Glycation and Glycosylation in Cardiovascular Remodeling: Focus on Advanced Glycation End Products and O-Linked Glycosylations as Glucose-Related Pathogenetic Factors and Disease Markers
Abstract
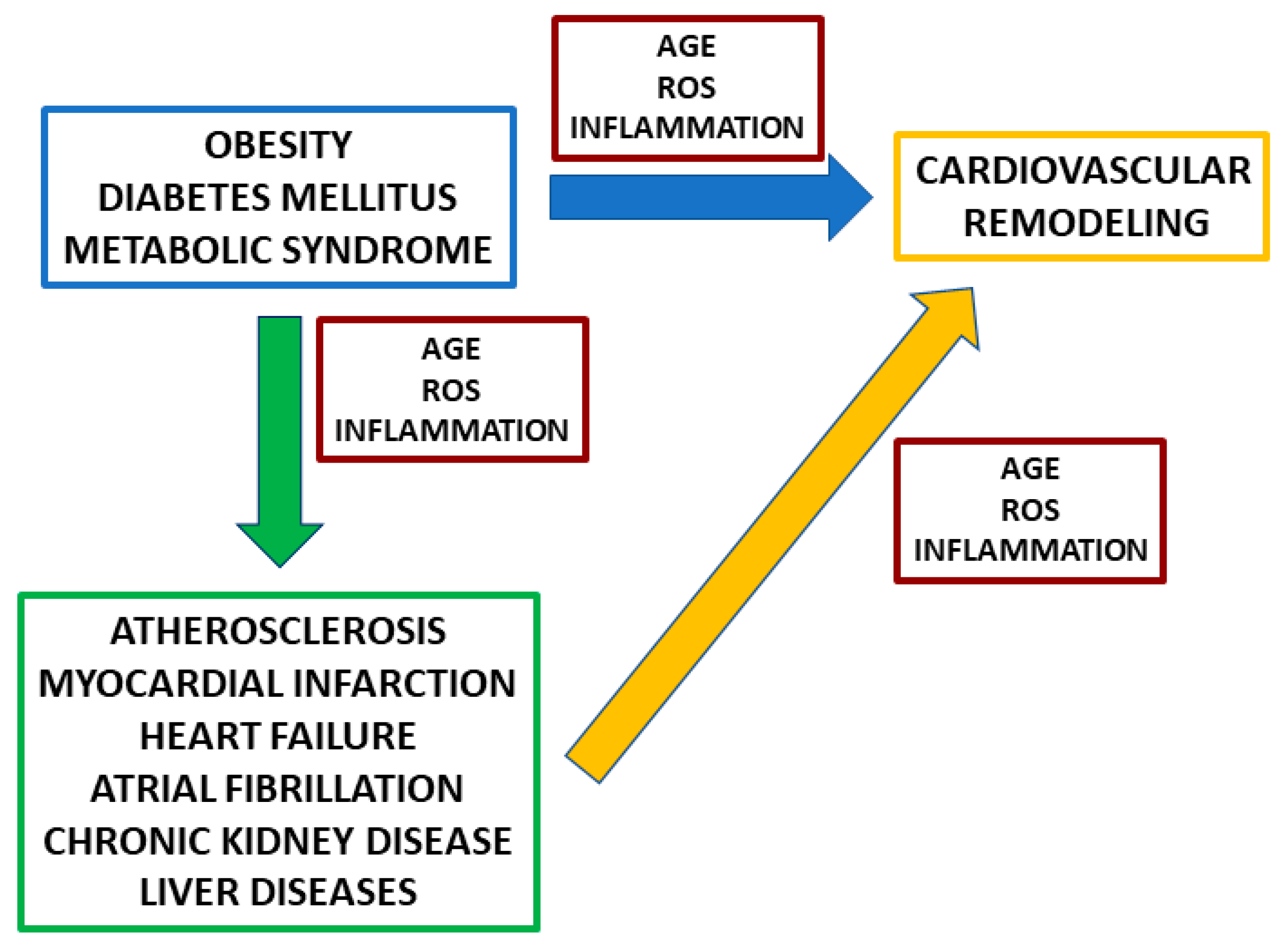
:1. Introduction
2. Advanced Glycation End Products (AGE) and Soluble Receptor for Advanced Glycation End Products (RAGE)
2.1. AGE–RAGE Pathway in Cardiac Remodeling in Different Diseases
2.1.1. AGE, RAGE, and Cardiac Remodeling in Diabetes Mellitus
2.1.2. AGE, RAGE, and Cardiac Remodeling in Chronic Kidney Disease (CKD)
2.1.3. AGE, RAGE, and Cardiac Remodeling after Myocardial Infarction
2.1.4. AGE, RAGE, and Atrial Fibrillation (AF)
2.1.5. AGE, RAGE, and Vascular Remodeling
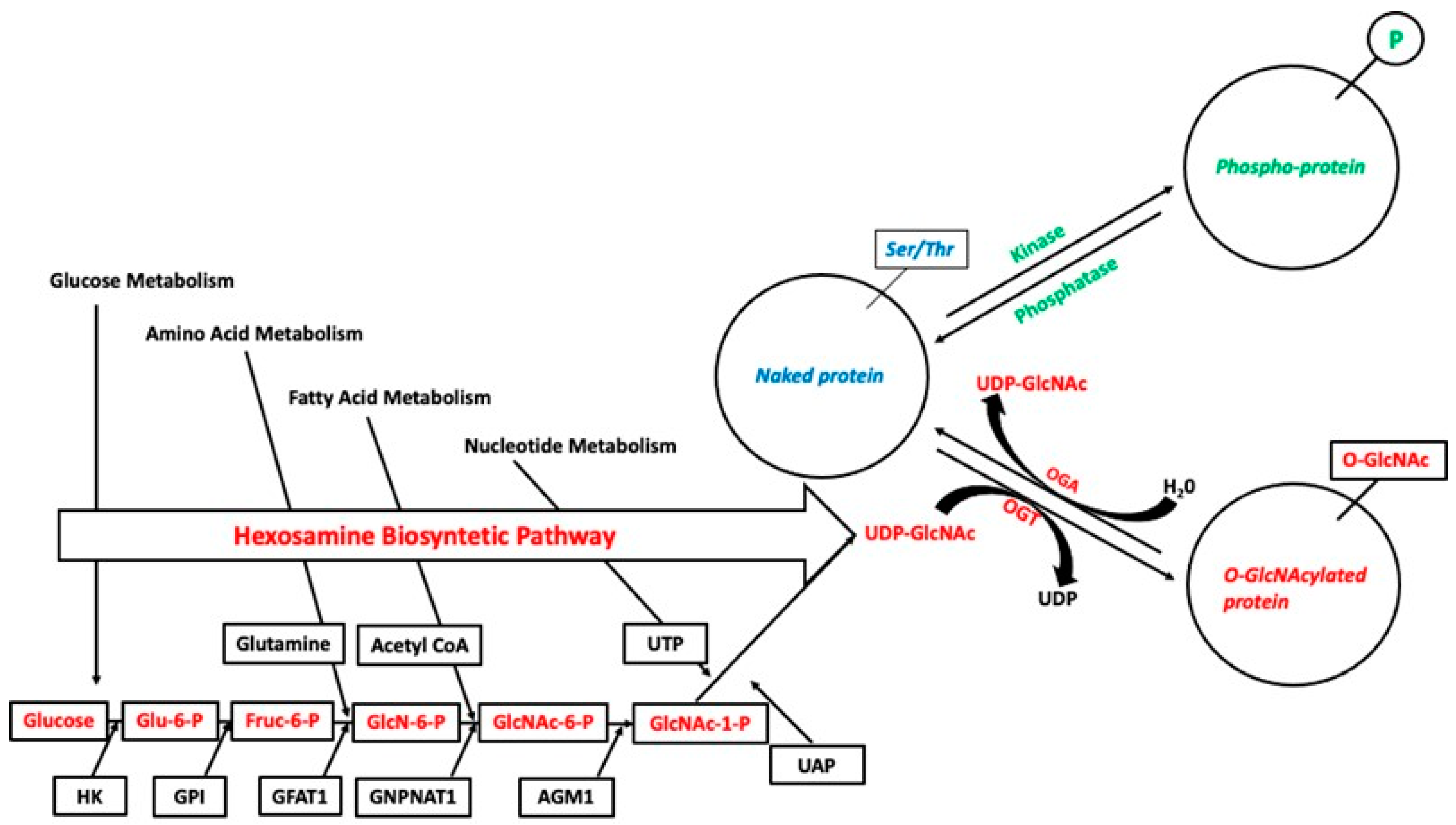
3. O-Linked Glycosylation
3.1. O-Linked Glycosylation in Cardiovascular Remodeling in Different Diseases
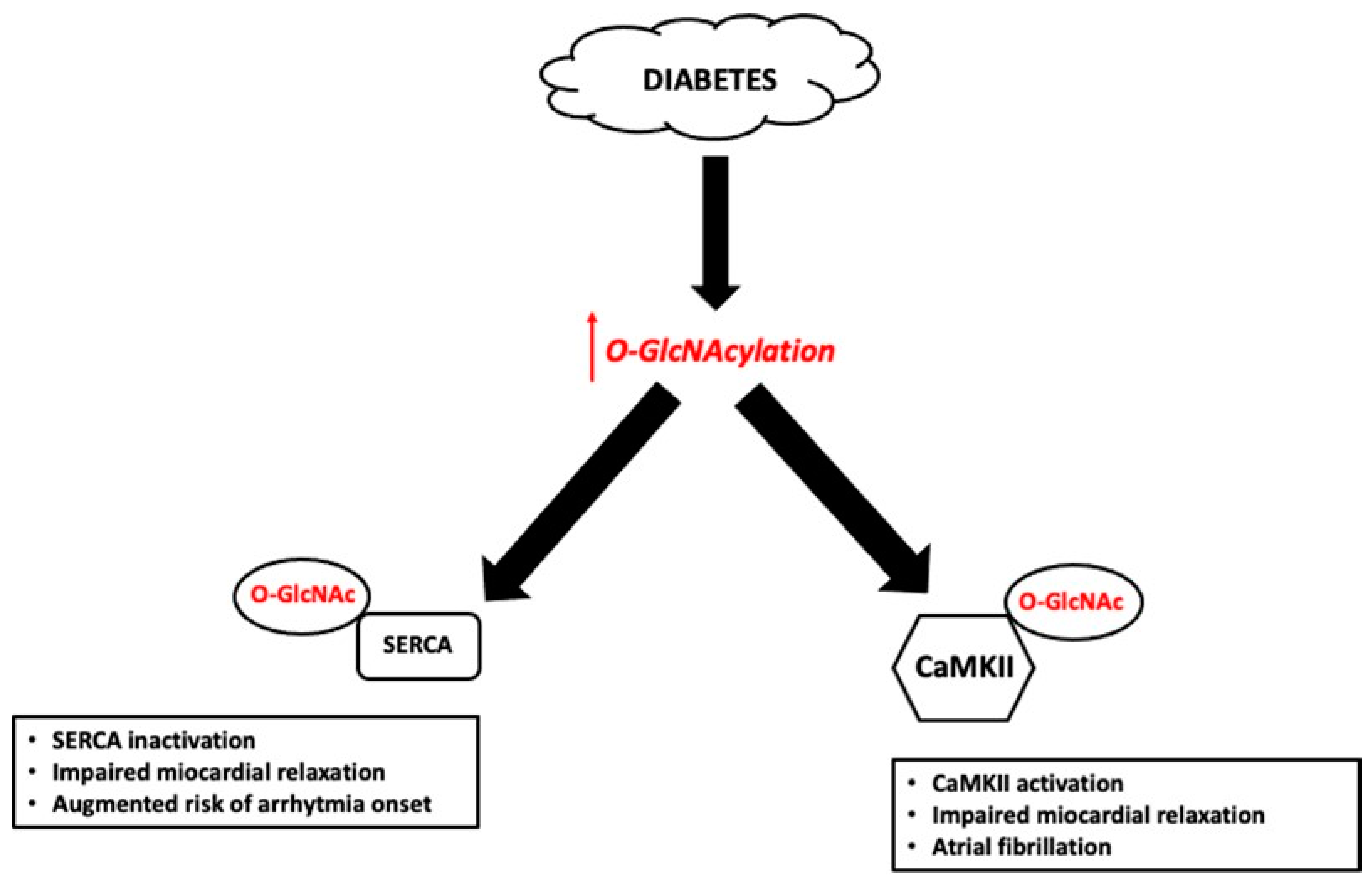
3.1.1. Effect of Hyperglycemia on O-GlcNAcylation in the Heart
3.1.2. O-GlcNAcylation and Ischemia-Reperfusion (I/R) Injury
3.1.3. O-GlcNAcylation and Cardiac Remodeling
3.1.4. O-GlcNAcylation and Atrial Fibrillation (AF)
3.1.5. O-GlcNAcylation and Vascular Remodeling
3.1.6. O-GlcNAcylation and Remodeling in CKD
4. Which Link between AGE-RAGE and O-Linked Glycosylation in Cardiovascular Remodeling?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.J.; Roman, G.; Yeboah, F.; Konishi, Y. The road to advanced glycation end products: A mechanistic perspective. Curr. Med. Chem. 2007, 14, 1653–1671. [Google Scholar] [CrossRef]
- Dozio, E.; Di Gaetano, N.; Findeisen, P.; Corsi Romanelli, M.M. Glycated albumin: From biochemistry and laboratory medicine to clinical practice. Endocrine 2017, 55, 682–690. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Pu, Q.; Yu, C. Glycosyltransferases, glycosylation and atherosclerosis. Glycoconj. J. 2014, 31, 605–611. [Google Scholar] [CrossRef]
- Deluyker, D.; Evens, L.; Bito, V. Advanced glycation end products (AGEs) and cardiovascular dysfunction: Focus on high molecular weight AGEs. Amino. Acids 2017, 49, 1535–1541. [Google Scholar] [CrossRef]
- Chen, P.H.; Chi, J.T.; Boyce, M. Functional crosstalk among oxidative stress and O-GlcNAc signaling pathways. Glycobiology 2018, 28, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chatterjee, S.; Cipollo, J. The Glycoproteomics-MS for Studying Glycosylation in Cardiac Hypertrophy and Heart Failure. Proteom. Clin. Appl. 2018, 12, e1700075. [Google Scholar] [CrossRef]
- Rojas, A.; Mercadal, E.; Figueroa, H.; Morales, M.A. Advanced Glycation and ROS: A link between diabetes and heart failure. Curr. Vasc. Pharmacol. 2008, 6, 44–51. [Google Scholar] [CrossRef]
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.; Zornoff, L.A. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq. Bras Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Hohn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Dietary AGEs and ALEs and risk to human health by their interaction with the receptor for advanced glycation endproducts (RAGE)--an introduction. Mol. Nutr. Food Res. 2007, 51, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Lam, J.K.; Shiu, S.W.; Wong, Y.; Betteridge, D.J.; Tan, K.C. Serum Level of Soluble Receptor for Advanced Glycation End Products Is Associated with A Disintegrin And Metalloproteinase 10 in Type 1 Diabetes. PLoS ONE 2015, 10, e0137330. [Google Scholar] [CrossRef] [Green Version]
- Katakami, N.; Matsuhisa, M.; Kaneto, H.; Matsuoka, T.A.; Sakamoto, K.; Nakatani, Y.; Ohtoshi, K.; Hayaishi-Okano, R.; Kosugi, K.; Hori, M.; et al. Decreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: Its possible association with diabetic vascular complications. Diabetes Care 2005, 28, 2716–2721. [Google Scholar] [CrossRef] [Green Version]
- Koyama, H.; Yamamoto, H.; Nishizawa, Y. Endogenous Secretory RAGE as a Novel Biomarker for Metabolic Syndrome and Cardiovascular Diseases. Biomark Insights 2007, 2, 331–339. [Google Scholar] [CrossRef]
- Koyama, H.; Yamamoto, H.; Nishizawa, Y. RAGE and soluble RAGE: Potential therapeutic targets for cardiovascular diseases. Mol. Med. 2007, 13, 625–635. [Google Scholar] [CrossRef]
- Vazzana, N.; Santilli, F.; Cuccurullo, C.; Davi, G. Soluble forms of RAGE in internal medicine. Intern. Emerg. Med. 2009, 4, 389–401. [Google Scholar] [CrossRef]
- Zhao, J.; Randive, R.; Stewart, J.A. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J. Diabetes 2014, 5, 860–867. [Google Scholar] [CrossRef]
- Zhang, X.; Stewart, J.A., Jr.; Kane, I.D.; Massey, E.P.; Cashatt, D.O.; Carver, W.E. Effects of elevated glucose levels on interactions of cardiac fibroblasts with the extracellular matrix. In Vitro Cell Dev. Biol. Anim. 2007, 43, 297–305. [Google Scholar] [CrossRef]
- Fowlkes, V.; Clark, J.; Fix, C.; Law, B.A.; Morales, M.O.; Qiao, X.; Ako-Asare, K.; Goldsmith, J.G.; Carver, W.; Murray, D.B.; et al. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts. Life Sci. 2013, 92, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flack, E.C.; Lindsey, M.L.; Squires, C.E.; Kaplan, B.S.; Stroud, R.E.; Clark, L.L.; Escobar, P.G.; Yarbrough, W.M.; Spinale, F.G. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J. Mol. Cell Cardiol. 2006, 40, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansode, S.B.; Gacche, R.N. Glycation-induced modification of tissue-specific ECM proteins: A pathophysiological mechanism in degenerative diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129411. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Wang, D.; Agarwal, R.; Dillender, S.; Ferrell, N. Glycation alters the mechanical behavior of kidney extracellular matrix. Matrix Biol. Plus 2020, 8, 100035. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef]
- Hutchinson, K.R.; Lord, C.K.; West, T.A.; Stewart, J.A., Jr. Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS ONE 2013, 8, e72080. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.; Zhang, X.; Leng, W.; Lei, X.; Chen, L.; Zhou, X.; Chow, K.; Shi, Y.; Dong, J.; Liang, Z.; et al. Increased fractal dimension of left ventricular trabeculations is associated with subclinical diastolic dysfunction in patients with type-2 diabetes mellitus. Int. J. Cardiovasc. Imaging 2019, 35, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Burr, S.D.; Stewart, J.A., Jr. Extracellular matrix components isolated from diabetic mice alter cardiac fibroblast function through the AGE/RAGE signaling cascade. Life Sci. 2020, 250, 117569. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Sigmon, V.K.; Babcock, S.A.; Ren, J. Advanced glycation endproduct induces ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes. Life Sci. 2007, 80, 1051–1056. [Google Scholar] [CrossRef]
- Kato, T.; Yamashita, T.; Sekiguchi, A.; Tsuneda, T.; Sagara, K.; Takamura, M.; Kaneko, S.; Aizawa, T.; Fu, L.T. AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat. J. Cardiovasc. Electrophysiol. 2008, 19, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, U.; Nagarajan, D. All-Trans Retinoic Acid supplementation prevents cardiac fibrosis and cytokines induced by Methylglyoxal. Glycoconj. J. 2017, 34, 255–265. [Google Scholar] [CrossRef]
- Umadevi, S.; Gopi, V.; Elangovan, V. Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chem. Biol. Interact. 2014, 208, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Leonardis, D.; Basta, G.; Mallamaci, F.; Cutrupi, S.; Pizzini, P.; Tripepi, R.; Tripepi, G.; De Caterina, R.; Zoccali, C. Circulating soluble receptor for advanced glycation end product (sRAGE) and left ventricular hypertrophy in patients with chronic kidney disease (CKD). Nutr. Metab. Cardiovasc. Dis. 2012, 22, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Boschetto, P.; Campo, I.; Stendardo, M.; Casimirri, E.; Tinelli, C.; Gorrini, M.; Ceconi, C.; Fucili, A.; Potena, A.; Papi, A.; et al. Plasma sRAGE and N-(carboxymethyl) lysine in patients with CHF and/or COPD. Eur. J. Clin. Investig. 2013, 43, 562–569. [Google Scholar] [CrossRef]
- Dozio, E.; Briganti, S.; Delnevo, A.; Vianello, E.; Ermetici, F.; Secchi, F.; Sardanelli, F.; Morricone, L.; Malavazos, A.E.; Corsi Romanelli, M.M. Relationship between soluble receptor for advanced glycation end products (sRAGE), body composition and fat distribution in healthy women. Eur. J. Nutr. 2016, 56, 2557–2564. [Google Scholar] [CrossRef] [Green Version]
- Wannamethee, S.G.; Welsh, P.; Papacosta, O.; Ellins, E.A.; Halcox, J.P.J.; Whincup, P.H.; Sattar, N. Circulating soluble receptor for advanced glycation end product: Cross-sectional associations with cardiac markers and subclinical vascular disease in older men with and without diabetes. Atherosclerosis 2017, 264, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Dozio, E.; Ambrogi, F.; de Cal, M.; Vianello, E.; Ronco, C.; Corsi Romanelli, M.M. Role of the Soluble Receptor for Advanced Glycation End Products (sRAGE) as a Prognostic Factor for Mortality in Hemodialysis and Peritoneal Dialysis Patients. Mediat. Inflamm. 2018, 2018, 1347432. [Google Scholar] [CrossRef] [Green Version]
- Dozio, E.; Vianello, E.; Bandera, F.; Longhi, E.; Brizzola, S.; Nebuloni, M.; Corsi Romanelli, M.M. Soluble Receptor for Advanced Glycation End Products: A Protective Molecule against Intramyocardial Lipid Accumulation in Obese Zucker Rats? Mediat. Inflamm. 2019, 2019, 2712376. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Yamagishi, S.; Adachi, H.; Kurita-Nakamura, Y.; Matsui, T.; Yoshida, T.; Imaizumi, T. Serum levels of sRAGE, the soluble form of receptor for advanced glycation end products, are associated with inflammatory markers in patients with type 2 diabetes. Mol. Med. 2007, 13, 185–189. [Google Scholar] [CrossRef]
- Dozio, E.; Corradi, V.; Vianello, E.; Scalzotto, E.; de Cal, M.; Corsi Romanelli, M.M.; Ronco, C. Increased Levels of sRAGE in Diabetic CKD-G5D Patients: A Potential Protective Mechanism against AGE-Related Upregulation of Fibroblast Growth Factor 23 and Inflammation. Mediat. Inflamm. 2017, 2017, 9845175. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Foley, R.N.; Chavers, B.; Gilbertson, D.; Herzog, C.; Johansen, K.; Kasiske, B.; Kutner, N.; Liu, J.; St Peter, W.; et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am. J. Kidney Dis. 2012, 59, A7, e1-420. [Google Scholar] [CrossRef]
- Bucala, R.; Vlassara, H. Advanced glycosylation end products in diabetic renal and vascular disease. Am. J. Kidney Dis. 1995, 26, 875–888. [Google Scholar] [CrossRef]
- Tezuka, Y.; Nakaya, I.; Nakayama, K.; Nakayama, M.; Yahata, M.; Soma, J. Methylglyoxal as a prognostic factor in patients with chronic kidney disease. Nephrology (Carlton) 2019, 24, 943–950. [Google Scholar] [CrossRef]
- Alpert, M.A. Cardiac performance and morphology in end-stage renal disease. Am. J. Med. Sci. 2003, 325, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Willemsen, S.; Hartog, J.W.; Heiner-Fokkema, M.R.; van Veldhuisen, D.J.; Voors, A.A. Advanced glycation end-products, a pathophysiological pathway in the cardiorenal syndrome. Heart Fail. Rev. 2012, 17, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Bowman, M.A. Chronic sustained inflammation links to left ventricular hypertrophy and aortic valve sclerosis: A new link between S100/RAGE and FGF23. Inflamm. Cell Signal. 2014, 1, e279. [Google Scholar] [CrossRef]
- Bar, L.; Wachter, K.; Wege, N.; Navarrete Santos, A.; Simm, A.; Foller, M. Advanced glycation end products stimulate gene expression of fibroblast growth factor 23. Mol. Nutr. Food Res. 2017, 61, 1601019. [Google Scholar] [CrossRef]
- Park, S.H.; Stenvinkel, P.; Lindholm, B. Cardiovascular biomarkers in chronic kidney disease. J. Ren. Nutr. 2012, 22, 120–127. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Asahia, K.; Benedetto, F.A.; Tripepi, G.; Tripepi, R.; Nicocia, G.; Buemi, M.; Miyata, T. Pentosidine, carotid atherosclerosis and alterations in left ventricular geometry in hemodialysis patients. J. Nephrol. 2001, 14, 293–298. [Google Scholar]
- Koyama, Y.; Takeishi, Y.; Niizeki, T.; Suzuki, S.; Kitahara, T.; Sasaki, T.; Kubota, I. Soluble Receptor for advanced glycation end products (RAGE) is a prognostic factor for heart failure. J. Card Fail. 2008, 14, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Lazo, M.; Halushka, M.K.; Shen, L.; Maruthur, N.; Rebholz, C.M.; Rawlings, A.M.; Hoogeveen, R.C.; Brinkley, T.E.; Ballantyne, C.M.; Astor, B.C.; et al. Soluble receptor for advanced glycation end products and the risk for incident heart failure: The Atherosclerosis Risk in Communities Study. Am. Heart J. 2015, 170, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Arch. Intern. Med. 2017, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling--concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Basta, G.; Lazzerini, G.; Massaro, M.; Simoncini, T.; Tanganelli, P.; Fu, C.; Kislinger, T.; Stern, D.M.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: A mechanism for amplification of inflammatory responses. Circulation 2002, 105, 816–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, N.J.R.; Vulesevic, B.; McNeill, B.; Cimenci, C.E.; Ahmadi, A.; Gonzalez-Gomez, M.; Ostojic, A.; Zhong, Z.; Brownlee, M.; Beisswenger, P.J.; et al. Methylglyoxal-derived advanced glycation end products contribute to negative cardiac remodeling and dysfunction post-myocardial infarction. Basic Res. Cardiol. 2017, 112, 57. [Google Scholar] [CrossRef]
- Daoud, S.; Schinzel, R.; Neumann, A.; Loske, C.; Fraccarollo, D.; Diez, C.; Simm, A. Advanced glycation endproducts: Activators of cardiac remodeling in primary fibroblasts from adult rat hearts. Mol. Med. 2001, 7, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Y.; Lu, L.; Wang, Y.N.; Jin, C.; Zhang, R.Y.; Zhang, Q.; Chen, Q.J.; Shen, W.F. Association of increased S100B, S100A6 and S100P in serum levels with acute coronary syndrome and also with the severity of myocardial infarction in cardiac tissue of rat models with ischemia-reperfusion injury. Atherosclerosis 2011, 217, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, Q.; Xu, Y.; Zhu, Z.B.; Geng, L.; Wang, L.J.; Jin, C.; Chen, Q.J.; Schmidt, A.M.; Shen, W.F. Intra-coronary administration of soluble receptor for advanced glycation end-products attenuates cardiac remodeling with decreased myocardial transforming growth factor-beta1 expression and fibrosis in minipigs with ischemia-reperfusion injury. Chin. Med. J. (Engl.) 2010, 123, 594–598. [Google Scholar] [PubMed]
- Hong, J.; Ku, S.H.; Lee, M.S.; Jeong, J.H.; Mok, H.; Choi, D.; Kim, S.H. Cardiac RNAi therapy using RAGE siRNA/deoxycholic acid-modified polyethylenimine complexes for myocardial infarction. Biomaterials 2014, 35, 7562–7573. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, B.M.; Rangel, J.O.; Machado, A.G.; Curuja, F.S.; Lopes, A.; Olsen, V.; Clausell, N.; Biolo, A.; Rohde, L.E.; Andrades, M. Characterization of advanced glycation end products and their receptor (RAGE) in an animal model of myocardial infarction. PLoS ONE 2019, 14, e0209964. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Sung, P.H.; Chang, L.T.; Sun, C.K.; Yeh, K.H.; Chung, S.Y.; Chua, S.; Chen, Y.L.; Wu, C.J.; Chang, H.W.; et al. Value and level of galectin-3 in acute myocardial infarction patients undergoing primary percutaneous coronary intervention. J. Atheroscler Thromb. 2012, 19, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Weir, R.A.; Petrie, C.J.; Murphy, C.A.; Clements, S.; Steedman, T.; Miller, A.M.; McInnes, I.B.; Squire, I.B.; Ng, L.L.; Dargie, H.J.; et al. Galectin-3 and cardiac function in survivors of acute myocardial infarction. Circ. Heart Fail. 2013, 6, 492–498. [Google Scholar] [CrossRef] [Green Version]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; Andre, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, L.Y.; Wu, Y.W.; Chien, K.L.; Lee, C.M.; Hsu, R.B.; Chao, C.L.; Wang, S.S.; Hsein, Y.C.; Liao, L.C.; et al. The relationship between serum galectin-3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin. Chim. Acta 2009, 409, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Pricci, F.; Leto, G.; Amadio, L.; Iacobini, C.; Romeo, G.; Cordone, S.; Gradini, R.; Barsotti, P.; Liu, F.T.; Di Mario, U.; et al. Role of galectin-3 as a receptor for advanced glycosylation end products. Kidney Int. Suppl. 2000, 77, S31–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volz, H.C.; Kaya, Z.; Katus, H.A.; Andrassy, M. The role of HMGB1/RAGE in inflammatory cardiomyopathy. Semin. Thromb. Hemost 2010, 36, 185–194. [Google Scholar] [CrossRef]
- Volz, H.C.; Seidel, C.; Laohachewin, D.; Kaya, Z.; Muller, O.J.; Pleger, S.T.; Lasitschka, F.; Bianchi, M.E.; Remppis, A.; Bierhaus, A.; et al. HMGB1: The missing link between diabetes mellitus and heart failure. Basic Res. Cardiol. 2010, 105, 805–820. [Google Scholar] [CrossRef]
- Toyota, E.; Warltier, D.C.; Brock, T.; Ritman, E.; Kolz, C.; O’Malley, P.; Rocic, P.; Focardi, M.; Chilian, W.M. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation 2005, 112, 2108–2113. [Google Scholar] [CrossRef] [Green Version]
- Rehn, T.A.; Borge, B.A.; Lunde, P.K.; Munkvik, M.; Sneve, M.L.; Grondahl, F.; Aronsen, J.M.; Sjaastad, I.; Prydz, K.; Kolset, S.O.; et al. Temporary fatigue and altered extracellular matrix in skeletal muscle during progression of heart failure in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R26–R33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Rana, J.S.; Wykrzykowska, J.; Du, Z.; Ke, Q.; Kang, P.; Li, J.; Laham, R.J. Exercise-induced expression of VEGF and salvation of myocardium in the early stage of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H389–H395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoporis, J.N.; Izhar, S.; Leong-Poi, H.; Desjardins, J.F.; Huttunen, H.J.; Parker, T.G. S100B interaction with the receptor for advanced glycation end products (RAGE): A novel receptor-mediated mechanism for myocyte apoptosis postinfarction. Circ. Res. 2010, 106, 93–101. [Google Scholar] [CrossRef]
- Tsoporis, J.N.; Izhar, S.; Proteau, G.; Slaughter, G.; Parker, T.G. S100B-RAGE dependent VEGF secretion by cardiac myocytes induces myofibroblast proliferation. J. Mol. Cell Cardiol. 2012, 52, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Raposeiras-Roubin, S.; Rodino-Janeiro, B.K.; Paradela-Dobarro, B.; Grigorian-Shamagian, L.; Garcia-Acuna, J.M.; Aguiar-Souto, P.; Jacquet-Hervet, M.; Reino-Maceiras, M.V.; Alvarez, E.; Gonzalez-Juanatey, J.R. Predictive value of advanced glycation end products for the development of post-infarction heart failure: A preliminary report. Cardiovasc. Diabetol. 2012, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Redondo, A.; Paradela-Dobarro, B.; Moscoso, I.; Moure-Alvarez, M.; Cebro-Marquez, M.; Gonzalez-Juanatey, J.R.; Garcia-Seara, J.; Alvarez, E. Galectin-3 and soluble RAGE as new biomarkers of post-infarction cardiac remodeling. J. Mol. Med. 2021, 99, 943–953. [Google Scholar] [CrossRef]
- Burstein, B.; Nattel, S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef] [Green Version]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ. Arrhythm. Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boos, C.J.; Lip, G.Y. Inflammation and atrial fibrillation: Cause or effect? Heart 2008, 94, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Hayashi, H.; Kawase, A.; Lin, S.F.; Li, H.; Weiss, J.N.; Chen, P.S.; Karagueuzian, H.S. Spontaneous atrial fibrillation initiated by triggered activity near the pulmonary veins in aged rats subjected to glycolytic inhibition. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H639–H648. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.P.; Sims, S.M.; Cook, M.A.; Karmazyn, M. Hydrogen peroxide-induced stimulation of L-type calcium current in guinea pig ventricular myocytes and its inhibition by adenosine A1 receptor activation. J. Pharmacol. Exp. Ther. 1998, 286, 1208–1214. [Google Scholar] [PubMed]
- Song, Y.; Shryock, J.C.; Wagner, S.; Maier, L.S.; Belardinelli, L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J. Pharmacol. Exp. Ther. 2006, 318, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Saba, S.; Janczewski, A.M.; Baker, L.C.; Shusterman, V.; Gursoy, E.C.; Feldman, A.M.; Salama, G.; McTiernan, C.F.; London, B. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-{alpha}. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1456–H1467. [Google Scholar] [CrossRef] [Green Version]
- Raposeiras-Roubin, S.; Rodino-Janeiro, B.K.; Grigorian-Shamagian, L.; Seoane-Blanco, A.; Moure-Gonzalez, M.; Varela-Roman, A.; Alvarez, E.; Gonzalez-Juanatey, J.R. Evidence for a role of advanced glycation end products in atrial fibrillation. Int. J. Cardiol. 2012, 157, 397–402. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Xu, Y. Decreased serum endogenous secretory receptor for advanced glycation endproducts and increased cleaved receptor for advanced glycation endproducts levels in patients with atrial fibrillation. Int. J. Cardiol. 2012, 158, 471–472. [Google Scholar] [CrossRef]
- Yan, X.; Shen, Y.; Lu, L.; Sano, M.; Fukuda, K.; Shen, W. Decreased endogenous secretory RAGE and increased hsCRP levels in serum are associated with atrial fibrillation in patients undergoing coronary angiography. Int. J. Cardiol. 2013, 166, 242–245. [Google Scholar] [CrossRef]
- Lancefield, T.F.; Patel, S.K.; Freeman, M.; Velkoska, E.; Wai, B.; Srivastava, P.M.; Horrigan, M.; Farouque, O.; Burrell, L.M. The Receptor for Advanced Glycation End Products (RAGE) Is Associated with Persistent Atrial Fibrillation. PLoS ONE 2016, 11, e0161715. [Google Scholar] [CrossRef] [Green Version]
- Al Rifai, M.; Schneider, A.L.; Alonso, A.; Maruthur, N.; Parrinello, C.M.; Astor, B.C.; Hoogeveen, R.C.; Soliman, E.Z.; Chen, L.Y.; Ballantyne, C.M.; et al. sRAGE, inflammation, and risk of atrial fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) Study. J. Diabetes Complicat. 2015, 29, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.S.; Kim, T.H.; Uhm, J.S.; Park, S.; Joung, B.; Lee, M.H.; Pak, H.N. High plasma level of soluble RAGE is independently associated with a low recurrence of atrial fibrillation after catheter ablation in diabetic patient. Europace 2016, 18, 1711–1718. [Google Scholar] [CrossRef]
- Begieneman, M.P.; Rijvers, L.; Kubat, B.; Paulus, W.J.; Vonk, A.B.; van Rossum, A.C.; Schalkwijk, C.G.; Stooker, W.; Niessen, H.W.; Krijnen, P.A. Atrial fibrillation coincides with the advanced glycation end product N(epsilon)-(carboxymethyl)lysine in the atrium. Am. J. Pathol. 2015, 185, 2096–2104. [Google Scholar] [CrossRef] [Green Version]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.H.; Son, W.R.; Lee, Y.S.; Lee, K.W. Glycolaldehyde-derived advanced glycation end products (glycol-AGEs)-induced vascular smooth muscle cell dysfunction is regulated by the AGES-receptor (RAGE) axis in endothelium. Cell Commun. Adhes. 2015, 22, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, F.; Leonov, S.; Howard, A.C.; Xiong, S.; Zhang, B.; Mei, L.; McNeil, P.; Simon, S.; Xiong, W.C. Receptor for advanced glycation end products (RAGE) prevents endothelial cell membrane resealing and regulates F-actin remodeling in a beta-catenin-dependent manner. J. Biol. Chem. 2011, 286, 35061–35070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vessieres, E.; Freidja, M.L.; Loufrani, L.; Fassot, C.; Henrion, D. Flow (shear stress)-mediated remodeling of resistance arteries in diabetes. Vasc. Pharmacol. 2012, 57, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Uekita, H.; Ishibashi, T.; Shiomi, M.; Koyama, H.; Ohtsuka, S.; Yamamoto, H.; Yamagishi, S.; Inoue, H.; Itabe, H.; Sugimoto, K.; et al. Integral role of receptor for advanced glycation end products (RAGE) in nondiabetic atherosclerosis. Fukushima J. Med. Sci. 2019, 65, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Du, R.; Zhang, R.Y.; Lu, L.; Shen, Y.; Pu, L.J.; Zhu, Z.B.; Zhang, Q.; Hu, J.; Yang, Z.K.; Ding, F.H.; et al. Increased glycated albumin and decreased esRAGE levels in serum are related to negative coronary artery remodeling in patients with type 2 diabetes: An Intravascular ultrasound study. Cardiovasc. Diabetol. 2018, 17, 149. [Google Scholar] [CrossRef] [Green Version]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Kass, D.A.; Shapiro, E.P.; Kawaguchi, M.; Capriotti, A.R.; Scuteri, A.; deGroof, R.C.; Lakatta, E.G. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 2001, 104, 1464–1470. [Google Scholar] [CrossRef] [Green Version]
- Tada, Y.; Yano, S.; Yamaguchi, T.; Okazaki, K.; Ogawa, N.; Morita, M.; Sugimoto, T. Advanced glycation end products-induced vascular calcification is mediated by oxidative stress: Functional roles of NAD(P)H-oxidase. Horm. Metab. Res. 2013, 45, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, M.R.; Bouvet, C.; Bouchard, S.; Moreau, S.; Leblond, J.; Deblois, D.; Moreau, P. Reduction of advanced-glycation end products levels and inhibition of RAGE signaling decreases rat vascular calcification induced by diabetes. PLoS ONE 2014, 9, e85922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Ren, X.; Jiang, Y.; Jin, H.; Liu, N.; Li, J. Advanced glycation end products accelerate rat vascular calcification through RAGE/oxidative stress. BMC Cardiovasc. Disord. 2013, 13, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.; Lafeber, F.P.; Baynes, J.W.; TeKoppele, J.M. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konova, E.; Baydanoff, S.; Atanasova, M.; Velkova, A. Age-related changes in the glycation of human aortic elastin. Exp. Gerontol. 2004, 39, 249–254. [Google Scholar] [CrossRef]
- Ren, X.; Shao, H.; Wei, Q.; Sun, Z.; Liu, N. Advanced glycation end-products enhance calcification in vascular smooth muscle cells. J. Int. Med. Res. 2009, 37, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Tsoporis, J.N.; Hatziagelaki, E.; Gupta, S.; Izhar, S.; Salpeas, V.; Tsiavou, A.; Rigopoulos, A.G.; Triantafyllis, A.S.; Marshall, J.C.; Parker, T.G.; et al. Circulating Ligands of the Receptor for Advanced Glycation End Products and the Soluble Form of the Receptor Modulate Cardiovascular Cell Apoptosis in Diabetes. Molecules 2020, 25, 5235. [Google Scholar] [CrossRef]
- Yoon, Y.W.; Kang, T.S.; Lee, B.K.; Chang, W.; Hwang, K.C.; Rhee, J.H.; Min, P.K.; Hong, B.K.; Rim, S.J.; Kwon, H.M. Pathobiological role of advanced glycation endproducts via mitogen-activated protein kinase dependent pathway in the diabetic vasculopathy. Exp. Mol. Med. 2008, 40, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Jang, E.J.; Baek, S.E.; Kim, E.J.; Park, S.Y.; Kim, C.D. HMGB1 enhances AGE-mediated VSMC proliferation via an increase in 5-LO-linked RAGE expression. Vascul. Pharmacol. 2019, 118-119, 106559. [Google Scholar] [CrossRef] [PubMed]
- Meloche, J.; Paulin, R.; Courboulin, A.; Lambert, C.; Barrier, M.; Bonnet, P.; Bisserier, M.; Roy, M.; Sussman, M.A.; Agharazii, M.; et al. RAGE-dependent activation of the oncoprotein Pim1 plays a critical role in systemic vascular remodeling processes. Arterioscler Thromb. Vasc. Biol. 2011, 31, 2114–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, T.; Zhang, L.; Yao, L.L.; Zheng, F.; Wang, L.; Yang, J.Y.; Guo, L.Y.; Li, X.Y.; Yan, Y.W.; Pan, Y.M.; et al. S100B promotes injury-induced vascular remodeling through modulating smooth muscle phenotype. Biochim. Biophys Acta Mol. Basis Dis. 2017, 1863, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.A.; Engelen, L.; Buijs, J.; Chaturvedi, N.; Fuller, J.H.; Jorsal, A.; Parving, H.H.; Tarnow, L.; Theilade, S.; Rossing, P.; et al. Circulating matrix metalloproteinases are associated with arterial stiffness in patients with type 1 diabetes: Pooled analysis of three cohort studies. Cardiovasc. Diabetol. 2017, 16, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, S.A.; Engelen, L.; Buijs, J.; Theilade, S.; Rossing, P.; Schalkwijk, C.G.; Stehouwer, C.D.A. Associations between advanced glycation endproducts and matrix metalloproteinases and its inhibitor in individuals with type 1 diabetes. J. Diabetes Complicat. 2018, 32, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.P.; Peterson, K.R.; Slawson, C. O-GlcNAcylation and O-GlcNAc Cycling Regulate Gene Transcription: Emerging Roles in Cancer. Cancers 2021, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Comer, F.I.; Hart, G.W. O-Glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J. Biol. Chem. 2000, 275, 29179–29182. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Shimoji, S.; Hart, G.W. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett 2010, 584, 2526–2538. [Google Scholar] [CrossRef] [Green Version]
- Butkinaree, C.; Park, K.; Hart, G.W. O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim. Biophys. Acta 2009, 1800, 96–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janetzko, J.; Walker, S. The making of a sweet modification: Structure and function of O-GlcNAc transferase. J. Biol. Chem. 2014, 289, 34424–34432. [Google Scholar] [CrossRef] [Green Version]
- Ong, Q.; Han, W.; Yang, X. O-GlcNAc as an Integrator of Signaling Pathways. Front. Endocrinol. 2018, 9, 599. [Google Scholar] [CrossRef]
- Kazemi, Z.; Chang, H.; Haserodt, S.; McKen, C.; Zachara, N.E. O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J. Biol. Chem. 2010, 285, 39096–39107. [Google Scholar] [CrossRef] [Green Version]
- Gambetta, M.C.; Oktaba, K.; Muller, J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science 2009, 325, 93–96. [Google Scholar] [CrossRef]
- Qian, K.; Wang, S.; Fu, M.; Zhou, J.; Singh, J.P.; Li, M.D.; Yang, Y.; Zhang, K.; Wu, J.; Nie, Y.; et al. Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer. J. Biol. Chem. 2018, 293, 13989–14000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.K.; Zhou, X.; Pendleton, K.E.; Hunter, O.V.; Kohler, J.J.; O’Donnell, K.A.; Conrad, N.K. A Conserved Splicing Silencer Dynamically Regulates O-GlcNAc Transferase Intron Retention and O-GlcNAc Homeostasis. Cell Rep. 2017, 20, 1088–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khidekel, N.; Ficarro, S.B.; Clark, P.M.; Bryan, M.C.; Swaney, D.L.; Rexach, J.E.; Sun, Y.E.; Coon, J.J.; Peters, E.C.; Hsieh-Wilson, L.C. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat. Chem. Biol. 2007, 3, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Federici, M.; Menghini, R.; Mauriello, A.; Hribal, M.L.; Ferrelli, F.; Lauro, D.; Sbraccia, P.; Spagnoli, L.G.; Sesti, G.; Lauro, R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 2002, 106, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.J.; McDonough, P.M.; Swanson, E.; Trost, S.U.; Suzuki, M.; Fukuda, M.; Dillmann, W.H. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J. Biol. Chem. 2003, 278, 44230–44237. [Google Scholar] [CrossRef] [Green Version]
- McNulty, P.H. Hexosamine biosynthetic pathway flux and cardiomyopathy in type 2 diabetes mellitus. Focus on “Impact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart”. Am. J. Physiol. Cell Physiol. 2007, 292, C1243–C1244. [Google Scholar] [CrossRef] [Green Version]
- Lunde, I.G.; Aronsen, J.M.; Kvaloy, H.; Qvigstad, E.; Sjaastad, I.; Tonnessen, T.; Christensen, G.; Gronning-Wang, L.M.; Carlson, C.R. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol. Genom. 2012, 44, 162–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dassanayaka, S.; Jones, S.P. O-GlcNAc and the cardiovascular system. Pharmacol. Ther. 2014, 142, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Roquemore, E.P.; Chevrier, M.R.; Cotter, R.J.; Hart, G.W. Dynamic O-GlcNAcylation of the small heat shock protein alpha B-crystallin. Biochemistry 1996, 35, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H.; Vogt, C.; Iozzo, P.; Pipek, R.; Daniels, M.C.; Virkamaki, A.; Makimattila, S.; Mandarino, L.; DeFronzo, R.A.; McClain, D.; et al. UDP-N-acetylglucosamine transferase and glutamine: Fructose 6-phosphate amidotransferase activities in insulin-sensitive tissues. Diabetologia 1997, 40, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Du, X.L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef] [Green Version]
- Raza, H.; Prabu, S.K.; John, A.; Avadhani, N.G. Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2011, 12, 3133–3147. [Google Scholar] [CrossRef] [Green Version]
- Oliveri, L.M.; Buzaleh, A.M.; Gerez, E.N. An increase in O-GlcNAcylation of Sp1 down-regulates the gene expression of pi class glutathione S-transferase in diabetic mice. Biochem. Biophys. Rep. 2021, 27, 101049. [Google Scholar]
- Fricovsky, E.S.; Suarez, J.; Ihm, S.H.; Scott, B.T.; Suarez-Ramirez, J.A.; Banerjee, I.; Torres-Gonzalez, M.; Wang, H.; Ellrott, I.; Maya-Ramos, L.; et al. Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R689–R699. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R.; Pereira, L.; Wang, L.; Han, G.; Ferguson, A.; Dao, K.; Copeland, R.J.; Despa, F.; Hart, G.W.; Ripplinger, C.M.; et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 2013, 502, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Sleaby, R.; Davidoff, A.J.; Bell, J.R.; De Blasio, M.J.; Delbridge, L.M.; Chatham, J.C.; Ritchie, R.H. Insights into the role of maladaptive hexosamine biosynthesis and O-GlcNAcylation in development of diabetic cardiac complications. Pharmacol. Res. 2017, 116, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, D.; Lim, S.Y.; Erickson, J.R.; Wallace, R.S.; Lees, J.G.; Tate, M.; Kiriazis, H.; Donner, D.G.; Henstridge, D.C.; Davey, J.R.; et al. Fine-tuning the cardiac O-GlcNAcylation regulatory enzymes governs the functional and structural phenotype of the diabetic heart. Cardiovasc. Res. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Hu, Y.; Belke, D.; Suarez, J.; Swanson, E.; Clark, R.; Hoshijima, M.; Dillmann, W.H. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ. Res. 2005, 96, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Champattanachai, V.; Marchase, R.B.; Chatham, J.C. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am. J. Physiol. Cell Physiol. 2007, 292, C178–C187. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhou, H.; Jin, Z.; Bi, S.; Yang, X.; Yi, D.; Liu, W. Cardioprotection of salidroside from ischemia/reperfusion injury by increasing N-acetylglucosamine linkage to cellular proteins. Eur. J. Pharmacol. 2009, 613, 93–99. [Google Scholar] [CrossRef]
- Veighey, K.; Macallister, R.J. Clinical applications of remote ischemic preconditioning. Cardiol. Res. Pract. 2012, 2012, 620681. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.P.; Zachara, N.E.; Ngoh, G.A.; Hill, B.G.; Teshima, Y.; Bhatnagar, A.; Hart, G.W.; Marban, E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 2008, 117, 1172–1182. [Google Scholar] [CrossRef] [Green Version]
- Vibjerg Jensen, R.; Johnsen, J.; Buus Kristiansen, S.; Zachara, N.E.; Botker, H.E. Ischemic preconditioning increases myocardial O-GlcNAc glycosylation. Scand. Cardiovasc. J. 2013, 47, 168–174. [Google Scholar] [CrossRef]
- Jensen, R.V.; Zachara, N.E.; Nielsen, P.H.; Kimose, H.H.; Kristiansen, S.B.; Botker, H.E. Impact of O-GlcNAc on cardioprotection by remote ischaemic preconditioning in non-diabetic and diabetic patients. Cardiovasc. Res. 2013, 97, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef]
- Hirose, K.; Tsutsumi, Y.M.; Tsutsumi, R.; Shono, M.; Katayama, E.; Kinoshita, M.; Tanaka, K.; Oshita, S. Role of the O-linked beta-N-acetylglucosamine in the cardioprotection induced by isoflurane. Anesthesiology 2011, 115, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Ngoh, G.A.; Watson, L.J.; Facundo, H.T.; Jones, S.P. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino. Acids 2011, 40, 895–911. [Google Scholar] [CrossRef] [Green Version]
- Ngoh, G.A.; Hamid, T.; Prabhu, S.D.; Jones, S.P. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1711–H1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafir, A.; Readnower, R.; Long, B.W.; McCracken, J.; Aird, A.; Alvarez, A.; Cummins, T.D.; Li, Q.; Hill, B.G.; Bhatnagar, A.; et al. Protein O-GlcNAcylation is a novel cytoprotective signal in cardiac stem cells. Stem Cells 2013, 31, 765–775. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, X.; Lee, S.H.; Chen, F.; Jiang, K.; Tu, Z.; Liu, Z.; Du, J.; Wang, L.; Yin, C.; et al. Diabetes Exacerbates Myocardial Ischemia/Reperfusion Injury by Down-Regulation of MicroRNA and Up-Regulation of O-GlcNAcylation. JACC 2018, 3, 350–362. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Li, M.; Yuan, Q.; Xue, M.; Xu, F.; Chen, Y. Inhibition of ALDH2 by O-GlcNAcylation contributes to the hyperglycemic exacerbation of myocardial ischemia/reperfusion injury. Oncotarget 2017, 8, 19413–19426. [Google Scholar] [CrossRef]
- Darley-Usmar, V.M.; Ball, L.E.; Chatham, J.C. Protein O-linked beta-N-acetylglucosamine: A novel effector of cardiomyocyte metabolism and function. J. Mol. Cell Cardiol. 2012, 52, 538–549. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Polyol pathway and RAGE: A central metabolic and signaling axis in diabetic complications. Expert Rev. Endocrinol. Metab. 2010, 5, 65–75. [Google Scholar] [CrossRef]
- Cannizzo, B.; Lujan, A.; Estrella, N.; Lembo, C.; Cruzado, M.; Castro, C. Insulin resistance promotes early atherosclerosis via increased proinflammatory proteins and oxidative stress in fructose-fed ApoE-KO mice. Exp. Diabetes Res. 2012, 2012, 941304. [Google Scholar] [CrossRef]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2013, 20, 2794–2814. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Correa, G.A.; Jin, W.; Wang, Z.; Zhong, X.; Gao, W.D.; Dias, W.B.; Vecoli, C.; Hart, G.W.; Murphy, A.M. O-linked GlcNAc modification of cardiac myofilament proteins: A novel regulator of myocardial contractile function. Circ. Res. 2008, 103, 1354–1358. [Google Scholar] [CrossRef] [Green Version]
- Gelinas, R.; Mailleux, F.; Dontaine, J.; Bultot, L.; Demeulder, B.; Ginion, A.; Daskalopoulos, E.P.; Esfahani, H.; Dubois-Deruy, E.; Lauzier, B.; et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat. Commun. 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois-Deruy, E.; Belliard, A.; Mulder, P.; Bouvet, M.; Smet-Nocca, C.; Janel, S.; Lafont, F.; Beseme, O.; Amouyel, P.; Richard, V.; et al. Interplay between troponin T phosphorylation and O-N-acetylglucosaminylation in ischaemic heart failure. Cardiovasc. Res. 2015, 107, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksnes, T.A.; Schmieder, R.E.; Kjeldsen, S.E.; Ghani, S.; Hua, T.A.; Julius, S. Impact of new-onset diabetes mellitus on development of atrial fibrillation and heart failure in high-risk hypertension (from the VALUE Trial). Am. J. Cardiol. 2008, 101, 634–638. [Google Scholar] [CrossRef]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef] [Green Version]
- Dahlqvist, S.; Rosengren, A.; Gudbjornsdottir, S.; Pivodic, A.; Wedel, H.; Kosiborod, M.; Svensson, A.M.; Lind, M. Risk of atrial fibrillation in people with type 1 diabetes compared with matched controls from the general population: A prospective case-control study. Lancet Diabetes Endocrinol. 2017, 5, 799–807. [Google Scholar] [CrossRef]
- Ramirez-Correa, G.A.; Ma, J.; Slawson, C.; Zeidan, Q.; Lugo-Fagundo, N.S.; Xu, M.; Shen, X.; Gao, W.D.; Caceres, V.; Chakir, K.; et al. Removal of Abnormal Myofilament O-GlcNAcylation Restores Ca2+ Sensitivity in Diabetic Cardiac Muscle. Diabetes 2015, 64, 3573–3587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.; Hu, L.; Xie, J.; Chen, S.; Huang, L.; Xu, Z.; Liu, X.; Zhou, Q.; Yuan, P.; Yan, X.; et al. O-GlcNAcylation of cardiac Nav1.5 contributes to the development of arrhythmias in diabetic hearts. Int. J. Cardiol. 2018, 260, 74–81. [Google Scholar] [CrossRef]
- Yokoe, S.; Asahi, M.; Takeda, T.; Otsu, K.; Taniguchi, N.; Miyoshi, E.; Suzuki, K. Inhibition of phospholamban phosphorylation by O-GlcNAcylation: Implications for diabetic cardiomyopathy. Glycobiology 2010, 20, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Grimm, M.; Brown, J.H. Beta-adrenergic receptor signaling in the heart: Role of CaMKII. J. Mol. Cell Cardiol. 2009, 48, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.E.; Brown, J.H.; Bers, D.M. CaMKII in myocardial hypertrophy and heart failure. J. Mol. Cell Cardiol. 2011, 51, 468–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purohit, A.; Rokita, A.G.; Guan, X.; Chen, B.; Koval, O.M.; Voigt, N.; Neef, S.; Sowa, T.; Gao, Z.; Luczak, E.D.; et al. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 2013, 128, 1748–1757. [Google Scholar] [CrossRef] [Green Version]
- Erickson, J.R.; Joiner, M.L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef] [Green Version]
- Sommese, L.; Valverde, C.A.; Blanco, P.; Castro, M.C.; Rueda, O.V.; Kaetzel, M.; Dedman, J.; Anderson, M.E.; Mattiazzi, A.; Palomeque, J. Ryanodine receptor phosphorylation by CaMKII promotes spontaneous Ca(2+) release events in a rodent model of early stage diabetes: The arrhythmogenic substrate. Int. J. Cardiol. 2016, 202, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Liao, Z.; Lu, X.; Katschinski, D.M.; Mercola, M.; Chen, J.; Heller Brown, J.; Molkentin, J.D.; Bossuyt, J.; Bers, D.M. Hyperglycemia Acutely Increases Cytosolic Reactive Oxygen Species via O-linked GlcNAcylation and CaMKII Activation in Mouse Ventricular Myocytes. Circ. Res. 2020, 126, e80–e96. [Google Scholar] [CrossRef]
- Mesubi, O.O.; Rokita, A.G.; Abrol, N.; Wu, Y.; Chen, B.; Wang, Q.; Granger, J.M.; Tucker-Bartley, A.; Luczak, E.D.; Murphy, K.R.; et al. Oxidized CaMKII and O-GlcNAcylation cause increased atrial fibrillation in diabetic mice by distinct mechanisms. J. Clin. Investig. 2021, 131, e95747. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Feng, W.; Not, L.G.; Miller, A.P.; Zhang, Y.; Chen, Y.F.; Majid-Hassan, E.; Chatham, J.C.; Oparil, S. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H335–H342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, D.; Gong, K.; Feng, W.; Nozell, S.E.; Chen, Y.F.; Chatham, J.C.; Oparil, S. O-GlcNAc modification of NFkappaB p65 inhibits TNF-alpha-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS ONE 2011, 6, e24021. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, R.H.; Xing, D.; Gong, K.; Chen, Y.F.; Chatham, J.C.; Oparil, S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H513–H522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, D.; Xu, L.; Xu, O.; Li, R.; Chen, M.; Shen, H.; Zhu, H.; Zhang, F.; Yao, D.; Chen, Y.F.; et al. O-Linked beta-N-Acetylglucosamine Modification of A20 Enhances the Inhibition of NF-kappaB (Nuclear Factor-kappaB) Activation and Elicits Vascular Protection After Acute Endoluminal Arterial Injury. Arterioscler Thromb. Vasc. Biol. 2018, 38, 1309–1320. [Google Scholar] [CrossRef] [Green Version]
- Heath, J.M.; Sun, Y.; Yuan, K.; Bradley, W.E.; Litovsky, S.; Dell’Italia, L.J.; Chatham, J.C.; Wu, H.; Chen, Y. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ. Res. 2014, 114, 1094–1102. [Google Scholar] [CrossRef] [Green Version]
- Lima, V.V.; Giachini, F.R.; Carneiro, F.S.; Carneiro, Z.N.; Saleh, M.A.; Pollock, D.M.; Fortes, Z.B.; Carvalho, M.H.; Ergul, A.; Webb, R.C.; et al. O-GlcNAcylation contributes to augmented vascular reactivity induced by endothelin 1. Hypertension 2010, 55, 180–188. [Google Scholar] [CrossRef] [Green Version]
- da Costa, R.M.; da Silva, J.F.; Alves, J.V.; Dias, T.B.; Rassi, D.M.; Garcia, L.V.; Lobato, N.S.; Tostes, R.C. Increased O-GlcNAcylation of Endothelial Nitric Oxide Synthase Compromises the Anti-contractile Properties of Perivascular Adipose Tissue in Metabolic Syndrome. Front. Physiol. 2018, 9, 341. [Google Scholar] [CrossRef] [Green Version]
- Du, X.L.; Edelstein, D.; Dimmeler, S.; Ju, Q.; Sui, C.; Brownlee, M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Investig. 2001, 108, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.V.; Giachini, F.R.; Hardy, D.M.; Webb, R.C.; Tostes, R.C. O-GlcNAcylation: A novel pathway contributing to the effects of endothelin in the vasculature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R236–R250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, A.; Dai, A.; Han, Y.; Youssef, K.D.; Wang, W.; Donthamsetty, R.; Scott, B.T.; Wang, H.; Dillmann, W.H. O-GlcNAcase overexpression reverses coronary endothelial cell dysfunction in type 1 diabetic mice. Am. J. Physiol. Cell Physiol. 2015, 309, C593–C599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.Y.; Kim, D.B.; Kim, M.J.; Kwon, B.J.; Chang, S.Y.; Jang, S.W.; Cho, E.J.; Rho, T.H.; Kim, J.H. Higher plasma thrombospondin-1 levels in patients with coronary artery disease and diabetes mellitus. Korean Circ. J. 2012, 42, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, R.; Sahu, S.; Chavez, R.J.; Raman, P. Trivalent chromium inhibits TSP-1 expression, proliferation, and O-GlcNAc signaling in vascular smooth muscle cells in response to high glucose in vitro. Am. J. Physiol. Cell Physiol. 2014, 308, C111–C122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byon, C.H.; Kim, S.W. Regulatory Effects of O-GlcNAcylation in Vascular Smooth Muscle Cells on Diabetic Vasculopathy. J. lipid Atheroscler. 2020, 9, 243–254. [Google Scholar] [CrossRef]
- Souza-Silva, L.; Alves-Lopes, R.; Silva Miguez, J.; Dela Justina, V.; Neves, K.B.; Mestriner, F.L.; Tostes, R.C.; Giachini, F.R.; Lima, V.V. Glycosylation with O-linked beta-N-acetylglucosamine induces vascular dysfunction via production of superoxide anion/reactive oxygen species. Can. J. Physiol. Pharmacol. 2018, 96, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Seok, Y.M.; Kim, I.K.; Lee, I.K.; Jeong, S.Y.; Jeoung, N.H. Glucosamine increases vascular contraction through activation of RhoA/Rho kinase pathway in isolated rat aorta. BMB Rep. 2011, 44, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Yahr, J.; Calle, J.; Taliercio, J.J. A renaissance in the treatment of diabetic kidney disease, hypertension in chronic kidney disease, and beyond. J. Osteopath. Med. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.H.; Burns, J.A.; Aguilar, F.G.; Beussink, L.; Shah, S.J. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail. 2014, 2, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Birn, H.; Storm, T.; Weyer, K.; Nielsen, R. Endocytic receptors in the renal proximal tubule. Physiology 2012, 27, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Degrell, P.; Cseh, J.; Mohas, M.; Molnar, G.A.; Pajor, L.; Chatham, J.C.; Fulop, N.; Wittmann, I. Evidence of O-linked N-acetylglucosamine in diabetic nephropathy. Life Sci. 2009, 84, 389–393. [Google Scholar] [CrossRef]
- Silva-Aguiar, R.P.; Bezerra, N.C.F.; Lucena, M.C.; Sirtoli, G.M.; Sudo, R.T.; Zapata-Sudo, G.; Takiya, C.M.; Pinheiro, A.A.S.; Dias, W.B.; Caruso-Neves, C. O-GlcNAcylation reduces proximal tubule protein reabsorption and promotes proteinuria in spontaneously hypertensive rats. J. Biol. Chem. 2018, 293, 12749–12758. [Google Scholar] [CrossRef] [Green Version]
- Li, S.Y.; Liu, Y.; Sigmon, V.K.; McCort, A.; Ren, J. High-fat diet enhances visceral advanced glycation end products, nuclear O-Glc-Nac modification, p38 mitogen-activated protein kinase activation and apoptosis. Diabetes Obes. Metab. 2005, 7, 448–454. [Google Scholar] [CrossRef]
- Zachara, N.E.; O’Donnell, N.; Cheung, W.D.; Mercer, J.J.; Marth, J.D.; Hart, G.W. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 2004, 279, 30133–30142. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.D.; Xu, C.; Feng, L.; Wang, F. The augmentation of O-GlcNAcylation reduces glyoxal-induced cell injury by attenuating oxidative stress in human retinal microvascular endothelial cells. Int. J. Mol. Med. 2015, 36, 1019–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dozio, E.; Vettoretti, S.; Lungarella, G.; Messa, P.; Corsi Romanelli, M.M. Sarcopenia in Chronic Kidney Disease: Focus on Advanced Glycation End Products as Mediators and Markers of Oxidative Stress. Biomedicines 2021, 9, 405. [Google Scholar] [CrossRef]
- Liu, J.; Marchase, R.B.; Chatham, J.C. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J. Mol. Cell Cardiol. 2007, 42, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Champattanachai, V.; Marchase, R.B.; Chatham, J.C. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am. J. Physiol. Cell Physiol. 2008, 294, C1509–C1520. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, J.; Maeng, M.; Mortensen, U.M.; Berg, J.; Rehling, M.; Nielsen, T.T. Lack of cardioprotection from metabolic support with glutamine or glutamate in a porcine coronary occlusion model. Scand. Cardiovasc. J. 2005, 39, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Fulop, N.; Mason, M.M.; Dutta, K.; Wang, P.; Davidoff, A.J.; Marchase, R.B.; Chatham, J.C. Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am. J. Physiol. Cell Physiol. 2007, 292, C1370–C1378. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dozio, E.; Massaccesi, L.; Corsi Romanelli, M.M. Glycation and Glycosylation in Cardiovascular Remodeling: Focus on Advanced Glycation End Products and O-Linked Glycosylations as Glucose-Related Pathogenetic Factors and Disease Markers. J. Clin. Med. 2021, 10, 4792. https://doi.org/10.3390/jcm10204792
Dozio E, Massaccesi L, Corsi Romanelli MM. Glycation and Glycosylation in Cardiovascular Remodeling: Focus on Advanced Glycation End Products and O-Linked Glycosylations as Glucose-Related Pathogenetic Factors and Disease Markers. Journal of Clinical Medicine. 2021; 10(20):4792. https://doi.org/10.3390/jcm10204792
Chicago/Turabian StyleDozio, Elena, Luca Massaccesi, and Massimiliano Marco Corsi Romanelli. 2021. "Glycation and Glycosylation in Cardiovascular Remodeling: Focus on Advanced Glycation End Products and O-Linked Glycosylations as Glucose-Related Pathogenetic Factors and Disease Markers" Journal of Clinical Medicine 10, no. 20: 4792. https://doi.org/10.3390/jcm10204792





