Organosilica-Based Membranes in Gas and Liquid-Phase Separation
Abstract
:1. Introduction
2. Pure Organosilica Membranes
2.1. Sol-Gel Method
2.1.1. Effect of the Composition Ratio
2.1.2. Effect of Calcination Temperature
2.1.3. Effect of Other Factors
2.2. CVD Method
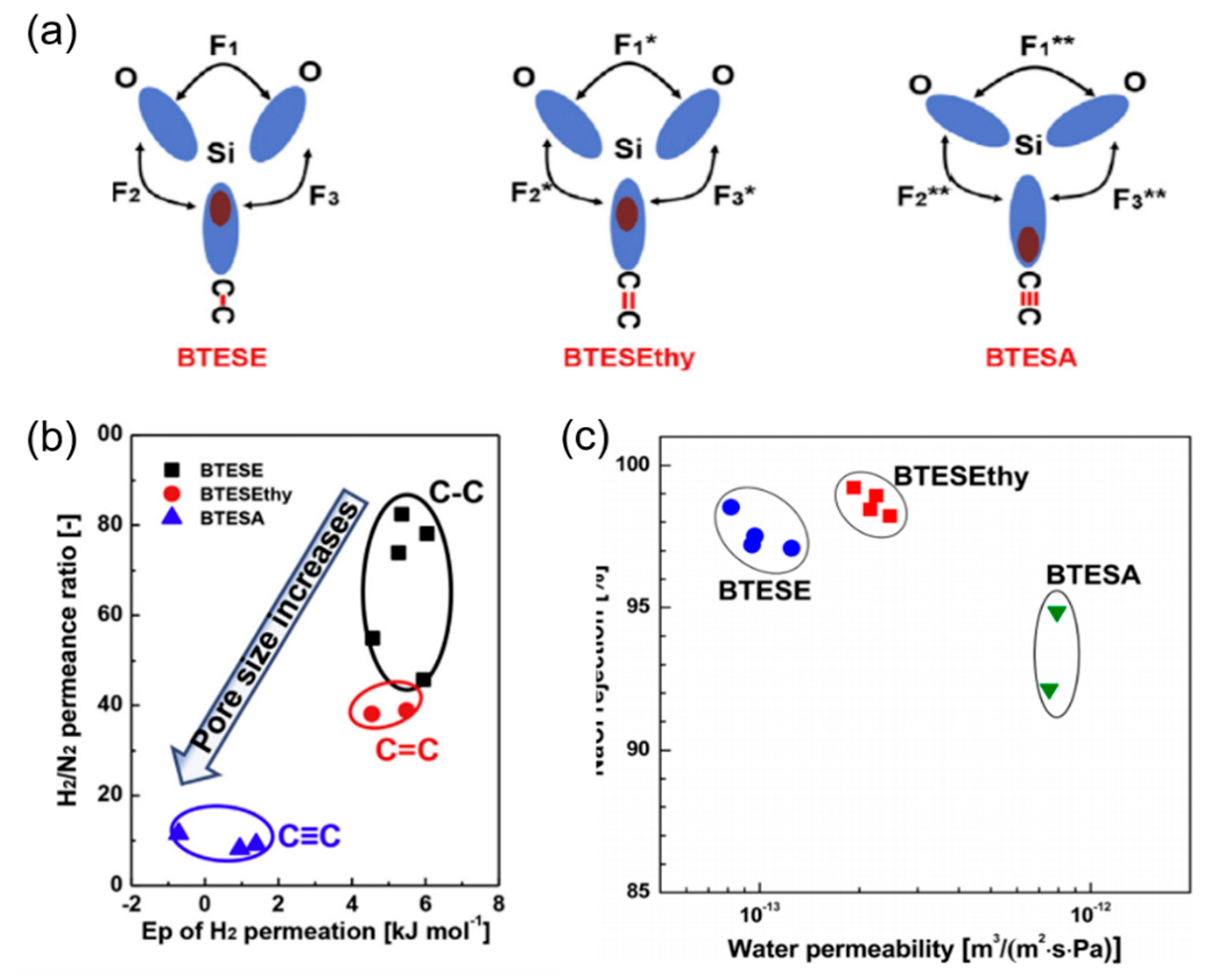
2.3. The Effect of Organic Groups
3. Hybrid Organosilica Membrane
3.1. Metal Doping
3.2. Alkoxysilane Co-Condensation
3.3. Other Types of Hybrid Organosilica
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APTES or PA | 3-aminopropyltriethoxysilane |
| BTESM | bis(triethoxysilyl)methane |
| BTESE | 1,2-bis(triethoxysilyl)ethane |
| BTESP | 1,3-bis(triethoxysilyl)propane |
| BTMSH | 1,6-bis(trimethoxysilyl)hexane |
| BTESB | bis(triethoxysilyl)benzene |
| BTESO | 1,8-bis(triethoxysilyl)octane |
| BTESEthy | 1,2-bis(triethoxysilyl)ethylene |
| BTESA | 1,2-bis(triethoxysilyl)acetylene |
| BTES-ED | 2,5-bis[2 -(triethoxysilyl)ethyl]-1,4-dioxane |
| BTES-MAz | 1,4-bis(triethoxysilylmethyl)-1,2,3-triazole |
| BTMES | bis(trimethoxysilyl)ethane |
| BTMSN or BTMS-Nor | bis(trimethoxysilyl)norbornane |
| BTPP | 4,6-bis(3-triethoxysilyl-1-propoxy)-1,3-pyrimidine |
| DMDMS | dimethoxydimethylsilane |
| DMDPS | dimethoxydiphenylsilane |
| HMDS or HMDSO | Hexamethyldisiloxane |
| HMTES | hydroxymethyl(triethoxy)silane |
| IM | N-(3-triethoxysilylpropyl)-4,5-dihydroimidazole |
| LDA | 3-(2-aminoethylamino)propyl-trimethoxysilane |
| MTES | methyltriethoxysilane |
| MTMS, or MTMOS | methyltrimethoxysilane |
| PhTES | phenyltriethoxysilane |
| PTMS | phenyltrimethoxysilane |
| QA | 3-(triethoxysilyl)-N, N-dimethylpropan-1-amine |
| SA | 4,6-bis(3-triethoxysilyl-1-propoxy)-1,3-pyrimidine |
| TA | 3-(triethoxysilyl)-N-methylpropan-1-amine |
| TEFS | triethoxyfluorosilane |
| TMMS, or TMMOS | trimethylmethoxysilane |
| TRIES | triethoxysilane |
| TTESPT | 2,4,6-tris[3-(triethoxysilyl)-1-propoxy]-1,3,5-triazine |
| TPMS | triphenylmethoxysilane |
References
- Kim, H.J.; Yang, H.C.; Chung, D.Y.; Yang, I.H.; Choi, Y.J.; Moon, J.K. Functionalized Mesoporous Silica Membranes for CO2 Separation Applications. J. Chem. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- De Vos, R.M.; Verweij, H. High-Selectivity, High-Flux Silica Membranes for Gas Separation. Science 1998, 279, 1710. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, K.; Sakamoto, S.; Saie, T.; Morooka, S. Pore structure of silica membranes formed by a sol–gel technique using tetraethoxysilane and alkyltriethoxysilanes. Sep. Purif. Technol. 1999, 16, 139–146. [Google Scholar] [CrossRef]
- Tsuru, T. Nano/subnano-tuning of porous ceramic membranes for molecular separation. J. Sol Gel Sci. Technol. 2008, 46, 349–361. [Google Scholar] [CrossRef]
- Lin, Y.S.; Kumakiri, I.; Nair, B.N.; Alsyouri, H. Microporous Inorganic Membranes. Sep. Purif. Methods 2007, 31, 229–379. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous inorganic membranes for CO2 capture: Present and prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef] [PubMed]
- Asaeda, M.; Yamasaki, S. Separation of inorganic/organic gas mixtures by porous silica membranes. Sep. Purif. Technol. 2001, 25, 151–159. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, R.; Song, C.; Zhang, B.; Liu, Q.; Wang, T. A Review on the Progress in Nanoparticle/C Hybrid CMS Membranes for Gas Separation. Membranes 2018, 8, 134. [Google Scholar] [CrossRef]
- Tsuru, T. Silica-Based Membranes with Molecular-Net-Sieving Properties: Development and Applications. J. Chem. Eng. Jpn. 2018, 51, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Battersby, S.; Smart, S.; Ladewig, B.; Liu, S.; Duke, M.C.; Rudolph, V.; Costa, J.C.D.D. Hydrothermal stability of cobalt silica membranes in a water gas shift membrane reactor. Sep. Purif. Technol. 2009, 66, 299–305. [Google Scholar] [CrossRef]
- Duke, M.C.; da Costa, J.C.D.; Do, D.D.; Gray, P.G.; Lu, G.Q. Hydrothermally Robust Molecular Sieve Silica for Wet Gas Separation. Adv. Funct. Mater. 2006, 16, 1215–1220. [Google Scholar] [CrossRef]
- Tsuru, T.; Wada, S.I.; Izumi, S.; Asaeda, M. Silica–zirconia membranes for nanofiltration. J. Membr. Sci. 1998, 149, 127–135. [Google Scholar] [CrossRef]
- Agirre, I.; Arias, P.L.; Castricum, H.L.; Creatore, M.; ten Elshof, J.E.; Paradis, G.G.; Ngamou, P.H.T.; van Veen, H.M.; Vente, J.F. Hybrid organosilica membranes and processes: Status and outlook. Sep. Purif. Technol. 2014, 121, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Kanezashi, M.; Yoneda, Y.; Nagasawa, H.; Tsuru, T.; Yamamoto, K.; Ohshita, J. Gas permeation properties for organosilica membranes with different Si/C ratios and evaluation of microporous structures. AIChE J. 2017, 63, 4491–4498. [Google Scholar] [CrossRef]
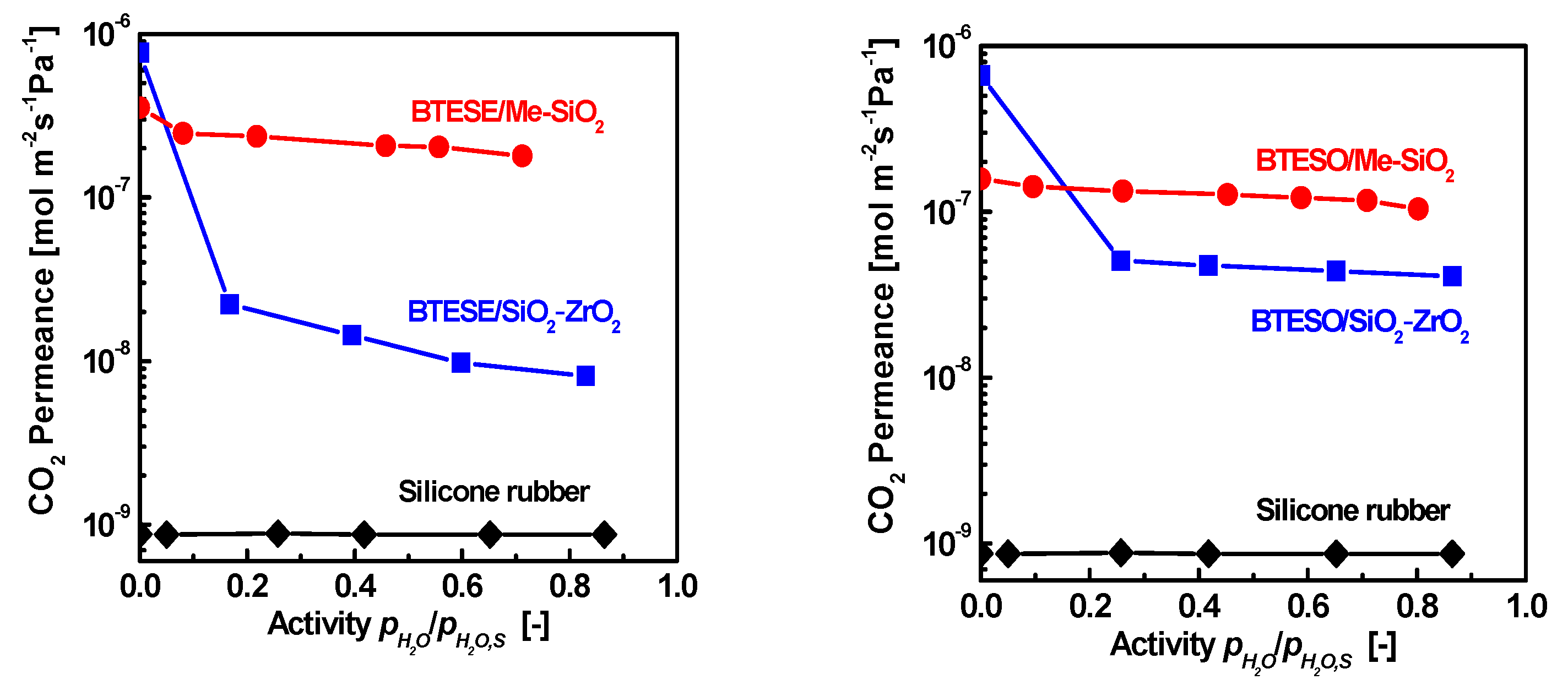
- Ren, X.; Nishimoto, K.; Kanezashi, M.; Nagasawa, H.; Yoshioka, T.; Tsuru, T. CO2 Permeation through Hybrid Organosilica Membranes in the Presence of Water Vapor. Ind. Eng. Chem. Res. 2014, 53, 6113–6120. [Google Scholar] [CrossRef]
- Castricum, H.L.; Paradis, G.G.; Mittelmeijer-Hazeleger, M.C.; Kreiter, R.; Vente, J.F.; ten Elshof, J.E. Tailoring the Separation Behavior of Hybrid Organosilica Membranes by Adjusting the Structure of the Organic Bridging Group. Adv. Funct. Mater. 2011, 21, 2319–2329. [Google Scholar] [CrossRef] [Green Version]
- Van Veen, H.M.; Rietkerk, M.D.A.; Shanahan, D.P.; van Tuel, M.M.A.; Kreiter, R.; Castricum, H.L.; ten Elshof, J.E.; Vente, J.F. Pushing membrane stability boundaries with HybSi (R) pervaporation membranes. J. Membr. Sci. 2011, 380, 124–131. [Google Scholar] [CrossRef]
- Xu, R.; Ibrahim, S.M.; Kanezashi, M.; Yoshioka, T.; Ito, K.; Ohshita, J.; Tsuru, T. New insights into the microstructure-separation properties of organosilica membranes with ethane, ethylene, and acetylene bridges. ACS Appl. Mater. Interfaces 2014, 6, 9357–9364. [Google Scholar] [CrossRef]
- Xu, R.; Kanezashi, M.; Yoshioka, T.; Okuda, T.; Ohshita, J.; Tsuru, T. Tailoring the affinity of organosilica membranes by introducing polarizable ethenylene bridges and aqueous ozone modification. ACS Appl. Mater. Interfaces 2013, 5, 6147–6154. [Google Scholar] [CrossRef]
- Kanezashi, M.; Matsutani, T.; Nagasawa, H.; Tsuru, T. Fluorine-induced microporous silica membranes: Dramatic improvement in hydrothermal stability and pore size controllability for highly permeable propylene/propane separation. J. Membr. Sci. 2018, 549, 111–119. [Google Scholar] [CrossRef]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Role of Amine Type in CO2 Separation Performance within Amine Functionalized Silica/Organosilica Membranes: A Review. Appl. Sci. 2018, 8, 32. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Tsai, C.Y.; Brinker, C.J. Microporous sol–gel derived aminosilicate membrane for enhanced carbon dioxide separation. Sep. Purif. Technol. 2005, 42, 249–257. [Google Scholar] [CrossRef]
- Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Effect of calcination temperature on the PV dehydration performance of alcohol aqueous solutions through BTESE-derived silica membranes. J. Membr. Sci. 2012, 415, 810–815. [Google Scholar] [CrossRef]
- Yu, X.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Improved thermal and oxidation stability of bis(triethoxysilyl)ethane (BTESE)-derived membranes, and their gas-permeation properties. J. Mater. Chem. A 2018, 6, 23378–23387. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Organic–inorganic hybrid silica membranes with controlled silica network size: Preparation and gas permeation characteristics. J. Membr. Sci. 2010, 348, 310–318. [Google Scholar] [CrossRef]
- Kanezashi, M.; Miyauchi, S.; Nagasawa, H.; Yoshioka, T.; Tsuru, T. Gas permeation properties through Al-doped organosilica membranes with controlled network size. J. Membr. Sci. 2014, 466, 246–252. [Google Scholar] [CrossRef]
- Kanezashi, M.; Murata, M.; Nagasawa, H.; Tsuru, T. Fluorine Doping of Microporous Organosilica Membranes for Pore Size Control and Enhanced Hydrophobic Properties. ACS Omega 2018, 3, 8612–8620. [Google Scholar] [CrossRef]
- Chai, S.; Du, H.; Zhao, Y.; Lin, Y.; Kong, C.; Chen, L. Fabrication of highly selective organosilica membrane for gas separation by mixing bis(triethoxysilyl)ethane with methyltriethoxysilane. Sep. Purif. Technol. 2019, 222, 162–167. [Google Scholar] [CrossRef]
- Gong, G.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Tailoring the Separation Behavior of Polymer-Supported Organosilica Layered-Hybrid Membranes via Facile Post-Treatment Using HCl and HN3 Vapors. ACS Appl. Mater. Interfaces 2016, 8, 11060–11069. [Google Scholar] [CrossRef]
- Kanezashi, M.; Sazaki, H.; Nagasawa, H.; Yoshioka, T.; Tsuru, T. Preparation and gas permeation properties of thermally stable organosilica membranes derived by hydrosilylation. J. Mater. Chem. A 2014, 2, 672–680. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ohshita, J.; Mizumo, T.; Kanezashi, M.; Tsuru, T. Preparation of hydroxyl group containing bridged organosilica membranes for water desalination. Sep. Purif. Technol. 2015, 156, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.L.; Li, C.L.; Wang, Y. In-Situ crosslinked PVA/organosilica hybrid membranes for pervaporation separations. J. Membr. Sci. 2016, 498, 263–275. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Beland, F.; Ilharco, L.M.; Pagliaro, M. The sol−gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef]
- Niimi, T.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Ito, K.; Tsuru, T. Preparation of BTESE-derived organosilica membranes for catalytic membrane reactors of methylcyclohexane dehydrogenation. J. Membr. Sci. 2014, 455, 375–383. [Google Scholar] [CrossRef]
- Kanezashi, M.; Matsugasako, R.; Tawarayama, H.; Nagasawa, H.; Tsuru, T. Pore size tuning of sol−gel-derived triethoxysilane (TRIES) membranes for gas separation. J. Membr. Sci. 2017, 524, 64–72. [Google Scholar] [CrossRef]
- Castricum, H.L.; Qureshi, H.F.; Nijmeijer, A.; Winnubst, L. Hybrid silica membranes with enhanced hydrogen and CO2 separation properties. J. Membr. Sci. 2015, 488, 121–128. [Google Scholar] [CrossRef]
- Moriyama, N.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Pervaporation dehydration of aqueous solutions of various types of molecules via organosilica membranes: Effect of membrane pore sizes and molecular sizes. Sep. Purif. Technol. 2018, 207, 108–115. [Google Scholar] [CrossRef]
- Yu, X.; Meng, L.; Niimi, T.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Network engineering of a BTESE membrane for improved gas performance via a novel pH-swing method. J. Membr. Sci. 2016, 511, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Wei, Y.; Wang, C.; Zhao, S.; Qi, H. Tuning sol size to optimize organosilica membranes for gas separation. Chin. J. Chem. Eng. 2018, 26, 53–59. [Google Scholar] [CrossRef]
- Kanezashi, M.; Shioda, T.; Gunji, T.; Tsuru, T. Gas permeation properties of silica membranes with uniform pore sizes derived from polyhedral oligomeric silsesquioxane. AIChE J. 2012, 58, 1733–1743. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Reverse osmosis performance of organosilica membranes and comparison with the pervaporation and gas permeation properties. AIChE J. 2013, 59, 1298–1307. [Google Scholar] [CrossRef]
- Kanezashi, M.; Shazwani, W.N.; Yoshioka, T.; Tsuru, T. Separation of propylene/propane binary mixtures by bis(triethoxysilyl) methane (BTESM)-derived silica membranes fabricated at different calcination temperatures. J. Membr. Sci. 2012, 415–416, 478–485. [Google Scholar] [CrossRef]
- Gong, G.; Wang, J.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Fabrication of a layered hybrid membrane using an organosilica separation layer on a porous polysulfone support, and the application to vapor permeation. J. Membr. Sci. 2014, 464, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Gong, G.; Wang, J.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Sol–gel spin coating process to fabricate a new type of uniform and thin organosilica coating on polysulfone film. Mater. Lett. 2013, 109, 130–133. [Google Scholar] [CrossRef]
- Nishibayashi, M.; Yoshida, H.; Uenishi, M.; Kanezashi, M.; Nagasawa, H.; Yoshioka, T.; Tsuru, T. Photo-induced sol–gel processing for low-temperature fabrication of high-performance silsesquioxane membranes for use in molecular separation. Chem. Commun. 2015, 51, 9932–9935. [Google Scholar] [CrossRef]
- Nagasawa, H.; Nishibayashi, M.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Photo-induced sol−gel synthesis of polymer-supported silsesquioxane membranes. RSC Adv. 2017, 7, 7150–7157. [Google Scholar] [CrossRef]
- Lee, H.R.; Shibata, T.; Kanezashi, M.; Mizumo, T.; Ohshita, J.; Tsuru, T. Pore-size-controlled silica membranes with disiloxane alkoxides for gas separation. J. Membr. Sci. 2011, 383, 152–158. [Google Scholar] [CrossRef]
- Akamatsu, K.; Suzuki, M.; Nakao, A.; Nakao, S.I. Development of hydrogen-selective dimethoxydimethylsilane-derived silica membranes with thin active separation layer by chemical vapor deposition. J. Membr. Sci. 2019, 580, 268–274. [Google Scholar] [CrossRef]
- Sea, B.K.; Kusakabe, K.; Morooka, S. Pore size control and gas permeation kinetics of silica membranes by pyrolysis of phenyl-substituted ethoxysilanes with cross-flow through a porous support wall. J. Membr. Sci. 1997, 130, 41–52. [Google Scholar] [CrossRef]
- Li, G.; Kanezashi, M.; Tsuru, T. Preparation of organic–inorganic hybrid silica membranes using organoalkoxysilanes: The effect of pendant groups. J. Membr. Sci. 2011, 379, 287–295. [Google Scholar] [CrossRef]
- Suzuki, S.; Messaoud, S.B.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Development of inorganic–organic hybrid membranes for carbon dioxide/methane separation. J. Membr. Sci. 2014, 471, 402–411. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yamada, H.; Saito, T.; Kai, T.; Murakami, K.; Nakashima, M.; Ohshita, J.; Akamatsu, K.; Nakao, S.I. Development of hydrogen-selective triphenylmethoxysilane-derived silica membranes with tailored pore size by chemical vapor deposition. J. Membr. Sci. 2016, 499, 28–35. [Google Scholar] [CrossRef]
- Ohta, Y.; Akamatsu, K.; Sugawara, T.; Nakao, A.; Miyoshi, A.; Nakao, S.I. Development of pore size-controlled silica membranes for gas separation by chemical vapor deposition. J. Membr. Sci. 2008, 315, 93–99. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Reis, R.; Chen, Z.; Milne, N.; Winther-Jensen, B.; Kong, L.; Dumee, L.F. Plasma Modification and Synthesis of Membrane Materials-A Mechanistic Review. Membranes 2018, 8. [Google Scholar] [CrossRef]
- Kafrouni, W.; Rouessac, V.; Julbe, A.; Durand, J. Synthesis and characterization of silicon carbonitride films by plasma enhanced chemical vapor deposition (PECVD) using bis(dimethylamino)dimethylsilane (BDMADMS), as membrane for a small molecule gas separation. Appl. Surf. Sci. 2010, 257, 1196–1203. [Google Scholar] [CrossRef]
- Ngamou, P.H.T.; Overbeek, J.P.; Kreiter, R.; van Veen, H.M.; Vente, J.F.; Wienk, I.M.; Cuperus, P.F.; Creatore, M. Plasma-deposited hybrid silica membranes with a controlled retention of organic bridges. J. Mater. Chem. A 2013, 1, 5567–5576. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, H.; Minamizawa, T.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Microporous organosilica membranes for gas separation prepared via PECVD using different O/Si ratio precursors. J. Membr. Sci. 2015, 489, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Tsuru, T.; Shigemoto, H.; Kanezashi, M.; Yoshioka, T. 2-Step plasma-enhanced CVD for low-temperature fabrication of silica membranes with high gas-separation performance. Chem. Commun. 2011, 47, 8070. [Google Scholar] [CrossRef]
- Nagasawa, H.; Yamamoto, Y.; Tsuda, N.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Atmospheric-pressure plasma-enhanced chemical vapor deposition of microporous silica membranes for gas separation. J. Membr. Sci. 2017, 524, 644–651. [Google Scholar] [CrossRef] [Green Version]
- Kreiter, R.; Rietkerk, M.D.A.; Castricum, H.L.; van Veen, H.M.; ten Elshof, J.E.; Vente, J.F. Evaluation of hybrid silica sols for stable microporous membranes using high-throughput screening. J. Sol Gel Sci. Technol. 2010, 57, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Kanezashi, M.; Kawano, M.; Yoshioka, T.; Tsuru, T. Organic-Inorganic Hybrid Silica Membranes with Controlled Silica Network Size for Propylene/Propane Separation. Ind. Eng. Chem. Res. 2011, 51, 944–953. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Xu, R.; Nagasawa, H.; Naka, A.; Ohshita, J.; Yoshioka, T.; Kanezashi, M.; Tsuru, T. Insight into the pore tuning of triazine-based nitrogen-rich organoalkoxysilane membranes for use in water desalination. RSC Adv. 2014, 4, 23759–23769. [Google Scholar] [CrossRef]
- Xu, R.; Lin, P.; Zhang, Q.; Zhong, J.; Tsuru, T. Development of Ethenylene-Bridged Organosilica Membranes for Desalination Applications. Ind. Eng. Chem. Res. 2016, 55, 2183–2190. [Google Scholar] [CrossRef]
- Yamamoto, K.; Muragishi, H.; Mizumo, T.; Gunji, T.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Diethylenedioxane-bridged microporous organosilica membrane for gas and water separation. Sep. Purif. Technol. 2018, 207, 370–376. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Xu, R.; Nagasawa, H.; Naka, A.; Ohshita, J.; Yoshioka, T.; Kanezashi, M.; Tsuru, T. A closer look at the development and performance of organic–inorganic membranes using 2,4,6-tris[3(triethoxysilyl)-1-propoxyl]-1,3,5-triazine (TTESPT). RSC Adv. 2014, 4, 12404. [Google Scholar] [CrossRef]
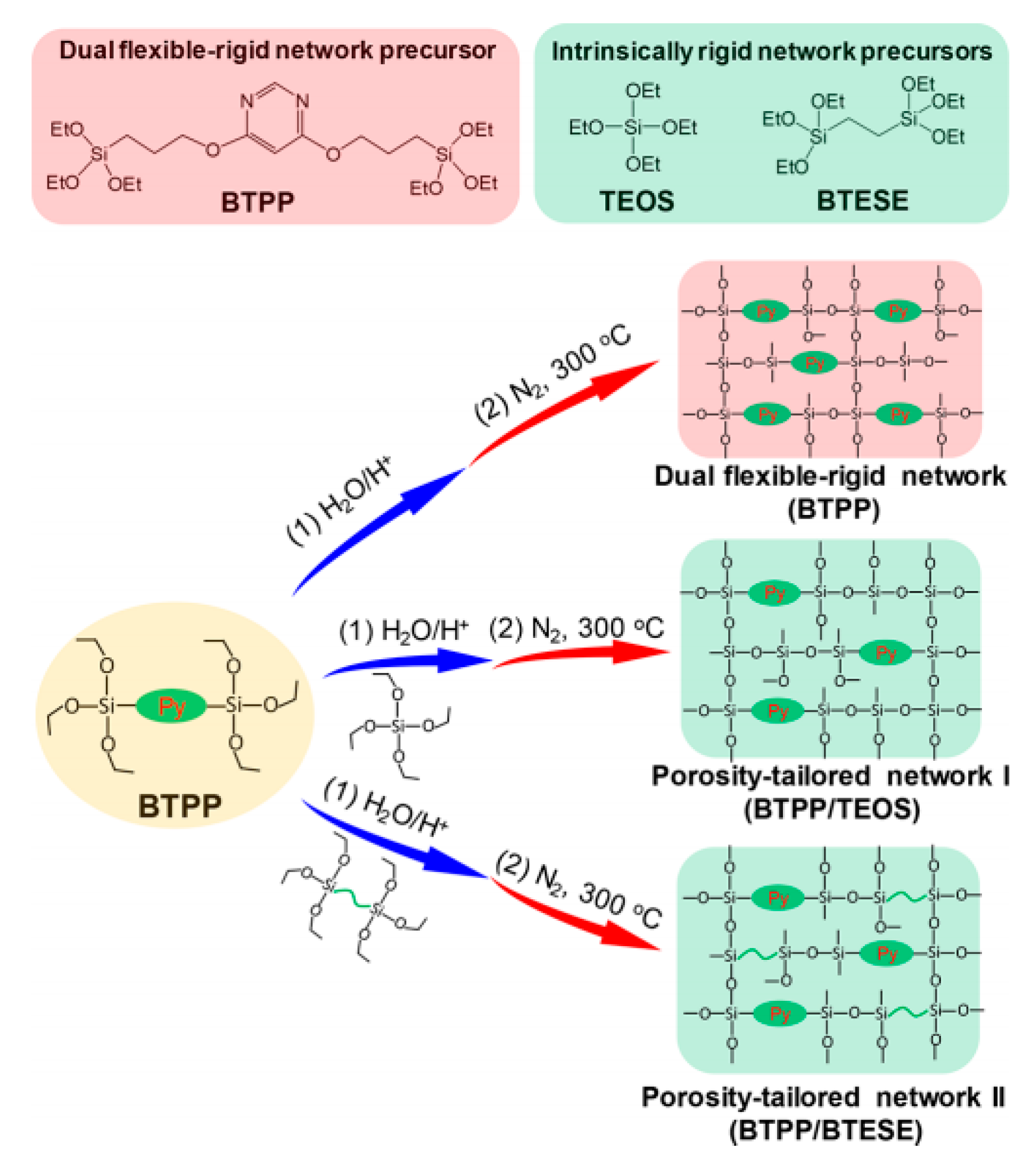
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Oshita, J.; Naka, A.; Tsuru, T. Pyrimidine-bridged organoalkoxysilane membrane for high-efficiency CO2 transport via mild affinity. Sep. Purif. Technol. 2017, 178, 232–241. [Google Scholar] [CrossRef]
- Wang, J.; Gong, G.; Kanezashi, M.; Yoshioka, T.; Ito, K.; Tsuru, T. Pervaporation performance and characterization of organosilica membranes with a tuned pore size by solid-phase HCl post-treatment. J. Membr. Sci. 2013, 441, 120–128. [Google Scholar] [CrossRef]
- Nagasawa, H.; Matsuda, N.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Pervaporation and vapor permeation characteristics of BTESE-derived organosilica membranes and their long-term stability in a high-water-content IPA/water mixture. J. Membr. Sci. 2016, 498, 336–344. [Google Scholar] [CrossRef]
- Castricum, H.L.; Kreiter, R.; van Veen, H.M.; Blank, D.H.A.; Vente, J.F.; ten Elshof, J.E. High-performance hybrid pervaporation membranes with superior hydrothermal and acid stability. J. Membr. Sci. 2008, 324, 111–118. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Robust organosilica membranes for high temperature reverse osmosis (RO) application: Membrane preparation, separation characteristics of solutes and membrane regeneration. J. Membr. Sci. 2015, 493, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Ohshita, J.; Muragishi, H.; Yamamoto, K.; Mizumo, T.; Kanezashi, M.; Tsuru, T. Preparation and separation properties of porous norbornane-bridged silica membrane. J. Sol Gel Sci. Technol. 2014, 73, 365–370. [Google Scholar] [CrossRef]
- Yamamoto, K.; Koge, S.; Sasahara, K.; Mizumo, T.; Kaneko, Y.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Preparation of Bridged Polysilsesquioxane Membranes from Bis[3-(triethoxysilyl)propyl]amine for Water Desalination. Bull. Chem. Soc. Jpn. 2017, 90, 1035–1040. [Google Scholar] [CrossRef] [Green Version]
- Paradis, G.G.; Kreiter, R.; van Tuel, M.M.A.; Nijmeijer, A.; Vente, J.F. Amino-functionalized microporous hybrid silica membranes. J. Mater. Chem. 2012, 22, 7258. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Design of silica networks for development of highly permeable hydrogen separation membranes with hydrothermal stability. J. Am. Chem. Soc. 2009, 131, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Wenten, I.G.; Dharmawijaya, P.T.; Aryanti, P.T.P.; Mukti, R.R.; Khoiruddin, K. LTA zeolite membranes: Current progress and challenges in pervaporation. RSC Adv. 2017, 7, 29520–29539. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Zhu, G.; Liu, J.; Yang, W. Hydrothermal stability of LTA zeolite membranes in pervaporation. J. Membr. Sci. 2007, 297, 10–15. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Development of robust organosilica membranes for reverse osmosis. Langmuir ACS J. Surf. Colloids 2011, 27, 13996–13999. [Google Scholar] [CrossRef]
- Guo, M.; Kanezashi, M.; Nagasawa, H.; Yu, L.; Yamamoto, K.; Gunji, T.; Ohshita, J.; Tsuru, T. Tailoring the microstructure and permeation properties of bridged organosilica membranes via control of the bond angles. J. Membr. Sci. 2019, 584, 56–65. [Google Scholar] [CrossRef]
- Song, H.; Zhao, S.; Lei, J.; Wang, C.; Qi, H. Pd-doped organosilica membrane with enhanced gas permeability and hydrothermal stability for gas separation. J. Mater. Sci. 2016, 51, 6275–6286. [Google Scholar] [CrossRef]
- Ten Hove, M.; Nijmeijer, A.; Winnubst, L. Facile synthesis of zirconia doped hybrid organic inorganic silica membranes. Sep. Purif. Technol. 2015, 147, 372–378. [Google Scholar] [CrossRef]
- Lei, J.; Song, H.; Wei, Y.; Zhao, S.; Qi, H. A novel strategy to enhance hydrothermal stability of Pd-doped organosilica membrane for hydrogen separation. Microporous Mesoporous Mater. 2017, 253, 55–63. [Google Scholar] [CrossRef]
- Qureshi, H.F.; Besselink, R.; Ten Elshof, J.E.; Nijmeijer, A.; Winnubst, L. Doped microporous hybrid silica membranes for gas separation. J. Sol-Gel Sci. Technol. 2015, 75, 180–188. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wen, J.L.; Shao, Q.; Yuan, A.; Ren, H.T.; Luo, F.Y.; Zhang, X.L. Fabrication of La/Y-codoped microporous organosilica membranes for high-performance pervaporation desalination. J. Membr. Sci. 2019, 584, 353–363. [Google Scholar] [CrossRef]
- Yang, X.; Fraser, T.; Myat, D.; Smart, S.; Zhang, J.; Diniz da Costa, J.C.; Liubinas, A.; Duke, M. A Pervaporation Study of Ammonia Solutions Using Molecular Sieve Silica Membranes. Membranes 2014, 4, 40–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boffa, V.; Parmeggiani, L.; Nielsen, A.H.; Magnacca, G. Hydrophilicity and surface heterogeneity of TiO 2 -doped silica materials for membrane applications. Microporous and Mesoporous Materials 2016, 221, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Kanezashi, M.; Sano, M.; Yoshioka, T.; Tsuru, T. Extremely thin Pd-silica mixed-matrix membranes with nano-dispersion for improved hydrogen permeability. Chem. Commun. 2010, 46, 6171–6173. [Google Scholar] [CrossRef] [PubMed]
- Karakiliç, P.; Huiskes, C.; Luiten-Olieman, M.W.J.; Nijmeijer, A.; Winnubst, L. Sol−gel processed magnesium-doped silica membranes with improved H2/CO2 separation. J. Membr. Sci. 2017, 543, 195–201. [Google Scholar] [CrossRef]
- Puthai, W.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Nanofiltration performance of SiO2-ZrO2 membranes in aqueous solutions at high temperatures. Sep. Purif. Technol. 2016, 168, 238–247. [Google Scholar] [CrossRef]
- Song, H.; Zhao, S.; Chen, J.; Qi, H. Hydrothermally stable Zr-doped organosilica membranes for H2 /CO2 separation. Microporous Mesoporous Mater. 2016, 224, 277–284. [Google Scholar] [CrossRef]
- Qi, H.; Chen, H.; Li, L.; Zhu, G.; Xu, N. Effect of Nb content on hydrothermal stability of a novel ethylene-bridged silsesquioxane molecular sieving membrane for H2/CO2 separation. J. Membr. Sci. 2012, 421–422, 190–200. [Google Scholar] [CrossRef]
- Qi, H.; Han, J.; Xu, N. Effect of calcination temperature on carbon dioxide separation properties of a novel microporous hybrid silica membrane. J. Membr. Sci. 2011, 382, 231–237. [Google Scholar] [CrossRef]
- Nagasawa, H.; Niimi, T.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Modified gas-translation model for prediction of gas permeation through microporous organosilica membranes. AIChE J. 2014, 60, 4199–4210. [Google Scholar] [CrossRef]
- Kanezashi, M.; Miyauchi, S.; Hayakawa, S.; Nagasawa, H.; Yoshioka, T.; Tsuru, T. Propylene/propane Permeation Properties of Metal-doped Organosilica Membranes with Controlled Network Sizes and Adsorptive Properties. J. Jpn. Pet. Inst. 2016, 59, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Sheridan, S.; Ding, L.; Wang, D.K.; Smart, S.; Diniz da Costa, J.C.; Liubinas, A.; Duke, M. Inter-layer free cobalt-doped silica membranes for pervaporation of ammonia solutions. J. Membr. Sci. 2018, 553, 111–116. [Google Scholar] [CrossRef]
- De Vos, R.M.; Maier, W.F.; Verweij, H. Hydrophobic silica membranes for gas separation. J. Membr. Sci. 1999, 158, 12. [Google Scholar] [CrossRef]
- El-Feky, H.H.; Briceño, K.; Jardim, E.D.O.; Silvestre-Albero, J.; Gumí, T. Novel silica membrane material for molecular sieve applications. Microporous Mesoporous Mater. 2013, 179, 22–29. [Google Scholar] [CrossRef]
- Tsuru, T.; Nakasuji, T.; Oka, M.; Kanezashi, M.; Yoshioka, T. Preparation of hydrophobic nanoporous methylated SiO2 membranes and application to nanofiltration of hexane solutions. J. Membr. Sci. 2011, 384, 149–156. [Google Scholar] [CrossRef]
- Yang, J.; Fan, W.; Bell, C.M. Effect of calcination atmosphere on microstructure and H2/CO2 separation of palladium-doped silica membranes. Sep. Purif. Technol. 2019, 210, 659–669. [Google Scholar] [CrossRef]
- Campaniello, J.; Engelen, C.W.; Haije, W.G.; Pex, P.P.; Vente, J.F. Long-term pervaporation performance of microporous methylated silica membranes. Chem. Commun. 2004, 834–835. [Google Scholar] [CrossRef] [PubMed]
- Elma, M.; Yacou, C.; Diniz da Costa, J.C.; Wang, D.K. Performance and long term stability of mesoporous silica membranes for desalination. Membranes 2013, 3, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Plasma treatment of hydrophobic sub-layers to prepare uniform multi-layered films and high-performance gas separation membranes. Appl. Surf. Sci. 2015, 349, 415–419. [Google Scholar] [CrossRef]
- Ren, X.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Preparation of organosilica membranes on hydrophobic intermediate layers and evaluation of gas permeation in the presence of water vapor. J. Membr. Sci. 2015, 496, 156–164. [Google Scholar] [CrossRef]
- Ren, X.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Plasma-assisted multi-layered coating towards improved gas permeation properties for organosilica membranes. RSC Adv. 2015, 5, 59837–59844. [Google Scholar] [CrossRef]
- Castricum, H.L.; Sah, A.; Kreiter, R.; Blank, D.H.; Vente, J.F.; ten Elshof, J.E. Hybrid ceramic nanosieves: Stabilizing nanopores with organic links. Chem. Commun. 2008, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Castricum, H.L.; Sah, A.; Kreiter, R.; Blank, D.H.A.; Vente, J.F.; ten Elshof, J.E. Hydrothermally stable molecular separation membranes from organically linked silica. J. Mater. Chem. 2008, 18, 2150. [Google Scholar] [CrossRef]
- Zheng, F.T.; Yamamoto, K.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Preparation of bridged silica RO membranes from copolymerization of bis(triethoxysilyl)ethene/(hydroxymethyl)triethoxysilane. Effects of ethenylene-bridge enhancing water permeability. J. Membr. Sci. 2018, 546, 173–178. [Google Scholar] [CrossRef]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Guo, M.; Moriyama, N.; Ito, K.; Tsuru, T. Tailoring Ultramicroporosity To Maximize CO2 Transport within Pyrimidine-Bridged Organosilica Membranes. ACS Appl. Mater. Interfaces 2019, 11, 7164–7173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Teng, Z.; Wang, Z.F. Facile Method To Efficiently Fabricate Large-Size Mesoporous Organosilica Nanosheets with Uniform Tunable Pore Size for Robust Separation Membranes. Chem. Mater. 2019, 31, 3823–3830. [Google Scholar] [CrossRef]
- Meng, L.; Kanezashi, M.; Wang, J.; Tsuru, T. Permeation properties of BTESE–TEOS organosilica membranes and application to O2/SO2 gas separation. J. Membr. Sci. 2015, 496, 211–218. [Google Scholar] [CrossRef]
- Kong, C.; Du, H.; Chen, L.; Chen, B. Nanoscale MOF/organosilica membranes on tubular ceramic substrates for highly selective gas separation. Energy Environ. Sci. 2017, 10, 1812–1819. [Google Scholar] [CrossRef]
- Yang, H.; Elma, M.; Wang, D.K.; Motuzas, J.; da Costa, J.C.D. Interlayer-free hybrid carbon-silica membranes for processing brackish to brine salt solutions by pervaporation. J. Membr. Sci. 2017, 523, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Kanezashi, M.; Matsutani, T.; Wakihara, T.; Tawarayama, H.; Nagasawa, H.; Yoshioka, T.; Okubo, T.; Tsuru, T. Tailoring the Subnano Silica Structure via Fluorine Doping for Development of Highly Permeable CO2 Separation Membranes. ChemNanoMat 2016, 2, 264–267. [Google Scholar] [CrossRef]
- Yamamoto, K.; Koge, S.; Gunji, T.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Preparation of POSS-derived robust RO membranes for water desalination. Desalination 2017, 404, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Zou, L.; Lin, P.; Zhang, Q.; Zhong, J. Pervaporative desulfurization of model gasoline using PDMS/BTESE-derived organosilica hybrid membranes. Fuel Process. Technol. 2016, 154, 188–196. [Google Scholar] [CrossRef]
- Xu, R.; Jiang, W.; Qi, L.; Zhang, Q.; Zhong, J. Fabrication of PEG Crosslinked Organosilica Hybrid Membranes for Reverse Osmosis Desalination. J. Inorg. Mater. 2018, 33, 1154–1160. [Google Scholar] [CrossRef]
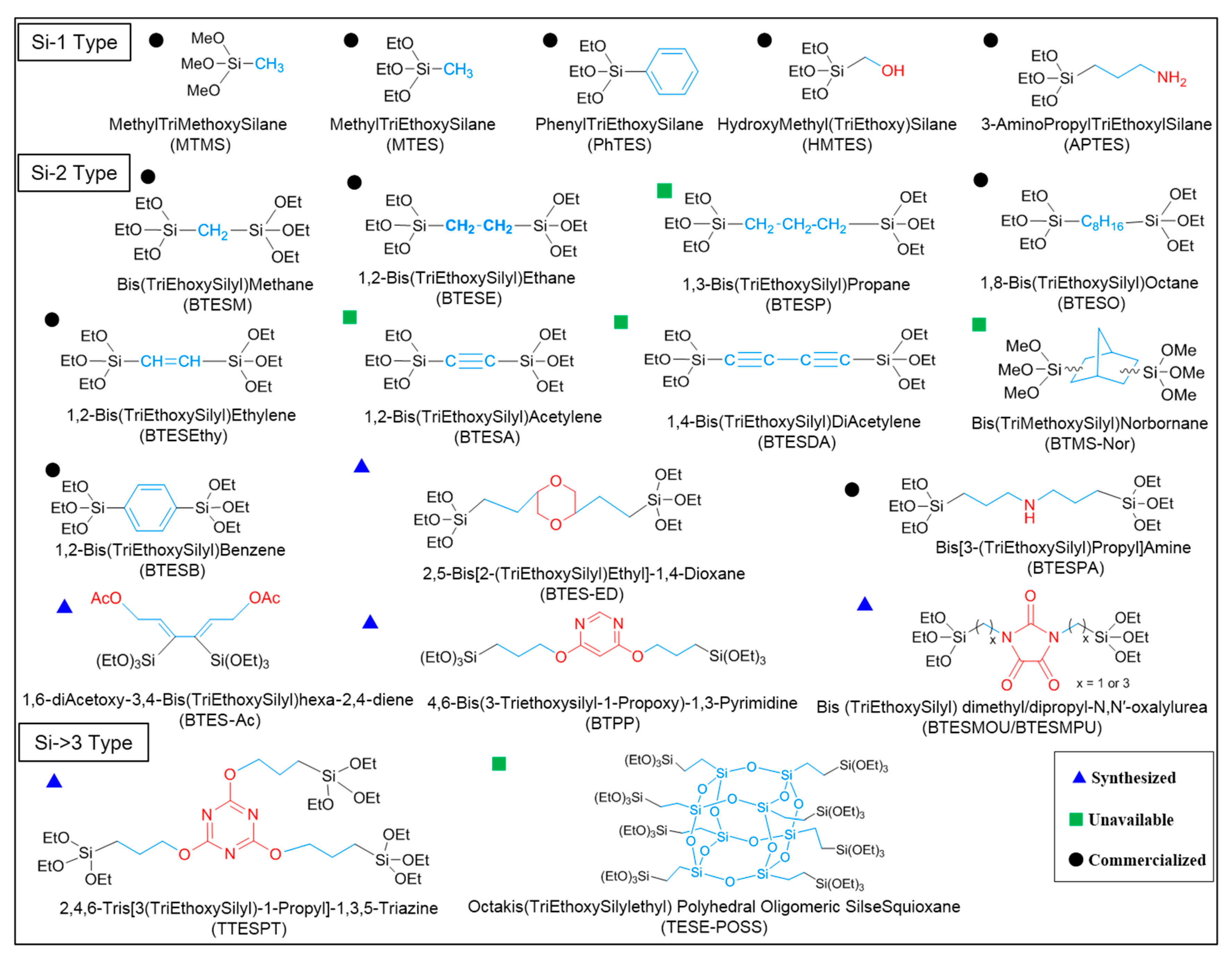
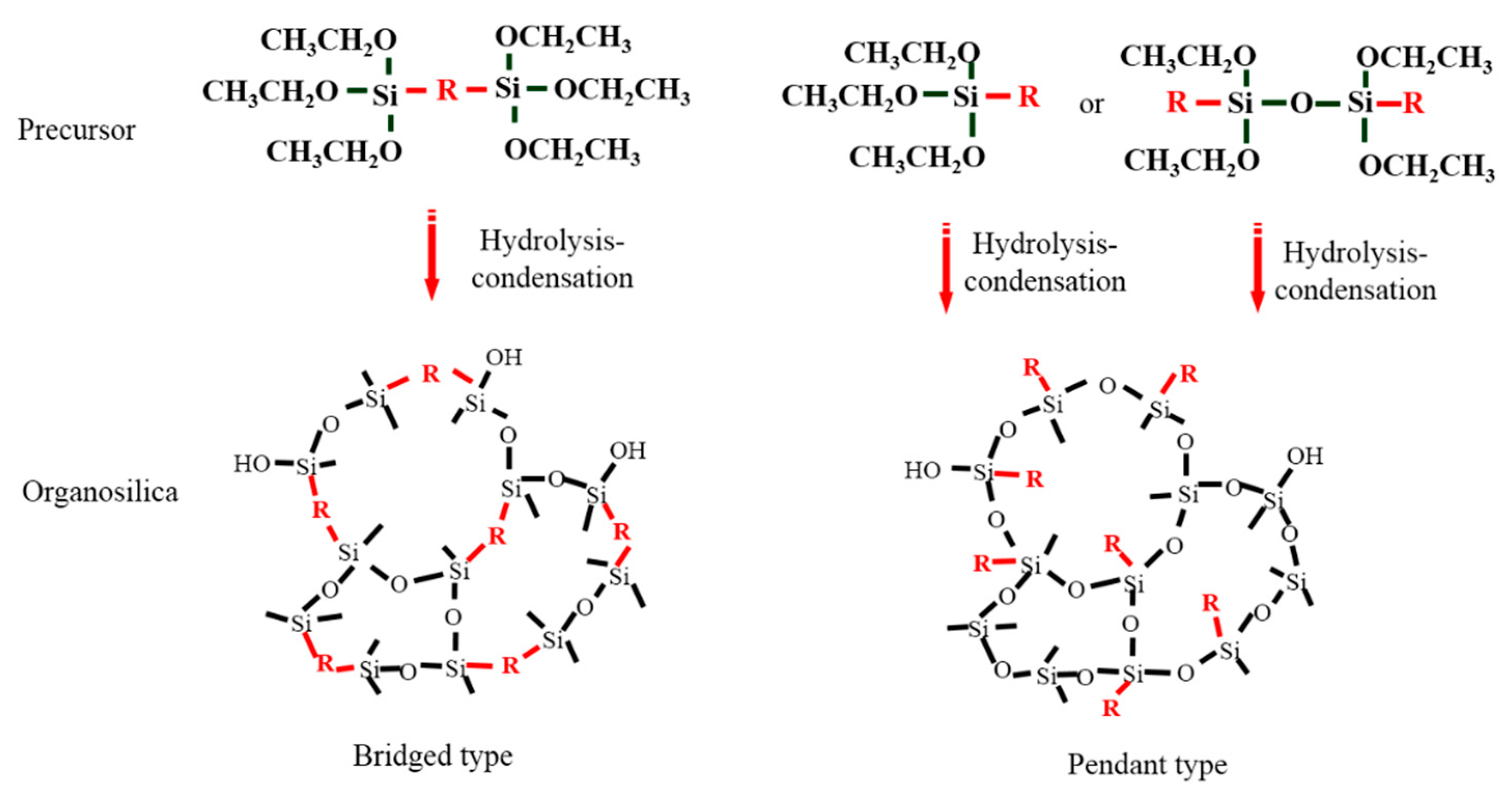

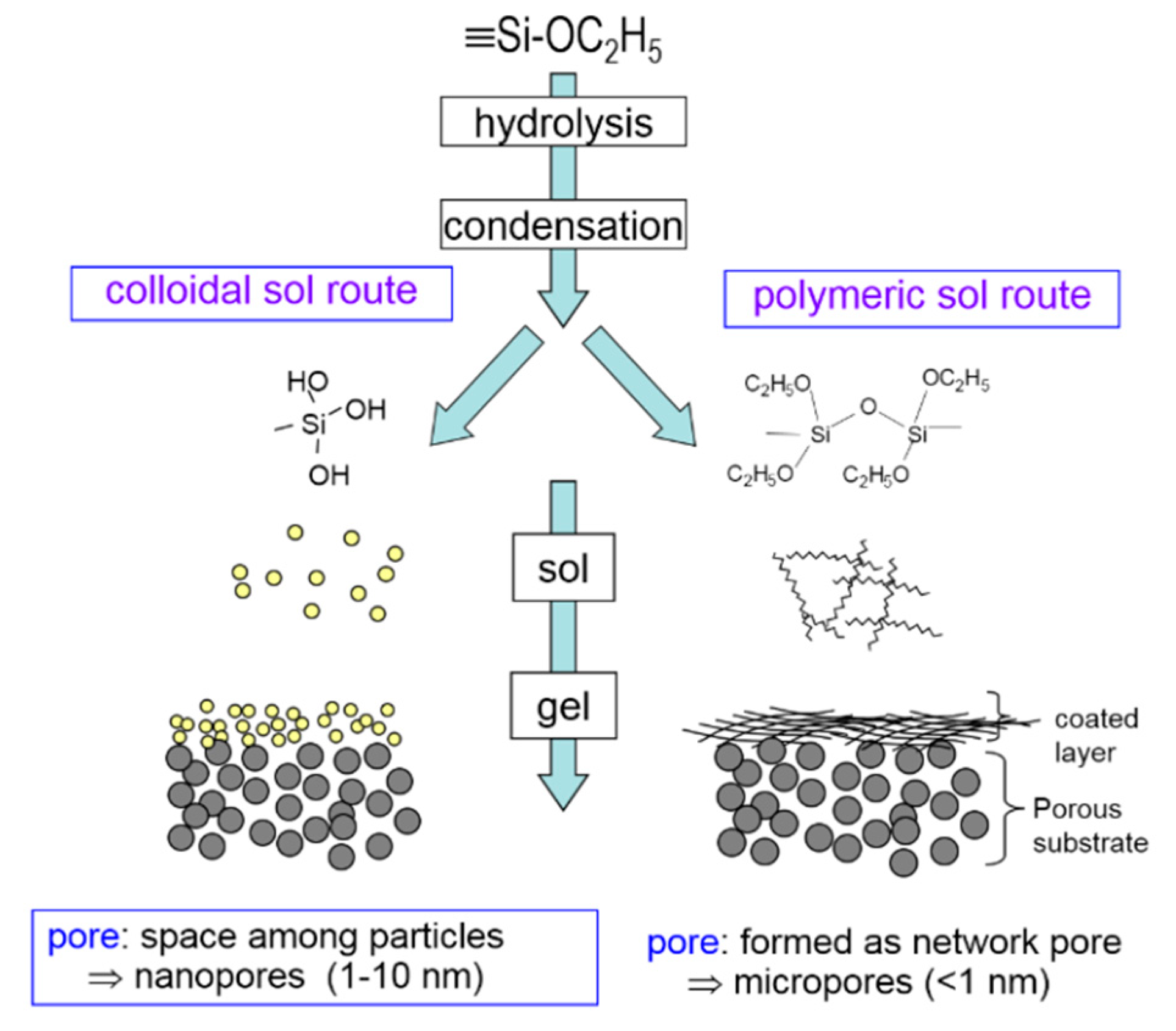

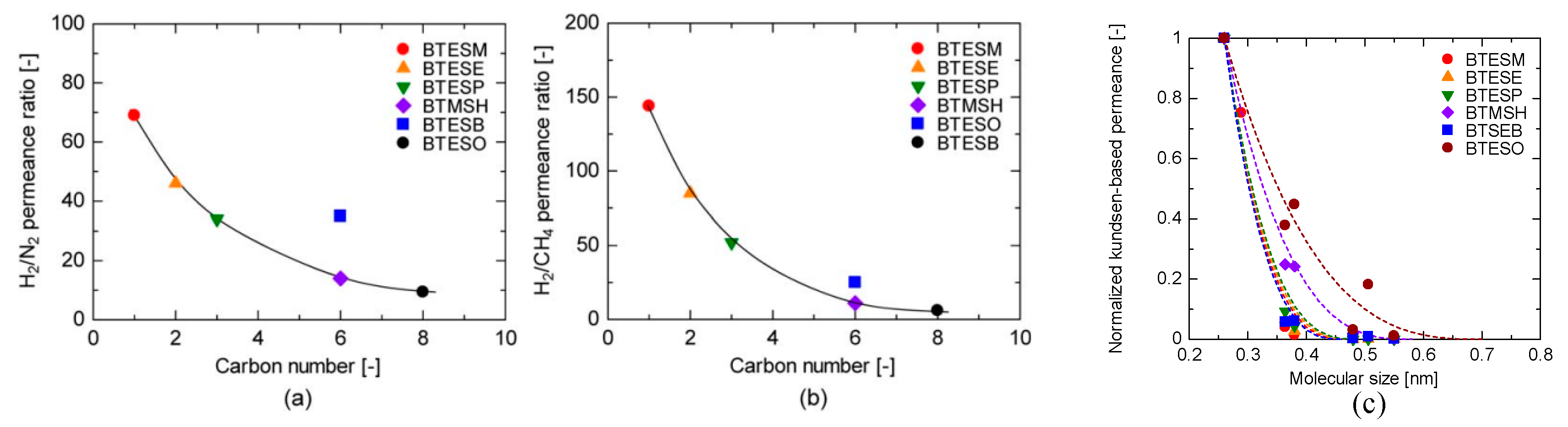

| Composition | Water Ratio, Acid Ratio, Solvent Ratio |
|---|---|
| Calcination temperature | 100–600 °C |
| Other method factors | Spin-coating, UV-irradiation |
| Precursor | Organic Group | T [°C] | H2 Permeance [10−7 mol m−2 s−1 Pa−1] | Selectivity | Ref. |
|---|---|---|---|---|---|
| BTESM |  | 200 | ~10 | H2/N2: 70 | [15] |
| H2/CH4: 150 | |||||
| 200 50 | 2.4 6.32 a 17.9 | H2/N2: 20.7 C3H6/C3H8: 8.8 H2/N2: 12 | [61] [62] [63] | ||
| BTESE | 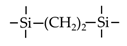 | 200 200 40 200 200 200 | 11 ~10 7.66 20–100 2.2 16–34 | H2/CH4: 150 CO2/N2: 36 H2/CH4: >400 H2/N2: ~20 H2/SF6: 1000–25500 CO2/N2: >100 H2/C3H8: 2600–5800 | [61] [15] [16] [26] [37] [39] |
| BTESP | 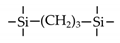 | 200 | ~8 | H2/CH4: ~50 | [15] |
| BTMSH | 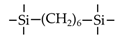 | 200 | ~2.5 | H2/CH4: ~10 | [15] |
| BTESB |  | 200 200 | 2 15.2 | H2/CH4: ~25 H2/N2: 8.4 | [15] [63] |
| BTESO | 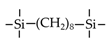 | 200 40 | ~7 6.63 23.2 | H2/CH4: ~5 CO2/N2: ~12 H2/N2: 5.3 | [15] [16] [17] |
| BTESEthy | 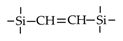 | 200 | 17 | H2/N2: 40 | [64] |
| BTSEA | 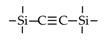 | 200 | 26.8 | H2/N2: 10.3 | [19] |
| BTMSN |  | 200 | 7 | H2/N2: 12 | [65] |
| BTES-ED | 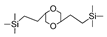 | 200 | 6 | H2/N2: 11 H2/SF6: 1160 | [65] |
| BTES-MAz | 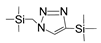 | 50 | 0.0295 | C3H6/C3H8: 37 | [66] |
| BTPP |  | 35 | ~0.6 | H2/N2: ~50CO2/N2: 25 | [67] |
| Precursor | Organic Group | Separation System | T [°C] | Flux [kg m−2 h−1] /Perm [10−13 m3 m−2 s−1 Pa−1] | Permeated H2O%/Rejection | Ref. |
|---|---|---|---|---|---|---|
| BTESM |  | 95%EtOH/H2O a | 70 | 1.18 | 92.2% | [61] |
| BTESE | 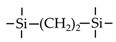 | 95%EtOH/H2O a 90%IPA/H2O a 90%IPA/H2O a 95%BuOH/H2O a 2% NaCl b | 70 75 75 70 80 | 0.73 3.54 1.9–3 20 10 | 89.2% 96% 96–99% 99% 98.1% | [61] [68] [69] [70] [71] |
| BTESB | 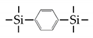 | 95%BuOH/H2O a | 95 | 3 | 32.6 | [17] |
| BTESO |  | 95%BuOH/H2O a | 95 | 3.2 | 92.7 | [17] |
| BTESEthy |  | 2%NaCl a 2% NaCl b | 70 25 | 14.2 2 | 99.6% 97% | [64] [19] |
| BTSEA | 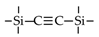 | 2% NaCl b | 25 | 8.5 | 95% | [19] |
| BTMSN |  | 2%NaCl b | 25 | 0.1 | 95% | [72] |
| BTES-ED |  | 2%NaCl b | 25 | 1.84 | 98.5% | [65] |
| BTES-MAz TTESPT | 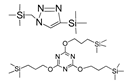 | 2%NaCl b 2%NaCl b | 25 60 | 3.7–5.4 >10 | 95–96% >98.5 | [73] [63] |
| Precursor | Organic Group | T [°C] | Permeance [10−7 mol m−2 s−1 Pa−1] | Selectivity | Ref. |
|---|---|---|---|---|---|
| MTES | 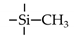 | 200 | H2: 5 | H2/N2: 15 H2/C3H8: 1300 | [51] |
| PhTES |  | 200 | H2: 2 | H2/N2: 7.4 H2/C3H8: 13.1 | [51] |
| MTMS | 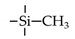 | 25 | H2: 0.09 | He/N2: 15 * | [58] |
| DMDMS |  | 500 | H2: 2.8 | H2/N2: 2000 * | [49] |
| TMMS | 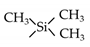 | 25 | H2: 0.1 | He/N2: 7.8 * | [58] |
| APTES | 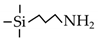 | 300 | H2: ~1.5 | H2/N2: 13.9 | [74] |
| SA | 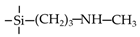 | 35 | CO2: 0.17 | CO2/N2: 11 | [22] |
| TA | 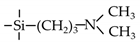 | 35 | CO2: 1.72 | CO2/N2: 21 | [22] |
| QA |  | 35 | CO2: 0.52 | CO2/N2: 24 | [22] |
| TEFS | 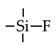 | 35 | C3H6: 2.2 | C3H6/C3H8: 42 | [21] |
| Precursor | Organic Group | Separation System | T [°C] | Flux [ kg m−2 h−1] /Perm [10−13 m3 m−2 s−1 Pa−1] | Permeated H2O%/Rejection | Ref. |
|---|---|---|---|---|---|---|
| APTES | 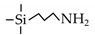 | 95%EtOH/H2O a | 70 | 0.2–2.1 | ~90% | [74] |
| HMTES |  | 2%NaCl b | 25 | 7.3 | 86.4% | [32] |
| IM | 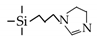 | 95%EtOH/H2O a | 70 | 1–3.4 | ~95% | [74] |
| LDA |  | 95%EtOH/H2O a | 70 | 2.2–4.1 | ~94% | [74] |
| Hybrid Material | Application | Separation System |
|---|---|---|
| MTES/TEOS | Gas separation; PV Nanofiltration | H2/other gas; Dehydration of alcohol/water Hexane/polyolefin oligomers |
| MTMS/TEOS | Intermediate layer | CO2/N2 in water vapor |
| BTESE/TEOS | Gas separation | O2/SO2 |
| BTPP/TEOS | Gas separation | CO2/other gas |
| BTPP/BTESE | Gas separation | CO2/other gas |
| MTES/BTESE | PV Gas separation | Dehydration of alcohol/water H2/other gas |
| HMTES/BTESE | RO | H2O/NaCl |
| HMTES/BTESEthy | RO | H2O/NaCl |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Tsuru, T. Organosilica-Based Membranes in Gas and Liquid-Phase Separation. Membranes 2019, 9, 107. https://doi.org/10.3390/membranes9090107
Ren X, Tsuru T. Organosilica-Based Membranes in Gas and Liquid-Phase Separation. Membranes. 2019; 9(9):107. https://doi.org/10.3390/membranes9090107
Chicago/Turabian StyleRen, Xiuxiu, and Toshinori Tsuru. 2019. "Organosilica-Based Membranes in Gas and Liquid-Phase Separation" Membranes 9, no. 9: 107. https://doi.org/10.3390/membranes9090107





