1. Introduction
Landfill leachates are one among the most polluted wastewaters, characterized by high chemical oxygen demand (COD) and ammonia content. The characteristics of organics and their concentration, as well as the concentration of N-NH
4+ depends on the landfill age [
1]. Additionally, depending on the type of waste deposited, they can contain high concentrations of heavy metals (e.g., Zn
2+, Cr
3+, Ni
2+, Cd
2+, Pb
2+, As
3+, or their speciations), other metal ions (Na
+, K
+, Ca
2+, Mg
2+, Fe
2+, and Al
3+) and anions like Cl
−, SO
42−, PO
43−, etc. Numerous technologies, in many possible combinations of them, have been studied and used for leachate treatment [
2,
3,
4].
Reverse osmosis is a state-of-the-art technology for the treatment of landfill leachates. RO process (using cellulose acetate membrane) was studied for treatment of landfill leachate within two decades from its discovery and was reported to be the most effective method for treating leachates [
5]. By the mid-1980s, RO systems had already penetrated significantly into the market of leachate treatment [
6,
7]. The technology is highly promising and efficient for the treatment of landfill leachates having rejection coefficients on an average greater than 0.98 [
3,
6,
8,
9] for all pollutants (esp. organics and heavy metals), thus providing high quality permeate. RO systems were installed in more than 100 landfill sites in northern Europe, North America, and the Far East during the last decade [
10].
Recirculation of leachate constituents by controlled re-injection of RO concentrate into the landfill has been researched and practiced widely. It has been shown to enhance the biodegradation rate or landfill gas production in young landfills, which contain a high portion of biodegradable organic matter [
11,
12,
13,
14]. However, it is also reported that constituents like ammonia, chloride, and metals are not removed by recirculation [
11,
15], due to which this cannot be a sustainable solution. Therefore, re-injection of RO retentate cannot be beneficial in the case of old landfills producing methanogenic leachate (containing no biodegradable COD) and would likely result in an increase in strength of the leachate. For this reason, recirculation of methanogenic leachates is forbidden by the German Landfill Ordinance 2009, unless the landfill operator can prove the gain of any benefit from doing so [
16].
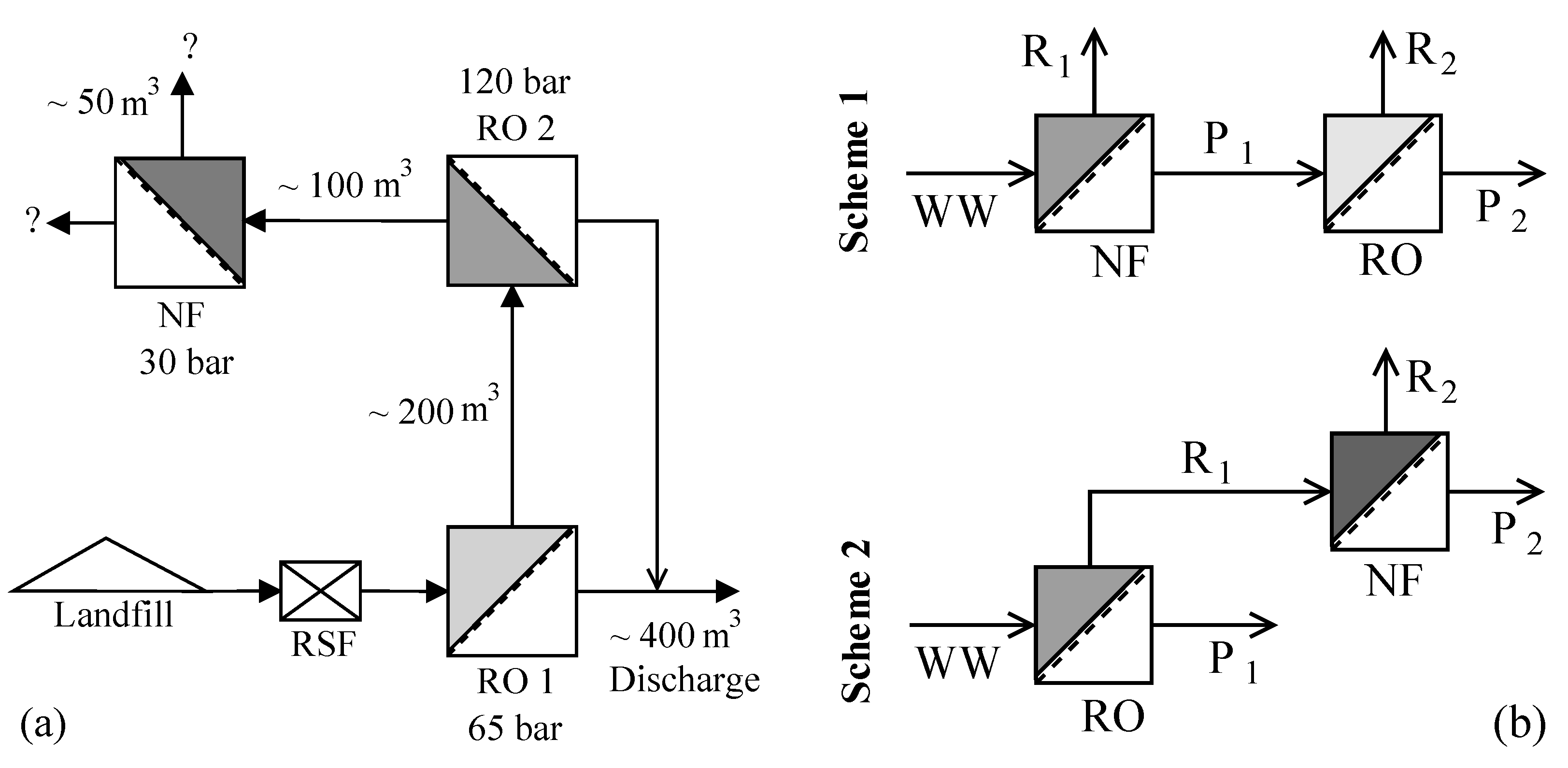
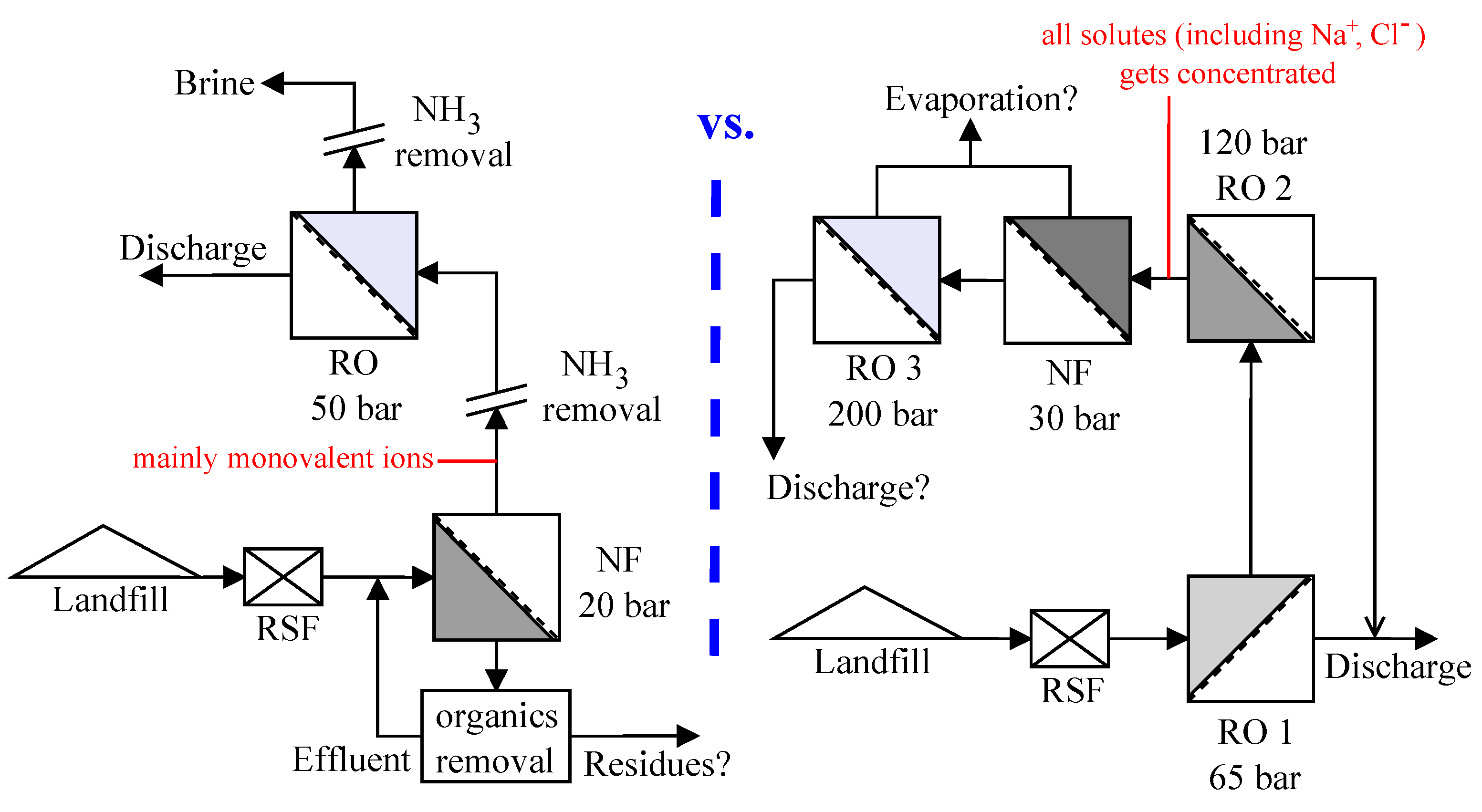
The leachate considered in this study is from the hazardous waste landfill site at Ihlenberg, Germany; one of the largest and most advanced landfills in Europe [
7]. The site with a landfill area of 113 hectares (1.13 km
2) was commissioned in 1983 with a total capacity of 26 million cubic meters [
17]. By the end of 1989, an RO system with 36 m
3/h capacity was installed for treating the raw leachate, recovering up to 80–85% clean water and re-injecting the retentate [
7]. Some studies at the Ihlenberg dumpsite in the past [
7,
9,
18,
19] have focused on minimizing the concentrate volume to be re-injected, as retentate disposal is very cost intensive. Some of them investigated the application of reverse osmosis at 200 bar while some investigated a combination of RO, high pressure RO and high pressure NF (as in
Figure 1a) for reducing the volume of concentrate as low as possible. To comply with the ban on retentate recirculation, the RO concentrate currently gets treated further as in
Figure 1a, the aptness of which (the process train) is questionable.
This study investigates the two integration options: NF-RO and RO-NF for this scenario, as illustrated in
Figure 1b. Scientists involved in the field of membrane technologies would typically recommend NF-RO over RO-NF [
20,
21]. However, landfill operators and leachate professionals, who do not necessarily possess a good knowhow of nanofiltration and reverse osmosis processes, will not be in a position to analyze these two schemes. The practice of nanofiltration of RO concentrate has been reported in literature [
19,
22] for landfill sites like Halle Lochau (Germany), Goerlitz (Germany), and Yachio Town (Japan). The first author of this paper came to know of similar other cases at the Sardinia Symposium 2017 (personal communication, leachate professional).
NF compared to RO can be operated at lower pressures; offers higher fluxes; rejects organics and multivalent ions selectively; and incurs lower investment, operation, and maintenance costs. Due to these reasons, numerous studies in the recent past have preferred NF over RO and explored its ability for the treatment of landfill leachates [
6,
23,
24,
25,
26,
27,
28,
29,
30,
31]. However, no study could be identified, which investigated a combination of NF and RO (NF-RO) for treating landfill leachates. Integration of technologies has been emphasized for achieving efficient and economic treatment of landfill leachates [
24,
28,
32,
33,
34,
35], which could also offer more flexibility and advantages for the individual processes in the process chain [
35].
This study aims to investigate the two combinations (NF-RO and RO-NF) for the treatment of old landfill leachates, and to identify the merits/demerits of the two process trains. Experiments were conducted in bench-scale to demonstrate the differences between the two schemes, with respect to water fluxes (energy requirements), rejection of solutes, and other operational advantages. This paper critically analyses the two integration options, and discusses some fundamental properties and transport characteristics of nanofiltration and reverse osmosis membranes applied to leachate treatment.
2. Materials and Methods
The experiments were carried out in the laboratories for drinking water research at the Institute of Water Resources and Water Supply, TUHH. For this reason the wastewater was prepared synthetically by dissolving known weights of the compounds (as in
Table 1) in deionized water to have a composition resembling that of the raw leachate from the Ihlenberg landfill site (
Table 2). This was done to avoid any kind of contamination of the laboratories from the hazardous landfill leachate. Sodium salt of humic acid, purchased from Carl Roth GmbH (Karlsruhe, Germany), had a humic acid (HA) content of about 45–65%. The HA salt had a sodium content of about 8–9% as Na
2O (Carl Roth GmbH, personal communication). The HA salt was measured to have a total organic carbon (TOC) content of 300 mg/g. All other solutes used were of analytical grade.
The HA salt had not dissolved completely, even over several hours of stirring. The synthetic leachate was filtered using a folded filter paper (Carl Roth 600P-500, 13 µm pore diameter) to remove the undissolved HA salt. The filtrate was analyzed for N-NH4+; total inorganic carbon (TIC), TOC, and COD concentrations; absorbance at 254 nm (Abs254); and conductivity, and used for all the experiments.
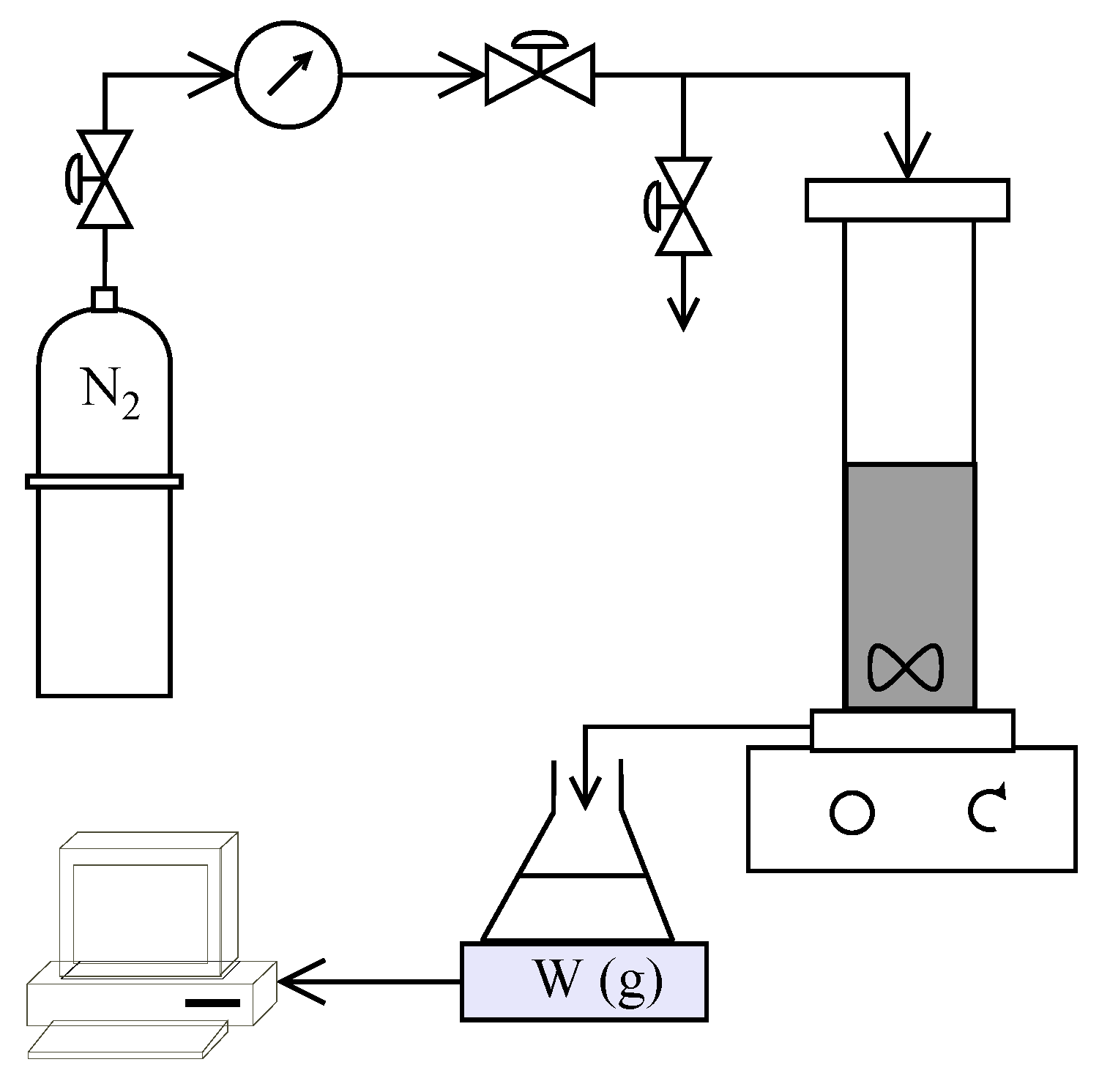
A schematic of the experimental setup used in the study is shown in
Figure 2. HP4750 Stirred Cell (Sterlitech Corporation, Kent, Washington, DC, USA) was used to carry out the filtration trials in dead-end mode providing an active membrane area of 14.6 cm
2. Nitrogen gas flowing through a digital manometer (Pressure Gauge Digital with Ceramic Sensor Element, Battery Powered MAN-SD from Kobold Messring GmbH, Hofheim, Germany) and pressure regulators (Swagelok, OH, USA) was used to apply the desired pressure inside the cell. The weight of the collected permeate was measured using an Acculab ATL-2202 balance (Sartorius AG, Goettingen, Germany) and recorded every 10 s. All experiments were carried out at ambient temperature (22 ± 1 °C) The recorded volume flow rate was used to estimate the temperature corrected permeate flux (using GE Water [
36]) at 25 °C.
Polyamide thin film composite membranes Dow Filmtec SW30HR and NF270 were used for performing the reverse osmosis and nanofiltration experiments, respectively. The membranes were cut in circles of 50 mm diameter and stored in pure water (type 1, 0.05 µS/cm conductivity, produced using a Direct-Q
® 5UV-R system, Merck Millipore, Darmstadt, Germany) at 4 °C for more than 24 h to allow swelling. Two types of experiments were conducted, as illustrated in
Figure 1b, to study the individual processes and their combination for treating the synthetic leachate. A total of eight trials were carried out as in
Table 3 using virgin membranes for each experiment. Before each experiment, pure water was filtered through the respective membrane at the desired operating pressure + 5 bar (OP + 5 bar) until a steady permeate flux was achieved. This would result in a stable membrane structure (after subjecting the membrane to possible compaction at that pressure) before operation and also enable the determination of pure water permeability of the membranes.
The trials were planned such that about 50% water recovery was achieved from each stage of the schemes. Feed volume for second stage in each scheme was fixed at 100 mL. Therefore, the first stage was operated so as to obtain 120 mL of desired feed (NF permeate in scheme 1 or RO retentate in scheme 2) for the second stage. 20 mL of the collected 120 mL was reserved for making the analyses. Samples of the different streams (F, R, and P) from each experiment were analyzed for N-NH
4+, TIC, and TOC concentrations; Abs254; and conductivity. All analyses were carried out as in
Table 4 following the German standard methods [
37]. The parameters chosen to be analyzed would enable the characterization of rejection capacities of the membranes for organic (using TOC and Abs254 values) and inorganic solutes (using conductivity and N-NH
4+ values).