Impact on CO2/N2 and CO2/CH4 Separation Performance Using Cu-BTC with Supported Ionic Liquids-Based Mixed Matrix Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Preparation of IL@MOF
2.4. Membranes Preparation
2.5. Pure Gas Permeation Experiments
3. Results
3.1. Composite Characterization
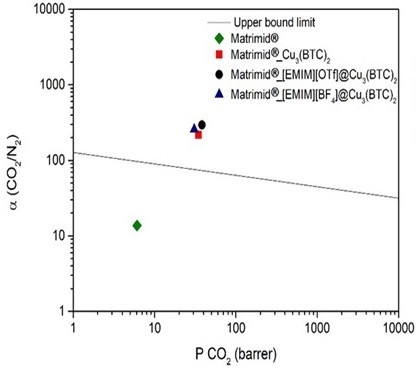
3.2. Gas Permeation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- D’Alessandro, D.M.; Smit, B.; Long, R.J. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-B.; Xiang, S.; Xing, H.; Zhou, W.; Chen, B. Exploration of porous metal–organic frameworks for gas separation and purification. Coord. Chem. Rev. 2017. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Snurr, R.Q. Development and Evaluation of Porous Materials for Carbon Dioxide Separation and Capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Z.; Gupta, K.M.; Jiang, J. Ionic Liquid/Metal–Organic Framework Composite for CO2 Capture: A Computational Investigation. J. Phys. Chem. C 2011, 115, 21736–21742. [Google Scholar] [CrossRef]
- Kinik, F.P.; Uzun, A.; Keskin, S. Ionic Liquid/Metal–Organic Framework Composites: From Synthesis to Applications. ChemSusChem 2017, 10, 2842–2863. [Google Scholar] [CrossRef] [PubMed]
- Fujie, K.; Kitagawa, H. Ionic liquid transported into metal–organic frameworks. Coord. Chem. Rev. 2015, 307, 382–390. [Google Scholar] [CrossRef]
- Vicent-Luna, J.M.; Gutiérrez-Sevillano, J.J.; Anta, J.A.; Calero, S. Effect of Room-Temperature Ionic Liquids on CO2 Separation by a Cu-BTC Metal-Organic Framework. J. Phys. Chem. C 2013, 117, 20762–20768. [Google Scholar] [CrossRef]
- Monteiro, B.; Nabais, A.R.; Paz, F.A.A.; Cabrita, L.; Branco, L.C.; Marrucho, I.M.; Neves, L.A.; Pereira, C.C.L. Membranes with a low loading of Metal-Organic Framework-Supported Ionic Liquids for CO2/N-2 separation in CO2 capture. Energy Technol. 2017, 5, 2158–2162. [Google Scholar] [CrossRef]
- Weigelt, F.; Georgopanos, P.; Shishatskiy, S.; Filiz, V.; Brinkmann, T.; Abetz, V. Development and Characterization of Defect-Free Matrimid® Mixed-Matrix Membranes Containing Activated Carbon Particles for Gas Separation. Polymers 2018, 10, 51. [Google Scholar] [CrossRef]
- Lei, Z.; Yuan, J.; Zhu, J. Solubility of CO2 in Propanone, 1-Ethyl-3-methylimidazolium Tetrafluoroborate, and Their Mixtures. J. Chem. Eng. Data 2010, 55, 4190–4194. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Schindler, B.J.; Killops, K.L.; Browe, M.A.; Mahle, J.J. The effect of water adsorption on the structure of the carboxylate containing metal–organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66. J. Mater. Chem. A 2013, 1, 11922–11932. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Li, Y.J.; Kreider, P.B.; Chang, C.-H.; Wannenmacher, N.; Thallapally, P.K.; Ahn, H.-G. High-rate synthesis of Cu–BTC metal–organic frameworks. Chem. Commun. 2013, 49, 11518–11520. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.A.; Crespo, J.G.; Coelhoso, I.M. Gas permeation studies in supported ionic liquid membranes. J. Membr. Sci. 2010, 357, 160–170. [Google Scholar] [CrossRef]
- Cussler, E.L. Fundamentals of Mass Transfer, 3rd ed.; Cambridge University Press: New York, NY, USA, 2009; pp. 237–273. [Google Scholar] [CrossRef]
- Moelands, M.A.H.; Schamhart, D.J.; Folkertsma, E.; Lutz, M.; Spekb, A.L.; Gebbink, R.J.M.K. Facial triad modelling using ferrous pyridinyl prolinate complexes: Synthesis and catalytic applications. Dalton Trans. 2014, 43, 6769–6785. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Nijmeijer, K. Performance and plasticization behavior of polymer–MOF membranes for gas separation at elevated pressures. J. Membr. Sci. 2014, 470, 166–177. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Sezginel, K.B.; Keskin, S.; Uzun, A. Tuning the Gas Separation Performance of CuBTC by Ionic Liquid Incorporation. Langmuir 2016, 32, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. Asymmetric Matrimid®/[Cu3(BTC)2] mixed-matrix membranes for gas separations. J. Membr. Sci. 2010, 362, 478–487. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, B.; Nabais, A.R.; Casimiro, M.H.; Martins, A.P.S.; Francisco, R.O.; Neves, L.A.; Pereira, C.C.L. Impact on CO2/N2 and CO2/CH4 Separation Performance Using Cu-BTC with Supported Ionic Liquids-Based Mixed Matrix Membranes. Membranes 2018, 8, 93. https://doi.org/10.3390/membranes8040093
Monteiro B, Nabais AR, Casimiro MH, Martins APS, Francisco RO, Neves LA, Pereira CCL. Impact on CO2/N2 and CO2/CH4 Separation Performance Using Cu-BTC with Supported Ionic Liquids-Based Mixed Matrix Membranes. Membranes. 2018; 8(4):93. https://doi.org/10.3390/membranes8040093
Chicago/Turabian StyleMonteiro, Bernardo, Ana R. Nabais, Maria H. Casimiro, Ana P. S. Martins, Rute O. Francisco, Luísa A. Neves, and Cláudia C. L. Pereira. 2018. "Impact on CO2/N2 and CO2/CH4 Separation Performance Using Cu-BTC with Supported Ionic Liquids-Based Mixed Matrix Membranes" Membranes 8, no. 4: 93. https://doi.org/10.3390/membranes8040093
APA StyleMonteiro, B., Nabais, A. R., Casimiro, M. H., Martins, A. P. S., Francisco, R. O., Neves, L. A., & Pereira, C. C. L. (2018). Impact on CO2/N2 and CO2/CH4 Separation Performance Using Cu-BTC with Supported Ionic Liquids-Based Mixed Matrix Membranes. Membranes, 8(4), 93. https://doi.org/10.3390/membranes8040093