Blend Hybrid Solid Electrolytes Based on LiTFSI Doped Silica-Polyethylene Oxide for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Apareance of Solutions and Materials
3.2. Structural Characterization
3.3. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katerina, S.A.H.; Aifantis, E.; Vasant Kumar, R. High Energy Density Lithium Batteries: Materials, Engineering, Applications; WILEY-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2014, 14, 359–367. [Google Scholar]
- Wang, H.; Matsui, M.; Takeda, Y.; Yamamoto, O.; Im, D.; Lee, D.; Imanishi, N. Interface Properties between Lithium Metal and a Composite Polymer Electrolyte of PEO18Li(CF3SO2)2N-Tetraethylene Glycol Dimethyl Ether. Membranes 2013, 3, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Tadanaga, K. Sol-Gel Processing of Solid Electrolytes for Li-Ion Batteries. In Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications; Springer: Berlin/Heidelberg, Germany, 2018; pp. 2631–2648. [Google Scholar]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic solid-state electrolytes for lithium batteries: Mechanisms and properties governing ion conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Gao, C.; Li, Z.B.; Wang, X.L.; Zhao, X.B.; Han, W.Q. Recent advances in inorganic solid electrolytes for lithium batteries. Front. Energy Res. 2014, 2, 25. [Google Scholar]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium ion Batteries. J. Mater. Chem. A 2015, 3, 19218–19233. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Jow, T.R.; Xue, K.; Borodin, O.; Ue, M. Electrolytes for Lithium and Lithium-ion Batteries; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef] [Green Version]
- Ki, J.; Gm Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103–16117. [Google Scholar] [CrossRef]
- Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.; Baggetto, L.; Notten, P.H. All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Fan, L.; Nan, C.W.; Zhao, S. Effect of modified SiO2 on the properties of PEO-based polymer electrolytes. Solid State Ion. 2003, 164, 81–86. [Google Scholar] [CrossRef]
- Mosa, J.; Aparicio, M. Hybrid Materials for High Ionic Conductivity, Sol-Gel Processing for Conventional and Alternative Energy; Springer: Berlin/Heidelberg, Germany, 2012; pp. 99–122. [Google Scholar]
- Gómez-Romero, P.; Sanchez, C. Functional Hybrid Materials; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Walcarius, A. Electrochemical applications of silica-based organic-inorganic hybrid materials. Chem. Mater. 2001, 13, 1589–1592. [Google Scholar] [CrossRef]
- Dahmouche, K. Investigation of new ion-conducting ORMOLYTES: Structure and properties. J. Sol Gel Sci. Technol. 1997, 8, 711–715. [Google Scholar] [CrossRef]
- Popall, M. New polymer lithium secondary batteries based on ORMOCER electrolytes inorganic-organic polymers. Electrochim. Acta 2001, 46, 1499–1508. [Google Scholar] [CrossRef]
- Saikia, D. New highly conductive organic-inorganic hybrid electrolytes based on star-branched silica based architectures. Polymer 2012, 53, 6008–6020. [Google Scholar] [CrossRef]
- Vélez, J.F.; Aparicio, M.; Mosa, J. Effect of lithium salt in nanostructured silica–polyethylene glycol solid electrolytes for Li-ion battery applications. J. Phys. Chem. C 2016, 120, 22852–22864. [Google Scholar] [CrossRef]
- Vélez, J.F.; Procaccini, R.A.; Aparicio, M.; Mosa, J. Epoxy-silica hybrid organic–inorganic electrolytes with a high Li-ion conductivity. Electrochim. Acta 2013, 110, 200–207. [Google Scholar] [CrossRef]
- Vélez, J.F.; Aparicio, M.; Mosa, J. Covalent silica-PEO-LiTFSI hybrid solid electrolytes via sol-gel for Li-ion battery applications. Electrochim. Acta 2016, 213, 831–841. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.Y. Synthesis, structure characterization and ionic conductivity of star-branched organiceinorganic hybrid electrolytes based on cyanuric chloride, diamine-capped poly(oxyalkylene) and alkoxysilane. Polymer 2010, 521, 4351–4361. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
- Cha, E.H. Ionic conductivity studies of polymeric electrolytes containing lithium salt with plasticizer. Electrochim. Acta 2004, 50, 335–338. [Google Scholar] [CrossRef]
- Rey, I. Infrared and Raman study of the PEO-LiTFSI polymer electrolyte. Electrochim. Acta 1998, 43, 1505–1510. [Google Scholar] [CrossRef]
- Stefanescu, M.; Stoia, M.; Stefanescu, O. Thermal and FT-IR study of the hybrid ethylene-glycol-silica matrix. J. Sol Gel Sci. Technol. 2007, 41, 71–78. [Google Scholar] [CrossRef]
- Jung, H.Y. Vibrational spectroscopic studies of sol-gel derived physical and chemical bonded ORMOSILs. J. Non Cryst. Solids 2005, 351, 372–379. [Google Scholar] [CrossRef]
- Ramesh, S.; Yuen, T.F.; Shen, C.J. Conductivity and FTIR studies on PEO–LiX [X: CF3SO3−, SO42−] polymer electrolytes. Spectrochim. Acta Part A 2008, 69, 670–675. [Google Scholar] [CrossRef]
- Bakker, A.; Gejji, S.; Lindgren, J.; Hermansson, K.; Probst, M.M. Contact ion pair formation and ether oxygen coordination in the polymer electrolytes M[N(CF3SO2)2]2PEOn for M = Mg, Ca, Sr and Ba. Polymer 1995, 36, 4371–4378. [Google Scholar] [CrossRef]
- Zelazowska, E.; Rysiakiewicz-Pasek, E. Hybrid materials doped with lithium ions. Opt. Appl. 2010, XL, 383–396. [Google Scholar]
- Deepa, M.; Agnihotry, S.A.; Gupta, D.; Chandra, R. Ion-pairing effects and ion–solvent–polymer interactions in LiN(CF3SO2)2–PC–PMMA electrolytes: A FTIR study. Electrochim. Acta 2004, 49, 373–383. [Google Scholar] [CrossRef]
- Vargas, G.; Acevedo, J.L.; López, J.; Romero, J. Study of cross-linking of gelatin by ethylene glycol diglycidyl ether. Mater. Lett. 2008, 62, 3656–3658. [Google Scholar] [CrossRef]
- Huang, W.; Frech, R. Dependence of ionic association on polymer chain length in poly(ethylene oxide)-lithium triflate complexes. Polymer 1994, 35, 235–242. [Google Scholar] [CrossRef]
- Fischer, D.; Pospiech, D.; Scheler, U.; Navarro, R.; Messori, M.; Fabbri, P. Monitoring of the sol-gel synthesis of organic-inorganic hybrids by FTIR transmission, FTIR/ATR, NIR and Raman spectroscopy. Macromol. Symp. 2008, 265, 134–143. [Google Scholar] [CrossRef]
- Alía, J.M.; Edwards, H.G. Ion solvation and ion association in lithium trifluoromethanesulfonate solutions in three aprotic solvents. An FT-Raman spectroscopic study. Vib. Spectrosc. 2000, 24, 185–200. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.R. Spectroscopic characterization of GPTMS/DETA and GPTMS/EDA hybrid polymers. J. Non Cryst. Solids 2007, 353, 1598–1605. [Google Scholar] [CrossRef]
- Riegel, B.; Blittersdorf, S.; Kiefer, W.; Hofacker, S.; Müller, M.; Schottner, G. Kinetic investigations of hydrolysis and condensation of the glycidoxypropyltrimethoxysilaneraminopropyltriethoxy-silane system by means of FT-Raman spectroscopy I. J. Non Cryst. Solids 1998, 226, 76–84. [Google Scholar]
- Gnyba, M.; Keränen, M.; Kozanecki, M.; Bogdanowicz, R.; Kosmowski, B.; Wroczynski, P. Raman Investigation of sol-gel-derived hybrid polymers for optoelectronics. Optoelectron. Rev. 2002, 10, 137–143. [Google Scholar]
- Popall, M.; Andrei, M.; Kappel, J.; Krona, J.; Olma, K.; Olsowski, B. ORMOCERs as inorganic-organic electrolytes for new solid state lithium batteries and supercapacitors. Electrochim. Acta 1998, 43, 1155–1161. [Google Scholar] [CrossRef]
- Le Nest, J.F.; Gandini, A.; Cheradame, H.; Cohen-Addad, J.P. Influence of lithium perchlorate on the properties of polyether networks: Specific volume and glass transition temperature. Macromolecules 1988, 21, 1117–1120. [Google Scholar] [CrossRef]
- Queiroz, S.M.; Machado, J.C.; Porto, A.O.; Silva, G.G. Positron annihilation and differential scanning calorimetry studies of plasticized poly(ethylene oxide). Polymer 2001, 42, 3095–3101. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Fujinami, T.; Mehta, M.A.; Sugie, K.; Mori, K. Molecular design of inorganic–organic hybrid polyelectrolytes to enhance lithium ion conductivity. Electrochim. Acta 2000, 45, 1181–1186. [Google Scholar] [CrossRef]
- Xi, J.; Xiaomei, M.; Cui, M.; Huang, X.; Zheng, Z. Electrochemistry study on PEO-LiClO4-ZSM5 composite polymer electrolyte. Chin. Sci. Bull. 2004, 49, 785–789. [Google Scholar] [CrossRef]
- Watanabe, M.; Nishimoto, A. Effects of network structures and incorporated salt species on electrochemical properties of polyether-based polymer electrolytes. Solid State Ion. 1995, 79, 306–312. [Google Scholar] [CrossRef]
- Nishimoto, A.; Watanabe, M.; Ikeda, Y.; Kohjiya, S. High ionic conductivity of new polymer electrolytes based on high molecular weight polyether comb polymers. Electrochim. Acta 1998, 43, 1177–1184. [Google Scholar] [CrossRef]
- Nishimoto, A.; Agehara, K.; Furuya, N.; Watanabe, T.; Watanabe, M. High Ionic Conductivity of Polyether-Based Network Polymer Electrolytes with Hyperbranched Side Chains. Macromolecules 1999, 32, 1541–1548. [Google Scholar] [CrossRef]
- Xing, Y.; Xing, X.; Li, Y.; Yang, G.; Li, W. PVDF-based composite microporous gel polymer electrolytes containing a novelsingle ionic conductor SiO2(Li+). Electrochim. Acta 2013, 12, 183–190. [Google Scholar]
- Wu, C.G.; Lu, M.I.; Tsai, C.C.; Chuang, H.J. PVdF-HFP/metal oxide nanocomposites: The matrices for high-conducting, low-leakage porous polymer electrolytes. J. Power Sources 2006, 159, 295–300. [Google Scholar] [CrossRef]
- Angaiah, S.; Sundaran, N.; Jayakumar, S.P.; Kumar, G.V. Preparation of a microporous gel polymer electrolyte with a novel preferential polymer dissolution process for Li-ion batteries. J. Appl. Polym. Sci. 2005, 98, 1891–1896. [Google Scholar]
- Borghini, M.C.; Mastragostino, M.; Zanelli, A. Reliability of lithium batteries with crosslinked polymer electrolytes. Electrochim. Acta 1996, 41, 2369–2373. [Google Scholar] [CrossRef]
- Lobitz, P.; Füllbier, H.; Reiche, A.; Illner, J.C.; Reuter, H.; Höring, S. Ionic conductivity in poly (ethylene oxide)-poly (alkylmethacrylate)-block copolymer mixtures with LiI. Solid State Ion. 1992, 58, 41–48. [Google Scholar] [CrossRef]
- Geiculescu, O.E.; Rajagopal, R.; Creager, S.E.; DesMarteau, D.D.; Zhang, X.; Fedkiw, P. Transport properties of solid polymer electrolytes prepared from oligomeric fluorosulfonimide lithium salts dissolved in high molecular weight poly(ethylene oxide). J. Phys. Chem. B 2006, 110, 23130–23135. [Google Scholar] [CrossRef]








| Composition | Composition (mol) | ||||||
|---|---|---|---|---|---|---|---|
| GPTMS | TPTE | n-MI | EtOH | LiTFSI | [Li+]/[O] | GPTMS:TPTE %mol | |
| GTT-1 | 1.0 | 0.18 | 0.06 | 11.8 | 0.15 | 0.10 | 85:15 |
| GTT-2 | 1.0 | 0.43 | 0.09 | 14.3 | 0.23 | 0.10 | 70:30 |
| GTT-3 | 1.0 | 0.67 | 0.12 | 16.7 | 0.30 | 0.10 | 60:40 |
| GTT-4 | 1.0 | 1.00 | 0.16 | 20.0 | 0.40 | 0.10 | 50:50 |
| GTT-5 | 1.0 | 0.43 | 0.09 | 14.3 | 0.00 | 0.00 | 70:30 |
| GPTMS | TPTE | Hybrid Network | Assignments | Reference |
|---|---|---|---|---|
| 1643 | ν(C = C) (CH = CH of n-MI) | [37] | ||
| 1480–1370 | 1480–1370 | δCH3 of GPTMS | [34,37] | |
| 1462–1456 | 1454 | δasHCH/CH2 scissor/δ(CH2) | [34] | |
| 1420 | 1410–1414 | δCH2/νas(C-O-O) | [39] | |
| 1352 | CH2 wagg/νas(SO2) of LiTFSI | [38] | ||
| 1332 | νas(SO2) of LiTFSI | [35] | ||
| 1273–1294 | ν (C-Si-O) | [26,34] | ||
| 1254 | 1253 | 1253 | δ(Si-CH2) | [34] |
| 1260–1240 | 1250; 909–907 | ν epóxido | [26] | |
| 1227 | νsCF3 of LITFSI | [35,37] | ||
| 1194–1189 | νas(Si-O-Si)LO | [26,37] | ||
| 1136 | ν(-Si-O-Si-O-) (crosslinking)/νs(SO2) of LiTFSI | [32,34,37] | ||
| 1093 | 1092 | νas(Si-O)/νas(C-O)/νasCF3 of LiTFSI | [32,34,35] | |
| 1046 | νs(C-O-C)/νas(Si-O-Si) | [32,34] | ||
| 993–989 | 956 | ν(C-O-C) | [34,39] | |
| 884–880 | δCH2/ν(C-OH) | [35,37] | ||
| 854–852 | δ(Si-O) | [37] | ||
| 846 | ν(-Si-O-Si-) (crosslinking) | [37] | ||
| 816 | ν(Si-O-CH3) | [26,36] | ||
| 795–787 | νs(Si-O-Si) | [36] | ||
| 756* | ν(S-N) of LiTFSI | [35,37] | ||
| 750 | δ(Si-O-CH3) | [39] | ||
| 740 | νs(S-N-S) of LiTFSI | [35,37] |
| Composition | σ25 °C (S/cm) | σ60 °C (S/cm) | Ea (eV) | tLi+ (25 °C) |
|---|---|---|---|---|
| GTT-1 | 4.9 × 10−6 | 4.3 × 10−5 | 0.62 | 0.36 |
| GTT-2 | 1.3 × 10−5 | 1.0 × 10−4 | 0.56 | 0.40 |
| GTT-3 | 1.8 × 10−5 | 1.4 × 10−4 | 0.51 | 0.45 |
| GTT-4 | 3.1 × 10−6 | 3.8 × 10−5 | 0.67 | 0.31 |
| GTT-5 | ~10−11 | ~10−11 | - | - |
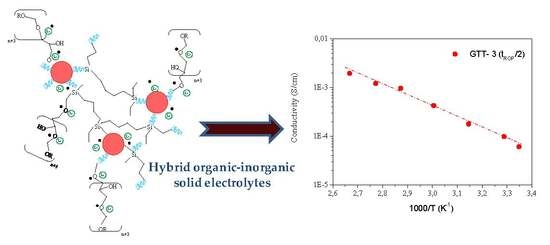
| GTT-3(TROP/2) | 1.3 × 10−4 | 1.4 × 10−3 | 0.49 | 0.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosa, J.; Vélez, J.F.; Aparicio, M. Blend Hybrid Solid Electrolytes Based on LiTFSI Doped Silica-Polyethylene Oxide for Lithium-Ion Batteries. Membranes 2019, 9, 109. https://doi.org/10.3390/membranes9090109
Mosa J, Vélez JF, Aparicio M. Blend Hybrid Solid Electrolytes Based on LiTFSI Doped Silica-Polyethylene Oxide for Lithium-Ion Batteries. Membranes. 2019; 9(9):109. https://doi.org/10.3390/membranes9090109
Chicago/Turabian StyleMosa, Jadra, Jonh Fredy Vélez, and Mario Aparicio. 2019. "Blend Hybrid Solid Electrolytes Based on LiTFSI Doped Silica-Polyethylene Oxide for Lithium-Ion Batteries" Membranes 9, no. 9: 109. https://doi.org/10.3390/membranes9090109