Three-Dimensional Quantification of Bone Mineral Density in the Distal Femur and Proximal Tibia Based on Computed Tomography: In Vitro Evaluation of an Extended Standardization Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
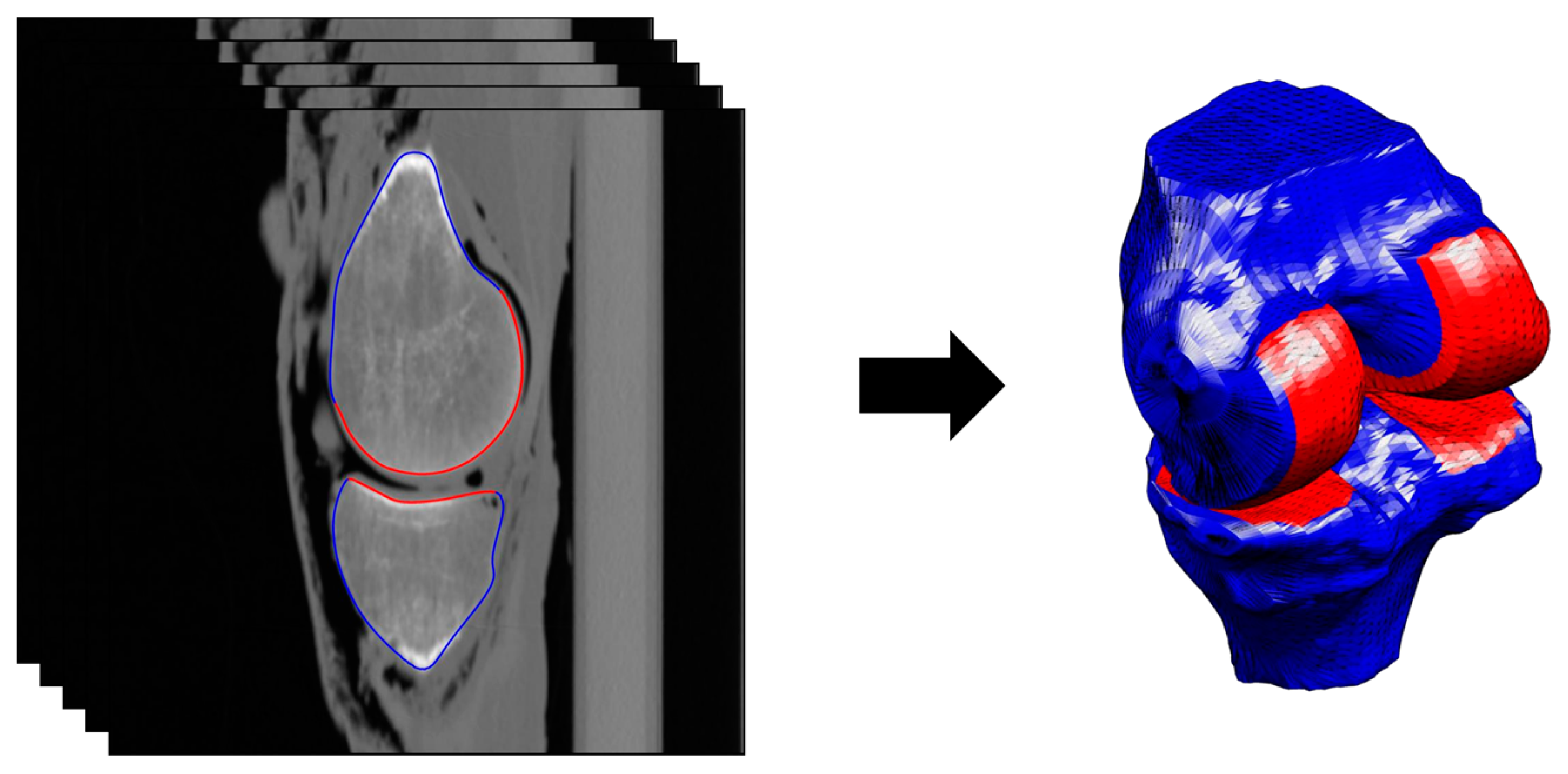
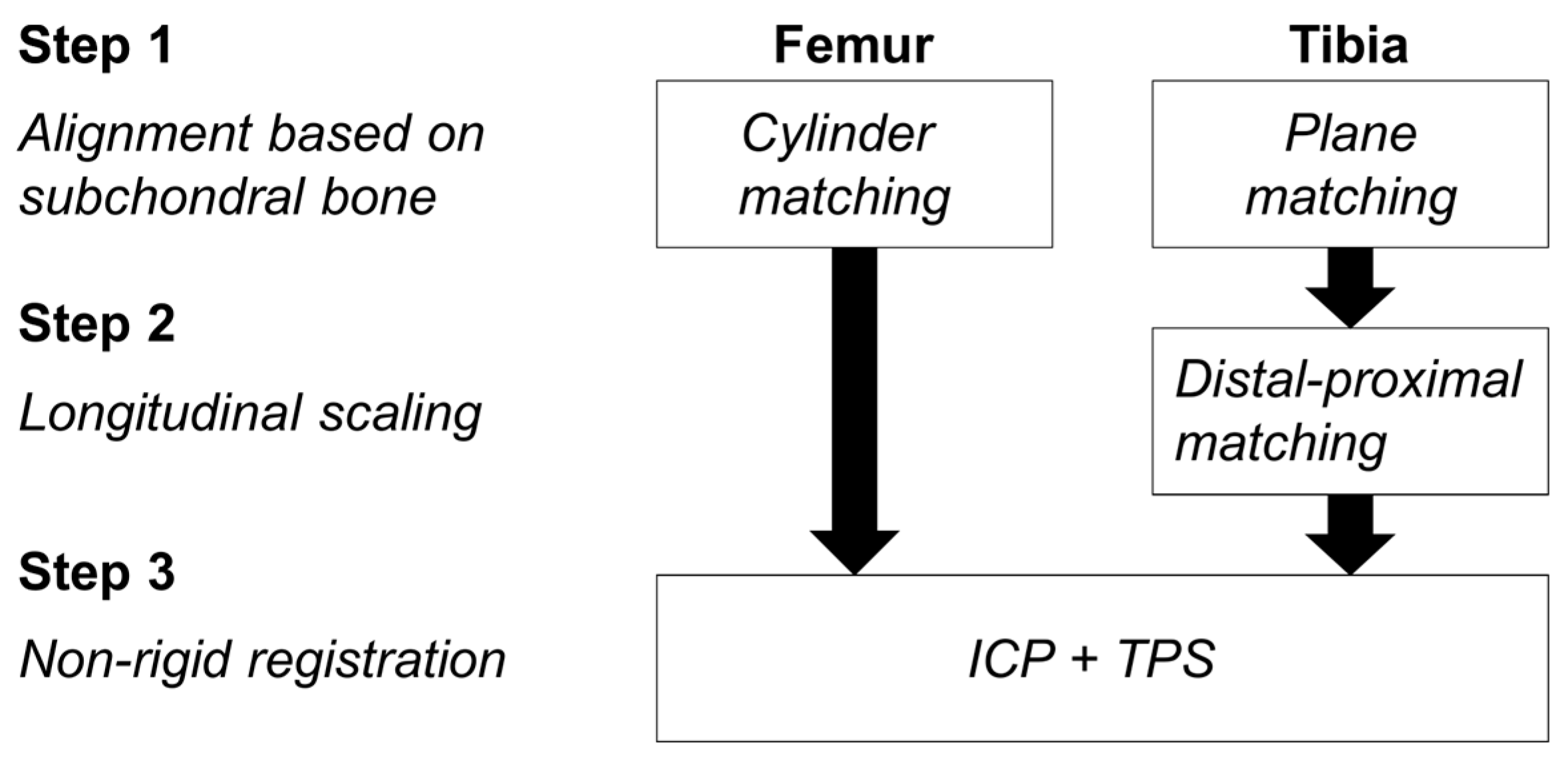
2.2. Segmentation and Registration
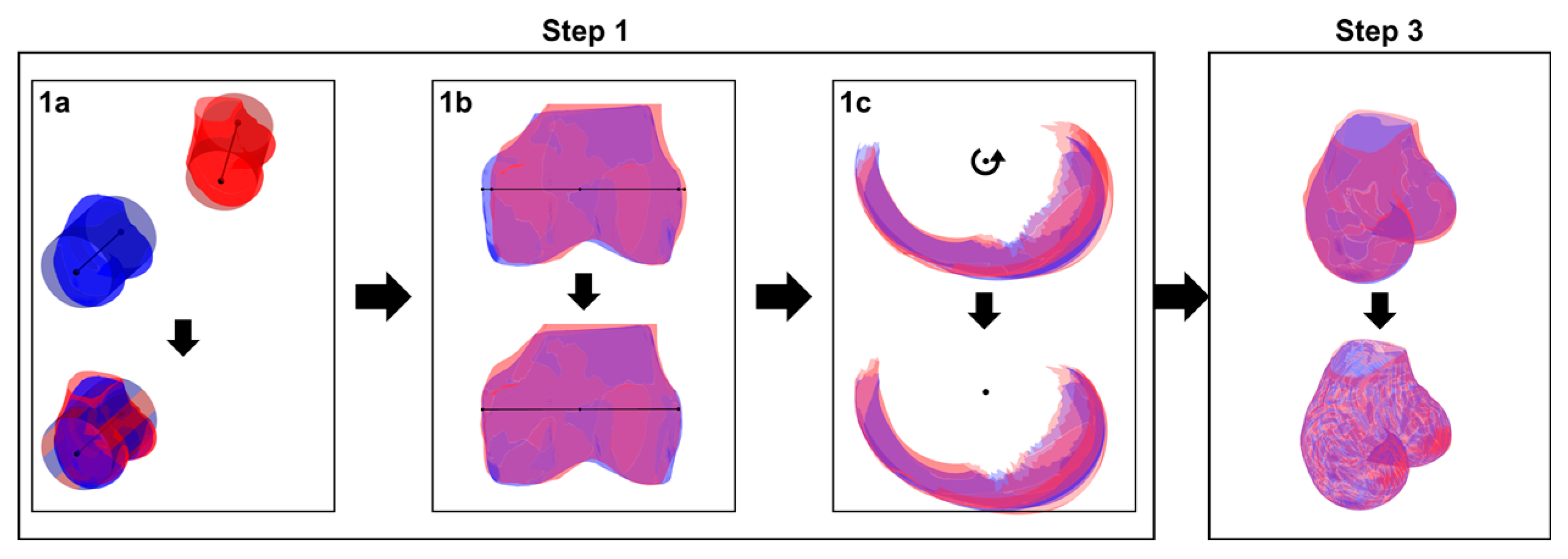
2.2.1. Phase 1
2.2.2. Phase 2
2.3. Evaluation of the Method
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edwards, W.B.; Schnitzer, T.J.; Troy, K.L. Bone mineral and stiffness loss at the distal femur and proximal tibia in acute spinal cord injury. Osteoporos. Int. 2014, 25, 1005–1015. [Google Scholar] [CrossRef]
- Garland, D.E.; Stewart, C.A.; Adkins, R.H.; Hu, S.S.; Rosen, C.; Liotta, F.J.; Weinstein, D.A. Osteoporosis after spinal cord injury. J. Orthop. Res. 1992, 10, 371–378. [Google Scholar] [CrossRef]
- Henderson, R.C.; Berglund, L.M.; May, R.; Zemel, B.S.; Grossberg, R.I.; Johnson, J.; Plotkin, H.; Stevenson, R.D.; Szalay, E.; Wong, B.; et al. The Relationship Between Fractures and DXA Measures of BMD in the Distal Femur of Children and Adolescents with Cerebral Palsy or Muscular Dystrophy. J. Bone Miner. Res. 2010, 25, 520–526. [Google Scholar] [CrossRef]
- Shi, M.; Chen, L.; Wu, H.; Wang, Y.; Wang, W.; Zhang, Y.; Yan, S. Effect of bisphosphonates on periprosthetic bone loss after total knee arthroplasty: A meta-analysis of randomized controlled trials. BMC Musculoskelet. Disord. 2018, 19, 177. [Google Scholar] [CrossRef]
- Winther, N.; Jensen, C.; Petersen, M.; Lind, T.; Schroder, H.; Petersen, M. Changes in bone mineral density of the proximal tibia after uncemented total knee arthroplasty. A prospective randomized study. Int. Orthop. 2016, 40, 285–294. [Google Scholar] [CrossRef]
- Petersen, M.M.; Olsen, C.; Lauritzen, J.B.; Lund, B. Changes in bone mineral density of the distal femur following uncemented total knee arthroplasty. J. Arthroplasty 1995, 10, 7–11. [Google Scholar] [CrossRef]
- Petersen, M.M.; Olsen, C.; Lauritzen, J.B.; Lund, B.; Hede, A. Late changes in bone mineral density of the proximal tibia following total or partial medial meniscectomy. A randomized study. J. Orthop. Res. 1996, 14, 16–21. [Google Scholar] [CrossRef]
- Lo, G.H.; Niu, J.; McLennan, C.E.; Kiel, D.P.; McLean, R.R.; Guermazi, A.; Genant, H.K.; McAlindon, T.E.; Hunter, D.J. Meniscal damage associated with increased local subchondral bone mineral density: A Framingham study. Osteoarthr. Cartil. 2008, 16, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Wal, R.J.P.; Attia, D.; Waarsing, E.H.; Thomassen, B.J.W.; van Arkel, E.R.A. Two-year follow-up of bone mineral density changes in the knee after meniscal allograft transplantation: Results of an explorative study. Knee 2018, 25, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Bayar, A.; Sarikaya, S.; Keser, S.; Ozdolap, S.; Tuncay, I.; Ege, A. Regional bone density changes in anterior cruciate ligament deficient knees: A DEXA study. Knee 2008, 15, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.K.; Ross, G.J.; Urso, J.A. Transient osteoporosis of the knee. Skeletal Radiol. 1997, 26, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Hulet, C.; Sabatier, J.P.; Souquet, D.; Locker, B.; Marcelli, C.; Vielpeau, C. Distribution of bone mineral density at the proximal tibia in knee osteoarthritis. Calcif. Tissue Int. 2002, 71, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.H.; Zhang, Y.; McLennan, C.; Niu, J.; Kiel, D.P.; McLean, R.R.; Aliabadi, P.; Felson, D.T.; Hunter, D.J. The ratio of medial to lateral tibial plateau bone mineral density and compartment-specific tibiofemoral osteoarthritis. Osteoarthr. Cartil. 2006, 14, 984–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, J.D.; Masri, B.A.; Wilson, D.R. Computed tomography topographic mapping of subchondral density (CT-TOMASD) in osteoarthritic and normal knees: Methodological development and preliminary findings. Osteoarthr. Cartil. 2009, 17, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Bousson, V.; Lowitz, T.; Laouisset, L.; Engelke, K.; Laredo, J.D. CT imaging for the investigation of subchondral bone in knee osteoarthritis. Osteoporos. Int. 2012, 23, 861–865. [Google Scholar] [CrossRef]
- Bennell, K.L.; Creaby, M.W.; Wrigley, T.V.; Hunter, D.J. Tibial subchondral trabecular volumetric bone density in medial knee joint osteoarthritis using peripheral quantitative computed tomography technology. Arthr. Rheum. 2008, 58, 2776–2785. [Google Scholar] [CrossRef]
- Omoumi, P.; Babel, H.; Jolles, B.M.; Favre, J. Quantitative regional and sub-regional analysis of femoral and tibial subchondral bone mineral density (sBMD) using computed tomography (CT): Comparison of non-osteoarthritic (OA) and severe OA knees. Osteoarthr. Cartil. 2017, 25, 1850–1857. [Google Scholar] [CrossRef] [Green Version]
- Babel, H.; Wageli, L.; Sonmez, B.; Thiran, J.-P.; Omoumi, P.; Jolles, B.; Favre, J. A Registration Method for Three-Dimensional Analysis of Bone Mineral Density in the Proximal Tibia. J. Biomech. Eng. 2020. [Google Scholar] [CrossRef]
- Biswas, D.; Bible, J.E.; Bohan, M.; Simpson, A.K.; Whang, P.G.; Grauer, J.N. Radiation exposure from musculoskeletal computerized tomographic scans. J. Bone Joint Surg. Am. 2009, 91, 1882–1889. [Google Scholar] [CrossRef]
- Bowes, M.; Lohmander, L.; Wolstenholme, C.; Vincent, G.; Conaghan, P.; Frobell, R. Marked and rapid change of bone shape in acutely ACL injured knees–An exploratory analysis of the Kanon trial. Osteoarthr. Cartilage 2019, 27, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Hunter, D.; Nevitt, M.; Lynch, J.; Kraus, V.B.; Katz, J.N.; Collins, J.E.; Bowes, M.; Guermazi, A.; Roemer, F.W.; Losina, E. Longitudinal validation of periarticular bone area and 3D shape as biomarkers for knee OA progression? Data from the FNIH OA Biomarkers Consortium. Annal. Rheum. Dis. 2016, 75, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Campanacci, M. Bone and Soft Tissue Tumors; Springer: New York, NY, USA, 2013. [Google Scholar]
- Koo, S.; Gold, G.E.; Andriacchi, T.P. Considerations in measuring cartilage thickness using MRI: Factors influencing reproducibility and accuracy. Osteoarthr. Cartil. 2005, 13, 782–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, W.; Eckstein, F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans. Med. Imaging 2008, 27, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, D.E.; Sumner, D.R.; Andriacchi, T.P.; Sugar, D.A. Dynamic knee loads during gait predict proximal tibial bone distribution. J. Biomech. 1998, 31, 423–430. [Google Scholar] [CrossRef]
- Wada, M.; Maezawa, Y.; Baba, H.; Shimada, S.; Sasaki, S.; Nose, Y. Relationships among bone mineral densities, static alignment and dynamic load in patients with medial compartment knee osteoarthritis. Rheumatology 2001, 40, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carballido-Gamio, J.; Nicolella, D.P. Computational anatomy in the study of bone structure. Curr. Osteoporos. Rep. 2013, 11, 237–245. [Google Scholar] [CrossRef]
- Grenander, U.; Miller, M.I. Computational anatomy: An emerging discipline. Q. Appl. Math. 1998, 56, 617–694. [Google Scholar] [CrossRef] [Green Version]
- Peterfy, C.G.; Guermazi, A.; Zaim, S.; Tirman, P.F.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Favre, J.; Erhart-Hledik, J.C.; Blazek, K.; Fasel, B.; Gold, G.E.; Andriacchi, T.P. Anatomically Standardized Maps Reveal Distinct Patterns of Cartilage Thickness With Increasing Severity of Medial Compartment Knee Osteoarthritis. J. Orthop. Res. 2017, 35, 2442–2451. [Google Scholar] [CrossRef] [Green Version]
- Scanlan, S.F.; Favre, J.; Andriacchi, T.P. The relationship between peak knee extension at heel-strike of walking and the location of thickest femoral cartilage in ACL reconstructed and healthy contralateral knees. J. Biomech. 2013, 46, 849–854. [Google Scholar] [CrossRef]
- Favre, J.; Scanlan, S.F.; Erhart-Hledik, J.C.; Blazek, K.; Andriacchi, T.P. Patterns of femoral cartilage thickness are different in asymptomatic and osteoarthritic knees and can be used to detect disease-related differences between samples. J. Biomech. Eng. 2013, 135, 101002–101010. [Google Scholar] [CrossRef] [Green Version]
- Amberg, B.; Romdhani, S.; Vetter, T. Optimal step nonrigid ICP algorithms for surface registration. In Proceedings of the Computer Vision and Pattern Recognition Conference, CVPR’07, Minneapolis, MN, USA, 17–22 June 2007; pp. 1–8. [Google Scholar]
- Donato, G.; Belongie, S.J. Approximation Methods for Thin Plate Spline Mappings and Principal Warps; Citeseer; Department of Computer Science and Engineering, University of California: San Diego, CA, USA, 2003. [Google Scholar]
- McGraw, K.O.; Wong, S.P. Forming inferences about some intraclass correlation coefficients. J. Psychol. Methods 1996, 1, 30. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Gluer, C.C.; Blake, G.; Lu, Y.; Blunt, B.A.; Jergas, M.; Genant, H.K. Accurate Assessment of Precision Errors–How to Measure the Reproducibility of Bone Densitometry Techniques. Osteoporos. Int. 1995, 5, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Wilcoxon, F. Individual comparisons by ranking methods. In Breakthroughs in Statistics; Springer: New York, NY, USA, 1992; pp. 196–202. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Ramme, A.J.; Criswell, A.J.; Wolf, B.R.; Magnotta, V.A.; Grosland, N.M. EM segmentation of the distal femur and proximal tibia: A high-throughput approach to anatomic surface generation. Ann. Biomed. Eng. 2011, 39, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.I.; Greenspan, M.; Ellis, R. Validation of bone segmentation and improved 3-D registration using contour coherency in CT data. IEEE Trans. Med. Imaging 2006, 25, 324–334. [Google Scholar] [CrossRef]
- Burnett, W.D.; Kontulainen, S.A.; McLennan, C.E.; Hazel, D.; Talmo, C.; Hunter, D.J.; Wilson, D.R.; Johnston, J.D. Knee osteoarthritis patients with severe nocturnal pain have altered proximal tibial subchondral bone mineral density. Osteoarthr. Cartilage 2015, 23, 1483–1490. [Google Scholar] [CrossRef] [Green Version]
- Johannesdottir, F.; Poole, K.E.; Reeve, J.; Siggeirsdottir, K.; Aspelund, T.; Mogensen, B.; Jonsson, B.Y.; Sigurdsson, S.; Harris, T.B.; Gudnason, V.G.; et al. Distribution of cortical bone in the femoral neck and hip fracture: A prospective case-control analysis of 143 incident hip fractures; the AGES-REYKJAVIK Study. Bone 2011, 48, 1268–1276. [Google Scholar] [CrossRef] [Green Version]
- Veitch, S.W.; Findlay, S.C.; Hamer, A.J.; Blumsohn, A.; Eastell, R.; Ingle, B.M. Changes in bone mass and bone turnover following tibial shaft fracture. Osteoporos. Int. 2006, 17, 364–372. [Google Scholar] [CrossRef]
- Li, W.J.; Kornak, J.; Harris, T.; Keyak, J.; Li, C.X.; Lu, Y.; Cheng, X.G.; Lang, T. Identify fracture-critical regions inside the proximal femur using statistical parametric mapping. Bone 2009, 44, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Carballido-Gamio, J.; Harnish, R.; Saeed, I.; Streeper, T.; Sigurdsson, S.; Amin, S.; Atkinson, E.J.; Therneau, T.M.; Siggeirsdottir, K.; Cheng, X.; et al. Proximal femoral density distribution and structure in relation to age and hip fracture risk in women. J. Bone Miner. Res. 2013, 28, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Carballido-Gamio, J.; Bonaretti, S.; Saeed, I.; Harnish, R.; Recker, R.; Burghardt, A.J.; Keyak, J.H.; Harris, T.; Khosla, S.; Lang, T.F. Automatic multi-parametric quantification of the proximal femur with quantitative computed tomography. Quant. Imaging Med. Surg. 2015, 5, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Kezele, I.; Collins, D.L.; Zijdenbos, A.; Keyak, J.; Kornak, J.; Koyama, A.; Saeed, I.; LeBlanc, A.; Harris, T.; et al. Voxel-based modeling and quantification of the proximal femur using inter-subject registration of quantitative CT images. Bone 2007, 41, 888–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, K.E.S.; Skingle, L.; Gee, A.H.; Turmezei, T.D.; Johannesdottir, F.; Blesic, K.; Rose, C.; Vindlacheruvu, M.; Donell, S.; Vaculik, J.; et al. Focal osteoporosis defects play a key role in hip fracture. Bone 2017, 94, 124–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treece, G.; Gee, A. Cortical Bone Mapping: Measurement and Statistical Analysis of Localised Skeletal Changes. Curr. Osteoporos. Rep. 2018, 16, 617–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haralick, R.M. Statistical and Structural Approaches to Texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar] [CrossRef]
- Lowitz, T.; Museyko, O.; Bousson, V.; Laouisset, L.; Kalender, W.A.; Laredo, J.D.; Engelke, K. Bone marrow lesions identified by MRI in knee osteoarthritis are associated with locally increased bone mineral density measured by QCT. Osteoarthr. Cartil. 2013, 21, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [Green Version]
- Topp, T.; Müller, T.; Huss, S.; Kann, P.H.; Weihe, E.; Ruchholtz, S.; Zettl, R.P. Embalmed and fresh frozen human bones in orthopedic cadaveric studies: Which bone is authentic and feasible? A mechanical study. Acta Orthopaedica 2012, 83, 543–547. [Google Scholar] [CrossRef] [Green Version]
- Stefan, U.; Michael, B.; Werner, S. Effects of three different preservation methods on the mechanical properties of human and bovine cortical bone. Bone 2010, 47, 1048–1053. [Google Scholar] [CrossRef]


| Intra-Operator Inter-Scan | Inter-Operator Intra-Scan | Inter-Operator Inter-Scan | ||
|---|---|---|---|---|
| Femur | ||||
| Reliability # | 0.97 (0.94, 0.98) | 0.97 (0.93, 0.98) | 0.96 (0.93, 0.98) | |
| Reproducibility # | 9.56 (7.10, 13.46) | 9.67 (6.61, 14.68) | 10.75 (8.16, 14.96) | |
| Tibia | ||||
| Reliability *,# | 0.97 (0.96, 0.98) | 0.99 (0.98, 1.0) | 0.97 (0.95, 0.98) | |
| 5 | 6.58 (5.70, 8.49) | 3.97 (2.39, 7.39) | 7.29 (5.92, 10.30) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babel, H.; Omoumi, P.; Cosendey, K.; Cadas, H.; Jolles, B.M.; Favre, J. Three-Dimensional Quantification of Bone Mineral Density in the Distal Femur and Proximal Tibia Based on Computed Tomography: In Vitro Evaluation of an Extended Standardization Method. J. Clin. Med. 2021, 10, 160. https://doi.org/10.3390/jcm10010160
Babel H, Omoumi P, Cosendey K, Cadas H, Jolles BM, Favre J. Three-Dimensional Quantification of Bone Mineral Density in the Distal Femur and Proximal Tibia Based on Computed Tomography: In Vitro Evaluation of an Extended Standardization Method. Journal of Clinical Medicine. 2021; 10(1):160. https://doi.org/10.3390/jcm10010160
Chicago/Turabian StyleBabel, Hugo, Patrick Omoumi, Killian Cosendey, Hugues Cadas, Brigitte M. Jolles, and Julien Favre. 2021. "Three-Dimensional Quantification of Bone Mineral Density in the Distal Femur and Proximal Tibia Based on Computed Tomography: In Vitro Evaluation of an Extended Standardization Method" Journal of Clinical Medicine 10, no. 1: 160. https://doi.org/10.3390/jcm10010160





