Antifouling Properties of Silver-Zinc Oxide Polyamide Thin Film Composite Membrane and Rejection of 2-Chlorophenol and 2,4-Dichlorophenol
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of Silver-Zinc Oxide Nanocomposites
2.3. Preparation of Membranes
2.4. Characterization
2.5. Hydrophilicity
2.6. Membrane Performance
Antifouling Properties
2.7. Silver Release
3. Results and Discussion
3.1. Characterization-Nanoparticles
3.1.1. FTIR
3.1.2. XRD
3.1.3. SEM and EDX analysis for Ag, ZnO NPs, and Ag-ZnO NCs
3.2. Membrane Characterization
3.2.1. FTIR
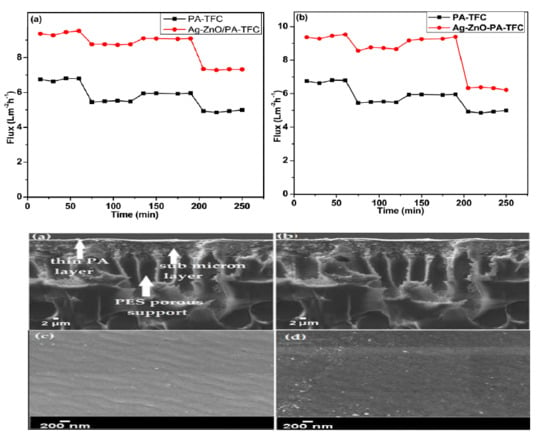
3.2.2. SEM and AFM Analysis
3.2.3. Surface Hydrophilicity of the Membranes Using Contact Angle
3.2.4. Water Flux and Permeation Test
3.2.5. Rejection Test
3.2.6. Evaluation of Antifouling Properties
3.2.7. Silver Release
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olujimi, O.; Fatoki, O.; Odendaal, J.; Okonkwo, J. Endocrine disrupting chemicals (phenol and phthalates) in the South African environment: A need for more monitoring. Water SA 2010, 36, 671–682. [Google Scholar] [CrossRef]
- Zendehdel, R.; Tayefeh-Rahimian, R.; Kabir, A. Chronic Exposure to Chlorophenol Related Compounds in the Pesticide Production Workplace and Lung Cancer: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2014, 15, 5149–5153. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Mohammad, A.; Teow, Y.; Ang, W.L.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Ouzzine, M.; Maciá-Agulló, J.A.; Lillo-Rodenas, M.A.; Quijada, C.; Linares-Solano, A. Synthesis of high surface area TiO2 nanoparticles by mild acid treatment with HCl or HI for hotocatalytic propene oxidation. Appl. Catal. B Environ. 2014, 154, 285–293. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Singh, T.; Srivastava, N.; Mishra, P.; Bhatiya, A. Application of TiO2 Nanoparticle in Photocatalytic Degradation of Organic Pollutants. In Materials Science Forum; Trans Tech Publications: Zurich, Switzerland, 2016. [Google Scholar]
- Al-Hobaib, A.S.; Al-Sheetan, K.M.; Shaik, M.R.; Al-Suhybani, M.S. Modification of thin-film polyamide membrane with multi-walled carbon nanotubes by interfacial polymerization. Appl. Water Sci. 2017, 7, 4341–4350. [Google Scholar] [CrossRef]
- Lind, M.L.; Ghosh, A.K.; Jawor, A.; Huang, X.; Hou, W.; Yang, Y.; Hoek, E.M.V. Influence of Zeolite Crystal Size on Zeolite-Polyamide Thin Film Nanocomposite Membranes. Langmuir 2009, 25, 10139–10145. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A.; Zarrabi, H.; Gholami, P.; Yekavalangi, M.E. High flux and fouling resistant reverse osmosis membrane modified with plasma treated natural zeolite. Desalination 2017, 411, 89–100. [Google Scholar] [CrossRef]
- Giwa, A.; Akther, N.; Dufour, V.; Hasan, S.W. A critical review on recent polymeric and nano-enhanced membranes for reverse osmosis. RSC Adv. 2016, 6, 8134–8163. [Google Scholar] [CrossRef]
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and Water Treatment Application of Carbon Nanotubes (CNTs)-Based Composite Membranes: A Review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; Park, H.-D. Effect of transmembrane pressure, linear velocity, and temperature on permeate water flux of high-density vertically aligned carbon nanotube membranes. Desalin. Water Treat. 2016, 57, 26706–26717. [Google Scholar] [CrossRef]
- Sweetman, M.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.; Hayball, J. Activated carbon, carbon nanotubes and graphene. J. Carbon Res. C 2017, 3, 18. [Google Scholar] [CrossRef]
- Lee, H.D.; Kim, H.W.; Cho, Y.H.; Park, H.B. Experimental Evidence of Rapid Water Transport through Carbon Nanotubes Embedded in Polymeric Desalination Membranes. Small 2014, 10, 2653–2660. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, M.Y.; Lee, H.D.; Roh, J.S.; Kim, H.W.; Park, H.B. Highly porous carbon nanotube/polysulfone nanocomposite supports for high-flux polyamide reverse osmosis membranes. J. Membr. Sci. 2017, 539, 441–450. [Google Scholar] [CrossRef]
- Makhetha, T.; Moutloali, R. Antifouling properties of Cu(tpa)@GO/PES composite membranes and selective dye rejection. J. Membr. Sci. 2018, 554, 195–210. [Google Scholar] [CrossRef]
- Mbuli, B.S.; Mhlanga, S.D.; Mamba, B.B.; Nxumalo, E.N. Fouling Resistance and Physicochemical Properties of Polyamide Thin-Film Composite Membranes Modified with Functionalized Cyclodextrins. Adv. Polym. Technol. 2017, 36, 249–260. [Google Scholar] [CrossRef]
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Dong, Y.; Zhao, X.; Jiang, Z.; Zhang, R.; Zhao, J. Separation performance of thin-film composite nanofiltration membrane through interfacial polymerization using different amine monomers. Desalination 2014, 333, 59–65. [Google Scholar] [CrossRef]
- Abdulkarima, A.A.; Ahmada, A.L.; Ismaila, S.; Ooia, B.S. Preparation and Characterization of Polyethersulfone Membrane Containing Zinc Oxide Nanoparticles and Polyvinylpyrrolidone. J. Teknol. 2013, 65, 13–16. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Braeken, L.; Liu, T.-Y.; Luis, P.; Wang, X.-L.; Van der Bruggen, B. Remarkable Anti-Fouling Performance of TiO2-Modified TFC Membranes with Mussel-Inspired Polydopamine Binding. Appl. Sci. 2017, 7, 81. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Gläser, R.; Schulze, A. Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J. Membr. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Ngo, T.H.A.; Nguyen, D.T.; Do, K.D.; Nguyen, T.T.M.; Mori, S.; Tran, D.T. Surface modification of polyamide thin film composite membrane by coating of titanium dioxide nanoparticles. J. Sci. Adv. Mater. Devices 2016, 1, 468–475. [Google Scholar] [CrossRef]
- He, M.; Wang, Q.; Wang, R.; Xie, Y.; Zhao, W.; Zhao, C. Design of Antibacterial Poly (ether sulfone) Membranes via Covalently Attaching Hydrogel Thin Layers Loaded with Ag Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 15962–15974. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, C.; Wang, Z.; Xu, Y.; Zhao, W.; Sun, S.; Zhao, C. Co-deposition towards mussel-inspired antifouling and antibacterial membranes by using zwitterionic polymers and silver nanoparticles. J. Mater. Chem. B 2017, 5, 7186–7193. [Google Scholar] [CrossRef]
- Sawada, I.; Fachrul, R.; Ito, T.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Development of a hydrophilic polymer membrane containing silver nanoparticles with both organic antifouling and antibacterial properties. J. Membr. Sci. 2012, 387, 1–6. [Google Scholar] [CrossRef]
- Koseoglu-Imer, D.Y.; Kose, B.; Altinbas, M.; Koyuncu, I. The production of polysulfone (PS) membrane with silver nanoparticles (AgNP): Physical properties, filtration performances, and biofouling resistances of membranes. J. Membr. Sci. 2013, 428, 620–628. [Google Scholar] [CrossRef]
- Dumée, L.F.; Maina, J.W.; Merenda, A.; Reis, R.; He, L.; Kong, L. Hybrid thin film nano-composite membrane reactors for simultaneous separation and degradation of pesticides. J. Membr. Sci. 2017, 528, 217–224. [Google Scholar] [CrossRef]
- Kargari, A.; Khazaali, F. Effect of operating parameters on 2-chlorophenol removal from wastewaters by a low-pressure reverse osmosis system. Desalin. Water Treat. 2015, 55, 114–124. [Google Scholar] [CrossRef]
- Li, J.H.; Yan, B.F.; Shao, X.S.; Wang, S.S.; Tian, H.Y.; Zhang, Q.Q. Influence of Ag/TiO2 nanoparticle on the surface hydrophilicity and visible-light response activity of polyvinylidene fluoride membrane. Appl. Surf. Sci. 2015, 324, 82–89. [Google Scholar] [CrossRef]
- Bueno, F.G.; Machareth, M.A.D.; Panizzon, G.P.; Lopes, G.C.; Mello, J.C.P.; Leite-Mello, E.V.S. Development of a UV/Vis spectrophotometric method for analysis of total polyphenols from Caesalpinia peltophoroides Benth. Química Nova 2012, 35, 822–826. [Google Scholar] [CrossRef]
- Gutul, T.; Rusu, E.; Condur, N.; Ursaki, V.; Goncearenco, E.; Vlazan, P.; Sidorenko, A.S. Preparation of poly(N-vinylpyrrolidone)-stabilized ZnO colloid nanoparticles. Beilstein J. Nanotechnol. 2014, 5, 402–406. [Google Scholar] [CrossRef]
- Gharibshahi, L.; Saion, E.; Gharibshahi, E.; Shaari, A.H.; Matori, K.A. Influence of Poly(vinylpyrrolidone) concentration on properties of silver nanoparticles manufactured by modified thermal treatment method. PLoS ONE 2017, 12, e0186094. [Google Scholar] [CrossRef]
- Shah, A.H.; Manikandan, E.; Ahmed, M.B.; Ganesan, V. Enhanced bioactivity of Ag/ZnO nanorods-A comparative antibacterial study. J. Nanomed. Nanotechol. 2013, 4, 2–6. [Google Scholar] [CrossRef]
- Kumar, R.; Anandan, S.; Hembram, K.; Rao, T.N. Efficient ZnO-Based Visible-Light-Driven Photocatalyst for Antibacterial Applications. ACS Appl. Mater. Interfaces 2014, 6, 13138–13148. [Google Scholar] [CrossRef]
- Thandavan, T.M.K.; Gani, S.M.A.; Wong, C.S.; Nor, R.M. Evaluation of Williamson–Hall Strain and Stress Distribution in ZnO Nanowires Prepared Using Aliphatic Alcohol. J. Nondestruct. Eval. 2015, 34, 14. [Google Scholar] [CrossRef]
- Song, C.; Lin, Y.; Wang, D.; Hu, Z. Facile synthesis of Ag/ZnO microstructures with enhanced photocatalytic activity. Mater. Lett. 2010, 64, 1595–1597. [Google Scholar] [CrossRef]
- Khoza, P.B.; Moloto, M.J.; Sikhwivhilu, L.M. The Effect of Solvents, Acetone, Water, and Ethanol, on the Morphological and Optical Properties of ZnO Nanoparticles Prepared by Microwave. J. Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Belfer, S. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Kim, S.; Lee, P.; Bano, S.; Park, Y.; Nam, S. Effective incorporation of TiO2 nanoparticles into polyamide thin-film composite membranes. J. Appl. Polym. Sci. 2016, 133, 43383. [Google Scholar] [CrossRef]
- Goh, P.S.; Ng, B.C.; Lau, W.J.; Ismail, A.F. Inorganic nanomaterials in polymeric ultrafiltrationmembranes for water treatment. Sep. Purif. Rev. 2015, 44, 216–249. [Google Scholar] [CrossRef]
- Baroña, G.N.B.; Lim, J.; Choi, M.; Jung, B. Interfacial polymerization of polyamide-aluminosilicate SWNT nanocomposite membranes for reverse osmosis. Desalination 2013, 325, 138–147. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, Y.; Boo, C.; Liu, Z.; Li, J.; Deng, L.; An, X. High-Performance Thin-Film Composite Membrane with an Ultrathin Spray-Coated Carbon Nanotube Interlayer. Environ. Sci. Technol. Lett. 2018, 5, 243–248. [Google Scholar] [CrossRef]
- Al Mayyahi, A. TiO2 Polyamide Thin Film Nanocomposite Reverses Osmosis Membrane for Water Desalination. Membranes 2018, 8, 66. [Google Scholar] [CrossRef]
- Isawi, H.; El-Sayed, M.H.; Feng, X.; Shawky, H.; Mottaleb, M.S.A. Surface nanostructuring of thin film composite membranes via grafting polymerization and incorporation of ZnO nanoparticles. Appl. Surf. Sci. 2016, 385, 268–281. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface Modification of Water Purification Membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, X.; Huang, X.; Waite, T.D.; Wen, X. Combined effect of membrane and foulant hydrophobicity and surface charge on adsorptive fouling during microfiltration. J. Membr. Sci. 2011, 373, 140–151. [Google Scholar] [CrossRef]
- Dipheko, T.D.; Matabola, K.P.; Kotlhao, K.; Moutloali, R.M.; Klink, M. Fabrication and Assessment of ZnO Modified Polyethersulfone Membranes for Fouling Reduction of Bovine Serum Albumin. Int. J. Polym. Sci. 2017, 2017. [Google Scholar] [CrossRef]
- Shen, L.; Bian, X.; Lu, X.; Shi, L.; Liu, Z.; Chen, L.; Hou, Z.; Fan, K. Preparation and characterization of ZnO/polyethersulfone (PES) hybrid membranes. Desalination 2012, 293, 21–29. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Balta, S.; Darvishmanash, S.; Kim, J.; Van Der Bruggen, B. Nanofiltration membranes enhanced with TiO 2 nanoparticles: A comprehensive study. Desalin. Water Treat. 2011, 34, 179–183. [Google Scholar] [CrossRef]
- Shon, H.K.; Phuntsho, S.; Chaudhary, D.S.; Vigneswaran, S.; Cho, J. Nanofiltration for water and wastewater treatment—A mini review. Drink. Water Eng. Sci. 2013, 6, 47–53. [Google Scholar] [CrossRef]
- Coday, B.D.; Yaffe, B.G.M.; Xu, P.; Cath, T.Y. Rejection of Trace Organic Compounds by Forward Osmosis Membranes: A Literature Review. Environ. Sci. Technol. 2014, 48, 3612–3624. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Binupriya, A.; Kavitha, D.; Selvakumar, R.; Jayabalan, R.; Choi, J.; Yun, S. Adsorption potential of maize cob carbon for 2,4-dichlorophenol removal from aqueous solutions: Equilibrium, kinetics and thermodynamics modeling. Chem. Eng. J. 2009, 147, 265–271. [Google Scholar] [CrossRef]
- Luján-Facundo, M.; Mendoza-Roca, J.; Cuartas-Uribe, B.; Alvarez-Blanco, S. Evaluation of cleaning efficiency of ultrafiltration membranes fouled by BSA using FTIR–ATR as a tool. J. Food Eng. 2015, 163, 1–8. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Huang, H.; Yu, J.; Guo, H.; Shen, Y.; Yang, F.; Wang, H.; Liu, R.; Liu, Y. Improved antifouling performance of ultrafiltration membrane via preparing novel zwitterionic polyimide. Appl. Surf. Sci. 2018, 427, 38–47. [Google Scholar] [CrossRef]
- Yin, J.; Yang, Y.; Hu, Z.; Deng, B. Attachment of silver nanoparticles (AgNPs) onto thin-film composite (TFC) membranes through covalent bonding to reduce membrane biofouling. J. Membr. Sci. 2013, 441, 73–82. [Google Scholar] [CrossRef]
- Zirehpour, A.; Rahimpour, A.; Shamsabadi, A.A.; Gh, M.S.; Soroush, M. Mitigation of Thin-Film Composite Membrane Biofouling via Immobilizing Nano-Sized Biocidal Reservoirs in the Membrane Active Layer. Environ. Sci. Technol. 2017, 51, 5511–5522. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Rahimpour, A. Interfacially polymerized thin film nanofiltration membranes on TiO2 coated polysulfone substrate. J. Ind. Eng. Chem. 2014, 20, 1261–1268. [Google Scholar] [CrossRef]













| PA-TFC/Ag-ZnO (wt%) | Organic Phase |  Aqueous Phase Aqueous Phase  | |||
|---|---|---|---|---|---|
| TMC (wt%) | Hexane (wt%) | Piperazine (wt%) | Water (wt%) | Nano-Composites (wt%) | |
| PA-TFC 0.0 | 0.4 | 99.6 | 2 | 98 | 0.0 |
| PA-TFC 0.5 | 0.4 | 99.6 | 2 | 98 | 0.5 |
| PA-TFC 1.0 | 0.4 | 99.6 | 2 | 98 | 1.0 |
| PA-TFC 1.5 | 0.4 | 99.6 | 2 | 98 | 1.5 |
| PA-TFC 2.0 | 0.4 | 99.6 | 2 | 98 | 2.0 |
| Membranes | Pure Water Hydraulic Permeability (L.m−2.h−1.bar−1) |
|---|---|
| PES | 10.0 |
| PA-TFC | 0.9 |
| Ag-ZnO/PA-TFC | 1.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotlhao, K.; Lawal, I.A.; Moutloali, R.M.; Klink, M.J. Antifouling Properties of Silver-Zinc Oxide Polyamide Thin Film Composite Membrane and Rejection of 2-Chlorophenol and 2,4-Dichlorophenol. Membranes 2019, 9, 96. https://doi.org/10.3390/membranes9080096
Kotlhao K, Lawal IA, Moutloali RM, Klink MJ. Antifouling Properties of Silver-Zinc Oxide Polyamide Thin Film Composite Membrane and Rejection of 2-Chlorophenol and 2,4-Dichlorophenol. Membranes. 2019; 9(8):96. https://doi.org/10.3390/membranes9080096
Chicago/Turabian StyleKotlhao, Kate, Isiaka A. Lawal, Richard M. Moutloali, and Michael J. Klink. 2019. "Antifouling Properties of Silver-Zinc Oxide Polyamide Thin Film Composite Membrane and Rejection of 2-Chlorophenol and 2,4-Dichlorophenol" Membranes 9, no. 8: 96. https://doi.org/10.3390/membranes9080096
APA StyleKotlhao, K., Lawal, I. A., Moutloali, R. M., & Klink, M. J. (2019). Antifouling Properties of Silver-Zinc Oxide Polyamide Thin Film Composite Membrane and Rejection of 2-Chlorophenol and 2,4-Dichlorophenol. Membranes, 9(8), 96. https://doi.org/10.3390/membranes9080096