Cholesterol Promotes Interaction of the Protein CLIC1 with Phospholipid Monolayers at the Air–Water Interface
Abstract
:1. Introduction
2. Results and Discussion
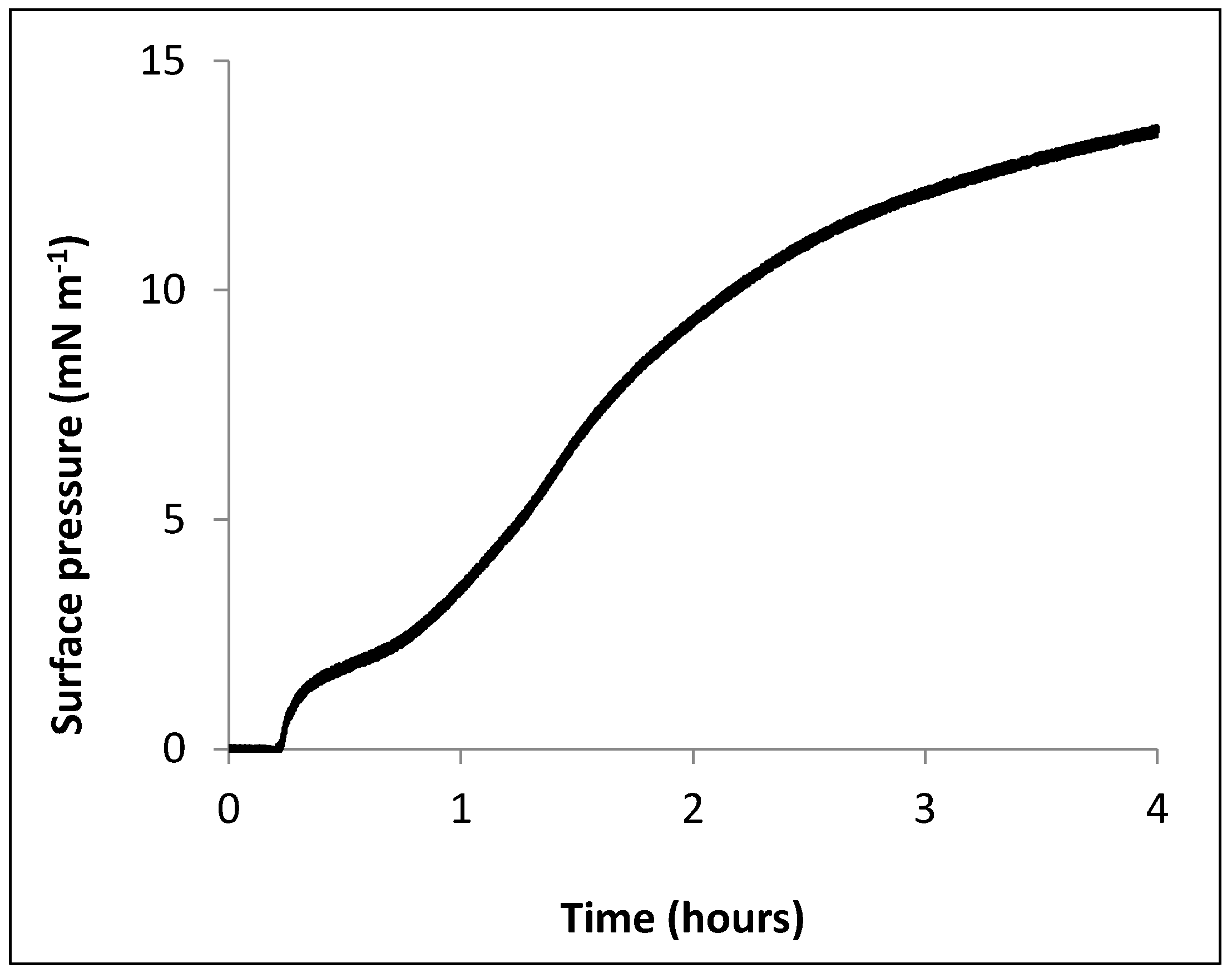
2.1. Surface Activity of CLIC1 Protein:
2.2. Interaction of CLIC1 with Phospholipid Monolayer
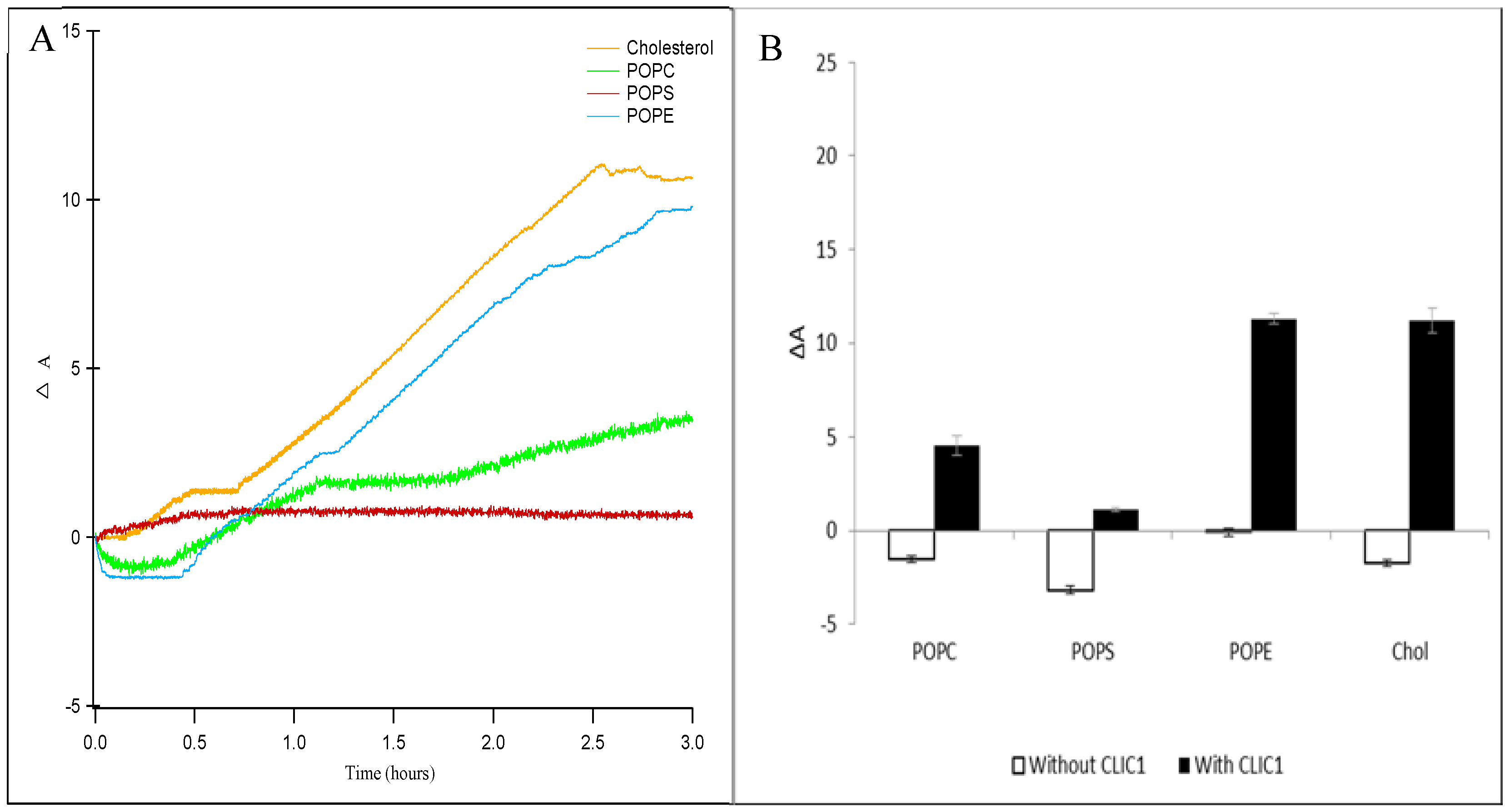
2.3. Interaction of CLIC1 with Phospholipid Monolayer in the Presence of Cholesterol

2.4. Interaction of CLIC1 with Mixed Lipid Monolayers

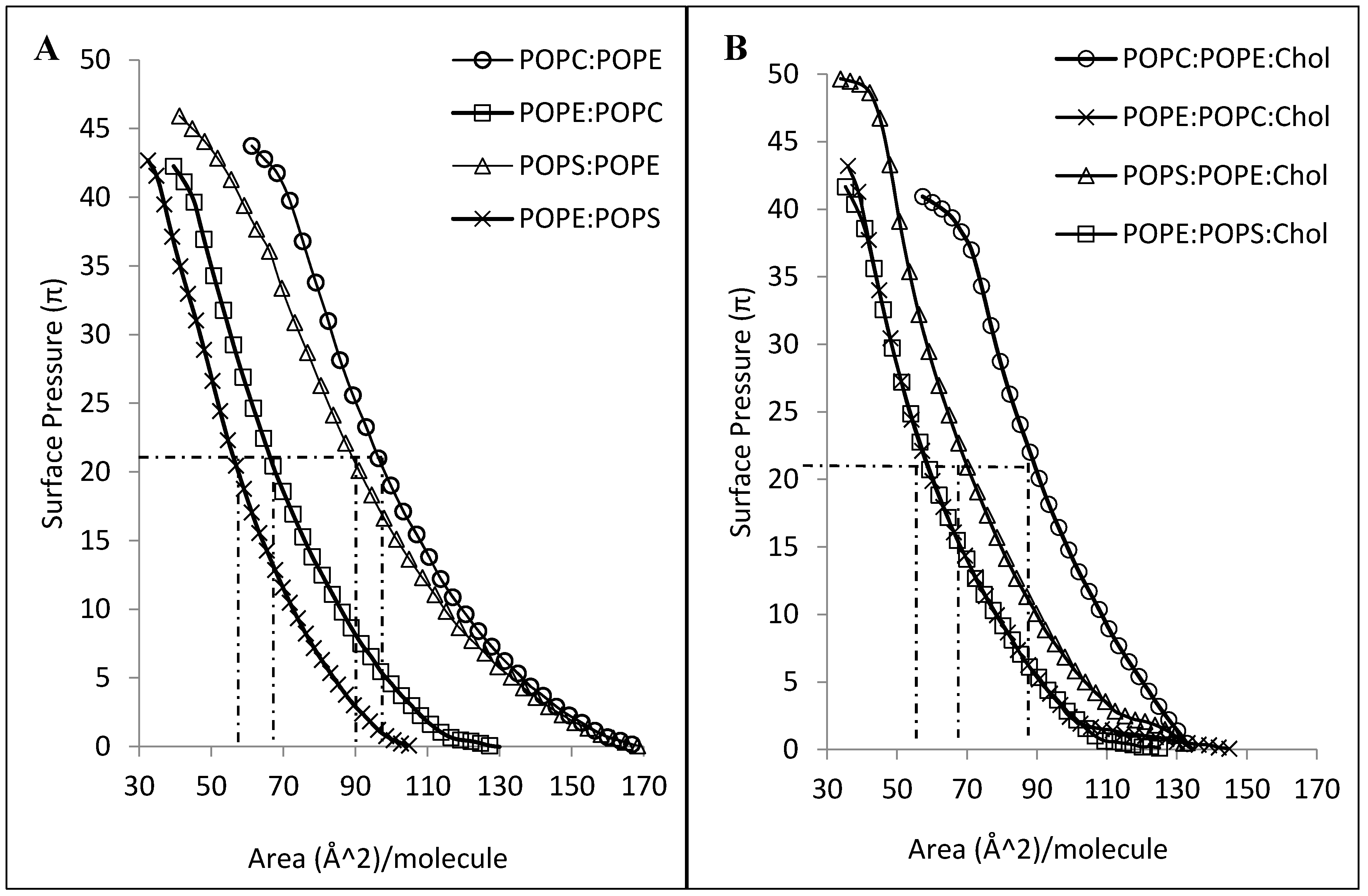
2.5. CLIC1–Cholesterol Interaction

3. Materials and Methods
3.1. Protein Expression and Purification
3.2. Surface Activity of CLIC1 Protein at the Air–Water Interface
3.3. Interaction of CLIC1 with Lipid Monolayers
3.4. Pre-incubation of CLIC1 with Cholesterol
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Liscum, L.; Underwood, K. Intracellular cholesterol transport and compartmentation. J. Biol. Chem. 1995, 270, 15443–15446. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. How cells handle cholesterol. Science 2000, 290, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.; Zuckermann, M. What’s so special about cholesterol? Lipids 2004, 39, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R. Cholesterol-dependent cytolysins. Adv. Exp. Med. Biol. 2010, 677, 56–66. [Google Scholar] [PubMed]
- Epand, R. Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 2006, 45, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.; Song, Y.; Van Horn, W.; Hustedt, E.; Schafer, J.; Hadziselimovic, A.; Beel, A.; Sanders, C. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science 2012, 336, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zheng, J. Cholesterol promotes the interaction of alzheimer β-amyloid monomer with lipid bilayer. J. Mol. Biol. 2012, 421, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, S.; Alkhamici, H.; Brown, L.; Almond, O.; Goodchild, S.; Carne, S.; Curmi, P.; Holt, S.; Cornell, B. Regulation of the membrane insertion and conductance activity of the metamorphic chloride intracellular channel protein clic1 by cholesterol. PLoS ONE 2013, 8, e56948. [Google Scholar] [CrossRef] [PubMed]
- Harrop, S.; DeMaere, M.; Fairlie, W.; Reztsova, T.; Valenzuela, S.; Mazzanti, M.; Tonini, R.; Qiu, M.; Jankova, L.; Warton, K.; et al. Crystal structure of a soluble form of the intracellular chloride ion channel clic1 (ncc27) at 1.4-a resolution. J. Biol. Chem. 2001, 276, 44993–45000. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, S.; Martin, D.; Por, S.; Robbins, J.; Warton, K.; Bootcov, M.; Schofield, P.; Campbell, T.; Breit, S. Molecular cloning and expression of a chloride ion channel of cell nuclei. J. Biol. Chem. 1997, 272, 12575–12582. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.; Harrop, S.; Goodchild, S.; Phang, J.; Mynott, A.; Jiang, L.; Valenzuela, S.; Mazzanti, M.; Brown, L.; Breit, S.; et al. The enigma of the clic proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010, 584, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Tulk, B.; Kapadia, S.; Edwards, J. Clic1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am. J. Physiol. Cell Physiol. 2002, 282, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Warton, K.; Tonini, R.; Fairlie, W.; Matthews, J.; Valenzuela, S.; Qiu, M.; Wu, W.; Pankhurst, S.; Bauskin, A.; Harrop, S.; et al. Recombinant clic1 (ncc27) assembles in lipid bilayers via a ph-dependent two-state process to form chloride ion channels with identical characteristics to those observed in chinese hamster ovary cells expressing clic1. J. Biol. Chem. 2002, 277, 26003–26011. [Google Scholar] [CrossRef] [PubMed]
- Tulk, B.; Schlesinger, P.; Kapadia, S.; Edwards, J. Clic-1 functions as a chloride channel when expressed and purified from bacteria. J. Biol. Chem. 2000, 275, 26986–26993. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, S.; Mazzanti, M.; Tonini, R.; Qiu, M.; Warton, K.; Musgrove, E.; Campbell, T.; Breit, S. The nuclear chloride ion channel ncc27 is involved in regulation of the cell cycle. J. Physiol. 2000, 529, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Novarino, G.; Fabrizi, C.; Tonini, R.; Denti, M.; Malchiodi-Albedi, F.; Lauro, G.; Sacchetti, B.; Paradisi, S.; Ferroni, A.; Curmi, P.; et al. Involvement of the intracellular ion channel clic1 in microglia-mediated beta-amyloid-induced neurotoxicity. J. Neurosci. 2004, 24, 5322–5330. [Google Scholar] [CrossRef] [PubMed]
- Al Khamici, H.; Brown, L.; Hossain, K.; Hudson, A.; Sinclair-Burton, A.; Ng, J.; Daniel, E.; Hare, J.; Cornell, B.; Curmi, P.; et al. Members of the chloride intracellular ion channel protein family demonstrate glutaredoxin-like enzymatic activity. PLoS ONE 2015, 10, e115699. [Google Scholar]
- Goodchild, S.; Howell, M.; Littler, D.; Mandyam, R.; Sale, K.; Mazzanti, M.; Breit, S.; Curmi, P.; Brown, L. Metamorphic response of the clic1 chloride intracellular ion channel protein upon membrane interaction. Biochemistry 2010, 49, 5278–5289. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.; Harrop, S.; Fairlie, W.; Brown, L.; Pankhurst, G.; Pankhurst, S.; DeMaere, M.; Campbell, T.; Bauskin, A.; Tonini, R.; et al. The intracellular chloride ion channel protein clic1 undergoes a redox-controlled structural transition. J. Biol. Chem. 2004, 279, 9298–9305. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, S.; Howell, M.; Cordina, N.; Littler, D.; Breit, S.; Curmi, P.; Brown, L. Oxidation promotes insertion of the clic1 chloride intracellular channel into the membrane. Eur. Biophys. J. 2009, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Ashley, R. Redox regulation of clic1 by cysteine residues associated with the putative channel pore. Biophys. J. 2006, 90, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Fanucchi, S.; Adamson, R.; Dirr, H. Formation of an unfolding intermediate state of soluble chloride intracellular channel protein clic1 at acidic ph. Biochemistry 2008, 47, 11674–11681. [Google Scholar] [CrossRef] [PubMed]
- Cromer, B.; Gorman, M.; Hansen, G.; Adams, J.; Coggan, M.; Littler, D.; Brown, L.; Mazzanti, M.; Breit, S.; Curmi, P.; et al. Structure of the janus protein human clic2. J. Mol. Biol. 2007, 374, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.; Assaad, N.; Harrop, S.; Brown, L.; Pankhurst, G.; Luciani, P.; Aguilar, M.; Mazzanti, M.; Berryman, M.; Breit, S.; et al. Crystal structure of the soluble form of the redox-regulated chloride ion channel protein clic4. FEBS J. 2005, 272, 4996–5007. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.; Harrop, S.; Brown, L.; Pankhurst, G.; Mynott, A.; Luciani, P.; Mandyam, R.; Mazzanti, M.; Tanda, S.; Berryman, M.; et al. Comparison of vertebrate and invertebrate clic proteins: The crystal structures of caenorhabditis elegans exc-4 and drosophila melanogaster dmclic. Proteins 2008, 71, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Stoychev, S.; Nathaniel, C.; Fanucchi, S.; Brock, M.; Li, S.; Asmus, K.; Woods, V.J.; Dirr, H. Structural dynamics of soluble chloride intracellular channel protein clic1 examined by amide hydrogen-deuterium exchange mass spectrometry. Biochemistry 2009, 48, 8413–8421. [Google Scholar] [CrossRef] [PubMed]
- García-Sáez, A.; Mingarro, I.; Pérez-Payá, E.; Salgado, J. Membrane-insertion fragments of bcl-xl, bax, and bid. Biochemistry 2004, 43, 10930–10943. [Google Scholar] [CrossRef] [PubMed]
- Tweten, R. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 2005, 73, 6199–6209. [Google Scholar] [CrossRef] [PubMed]
- Ege, C.; Lee, K. Insertion of alzheimer’s a beta 40 peptide into lipid monolayers. Biophys. J. 2004, 87, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Hauser, H.; Pascher, I.; Pearson, R.; Sundell, S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim. Biophys. Acta 1981, 650, 21–51. [Google Scholar] [CrossRef]
- Dill, K.; Stigter, D. Lateral interactions among phosphatidylcholine and phosphatidylethanolamine head groups in phospholipid monolayers and bilayers. Biochemistry 1988, 27, 3446–3453. [Google Scholar] [CrossRef] [PubMed]
- Domènech, O.; Torrent-Burgués, J.; Merino, S.; Sanz, F.; Montero, M.; Hernández-Borrell, J. Surface thermodynamics study of monolayers formed with heteroacid phospholipids of biological interest. Colloids Surf. B Biointerfaces 2005, 41, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Langner, M.; Kubica, K. The electrostatics of lipid surfaces. Chem. Phys. Lipids 1999, 101, 3–35. [Google Scholar] [CrossRef]
- Demel, R.; Jansen, J.; van Dijck, P.; van Deenen, L. The preferential interaction of cholesterol with different classes of phospholipids. Biochim. Biophys. Acta 1977, 465, 1–10. [Google Scholar] [CrossRef]
- McMullen, T.; Lewis, R.; McElhaney, R. Differential scanning calorimetric and fourier transform infrared spectroscopic studies of the effects of cholesterol on the thermotropic phase behavior and organization of a homologous series of linear saturated phosphatidylserine bilayer membranes. Biophys. J. 2000, 79, 2056–2065. [Google Scholar] [CrossRef]
- Van Dijck, P.; De Kruijff, B.; Van Deenen, L.; De Gier, J.; Demel, R. The preference of cholesterol for phosphatidylcholine in mixed phosphatidylcholine-phosphatidylethanolamine bilayers. Biochim. Biophys. Acta 1976, 455, 576–587. [Google Scholar] [CrossRef]
- Alouf, J.; Geoffroy, C.; Pattus, F.; Verger, R. Surface properties of bacterial sulfhydryl-activated cytolytic toxins. Interaction with monomolecular films of phosphatidylcholine and various sterols. Eur. J. Biochem. 1984, 141, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.; Darji, A.; Frahm, N.; Rohde, M.; Wehland, J.; Chakraborty, T.; Weiss, S. Listeriolysin o: Cholesterol inhibits cytolysis but not binding to cellular membranes. Mol. Microbiol. 1998, 28, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Janosi, L.; Doxastakis, M. Gxxxg motifs, phenylalanine, and cholesterol guide the self-association of transmembrane domains of erbb2 receptors. Biophys. J. 2011, 101, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Senes, A.; Engel, D.; DeGrado, W. Folding of helical membrane proteins: The role of polar, gxxxg-like and proline motifs. Curr. Opin. Struct. Biol. 2004, 14, 465–479. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, K.R.; Al Khamici, H.; Holt, S.A.; Valenzuela, S.M. Cholesterol Promotes Interaction of the Protein CLIC1 with Phospholipid Monolayers at the Air–Water Interface. Membranes 2016, 6, 15. https://doi.org/10.3390/membranes6010015
Hossain KR, Al Khamici H, Holt SA, Valenzuela SM. Cholesterol Promotes Interaction of the Protein CLIC1 with Phospholipid Monolayers at the Air–Water Interface. Membranes. 2016; 6(1):15. https://doi.org/10.3390/membranes6010015
Chicago/Turabian StyleHossain, Khondker R., Heba Al Khamici, Stephen A. Holt, and Stella M. Valenzuela. 2016. "Cholesterol Promotes Interaction of the Protein CLIC1 with Phospholipid Monolayers at the Air–Water Interface" Membranes 6, no. 1: 15. https://doi.org/10.3390/membranes6010015





