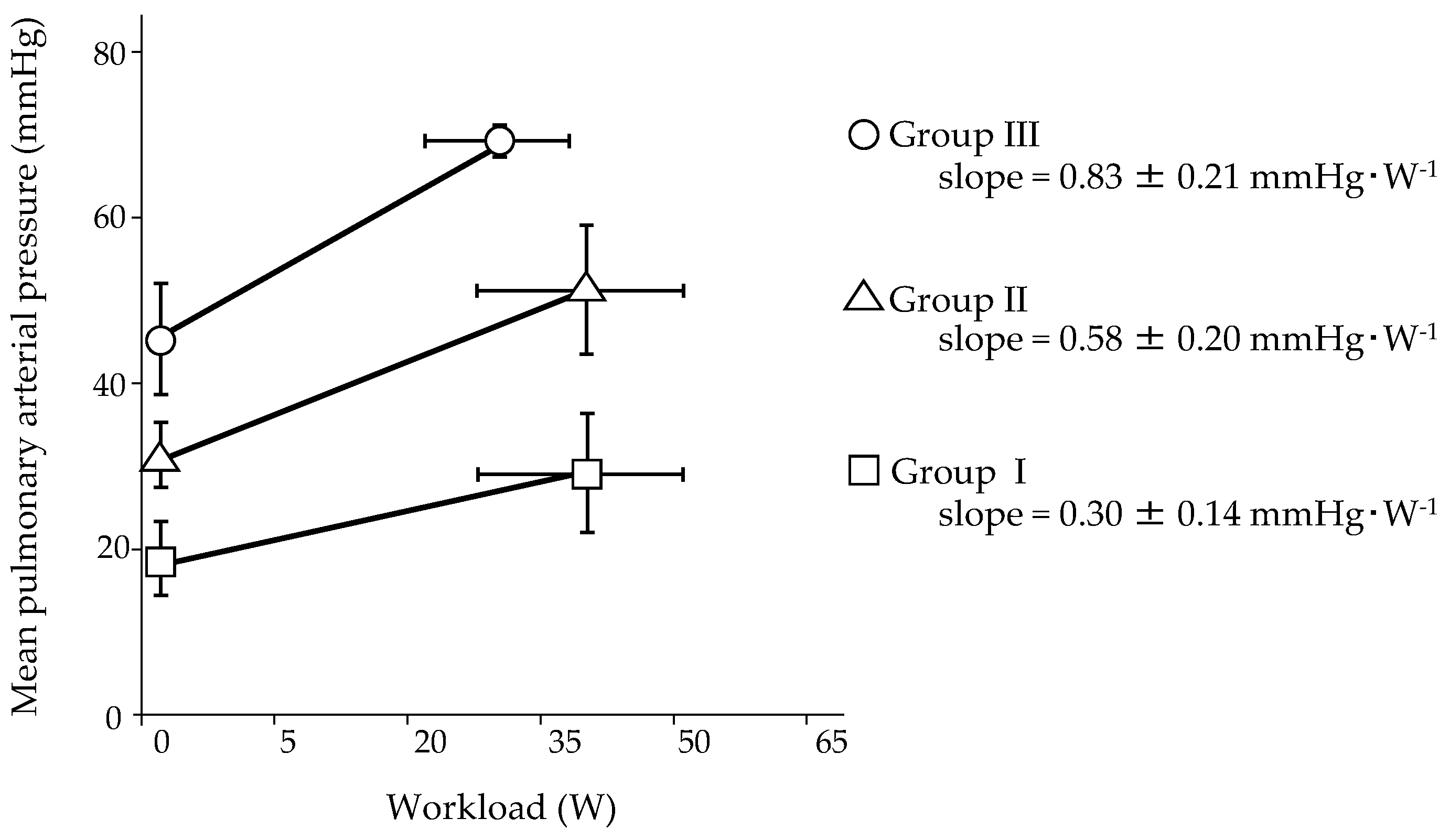
| First Author, Year [Ref.] | n | PAH, % | Age, y | WHO fc, n I/II/III/IV | Exercise Protocol | Workload, W | mPAP, mmHg | CO, L·min−1 (CI, L·min−1·m−2) | mPAP/CO Slope, mmHg·min·L−1 (mPAP/CI Slope, mmHg·min·L−1·m−2) | PAH-Specific Therapy | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest | Ex | Rest | Ex | |||||||||
| Tolle, 2008 [30] Lewis, 2013 [31] | 16 | 0 | 46 ± 15 | — | Cycle | 156 ± 43 | 14 ± 3 | 27 ± 4 | 5.8 ± 1.0 | 15.5 ± 3.2 | NA | — |
| Janicki, 1985 [41] | 9 | 56 | NA | NA | Treadmill | N/A | 43 ± 16 | 81 ± 16 | (1.9 ± 0.3) | (5.0 ± 1.5) | 13.4 ± 9.5 # | none |
| Blumberg, 2002 [42] | 16 | 63 | NA | 0/5/11/0 | 45°-Cycle | 25 or 50 | 45 ± 8 | 70 ± 13 | 3.7 ± 1.0 | 5.8 ± 2.4 | NA | none or aerosolized iloprost |
| Castelain, 2002 [43] | 7 | 100 | 46 ± 14 | 0/0/7/0 | Supine-Cycle | 0–60 | 52 ± 8 | NA | (2.6 ± 0.6) | NA | (13.1) ‡ | intravenous prostacyclin |
| Provencher, 2008 [44] | 42 | 100 | 48 ± 13 | 0/22/20/0 | Supine-Cycle | 0–40 | 52 ± 14 | 76 ± 17 | (2.9 ± 0.7) | (4.3 ± 1.3) | (21.5 ± 15.2) ‡ | ERA and/or intravenous prostacyclin |
| Chemla, 2013 [45] | 12 | 58 | 45 ± 14 | NA | Supine-Cycle | 0–60 | 57 ± 9 | 75 ± 10 | 4.4 ± 1.4 | 6.1 ± 2.1 | 11.7 ± 5.7 # | NA |
| Chaout, 2014 [46] | 55 | 100 | 54 ± 16 | 8(I/II)/32/15 | Supine-Cycle | 20 * | 52 ± 13 | 70 ± 17 | (2.0 ± 0.6) | (2.8 ± 1.1) | NA | NA |
| Hasler, 2016 [47] | 70 | 77 | 65 * | 20(I/II)/35/15 | Supine-Cycle | 30 * | 34 * | 55 * | 5.2 * (2.8 *) | 6.4 * (3.5 *) | 14.2 *,# 17.0 *,## | dual: 31%, triple: 4% |
| Ehlken, 2016 [48] | 41 | 63 | 57 ± 15 | 1/6/30/4 | Supine-Cycle | 72 ± 23 | 38 ± 12 | 58 ± 18 | 5.1 ± 1.8 (2.7 ± 0.9) | 9.1 ± 2.3 (4.7 ± 1.1) | NA | mono: 35%, dual: 55%, triple: 10% |
| Nishizaki, 2020 [9] | 32 | 100 | 33 ± 10 | 7/25/0/0 | 40°-Cycle | 38 ± 11 | 28 ± 11 | 46 ± 17 | (3.7 ± 0.9) | (5.4 ± 1.2) | 10.0 ± 6.7 ## | dual: 41%, triple: 59% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishizaki, M.; Ogawa, A.; Matsubara, H. High Right Ventricular Afterload during Exercise in Patients with Pulmonary Arterial Hypertension. J. Clin. Med. 2021, 10, 2024. https://doi.org/10.3390/jcm10092024
Nishizaki M, Ogawa A, Matsubara H. High Right Ventricular Afterload during Exercise in Patients with Pulmonary Arterial Hypertension. Journal of Clinical Medicine. 2021; 10(9):2024. https://doi.org/10.3390/jcm10092024
Chicago/Turabian StyleNishizaki, Mari, Aiko Ogawa, and Hiromi Matsubara. 2021. "High Right Ventricular Afterload during Exercise in Patients with Pulmonary Arterial Hypertension" Journal of Clinical Medicine 10, no. 9: 2024. https://doi.org/10.3390/jcm10092024






