Free Volume and Gas Permeation in Anthracene Maleimide-Based Polymers of Intrinsic Microporosity
Abstract
:1. Introduction
2. Experimental Section
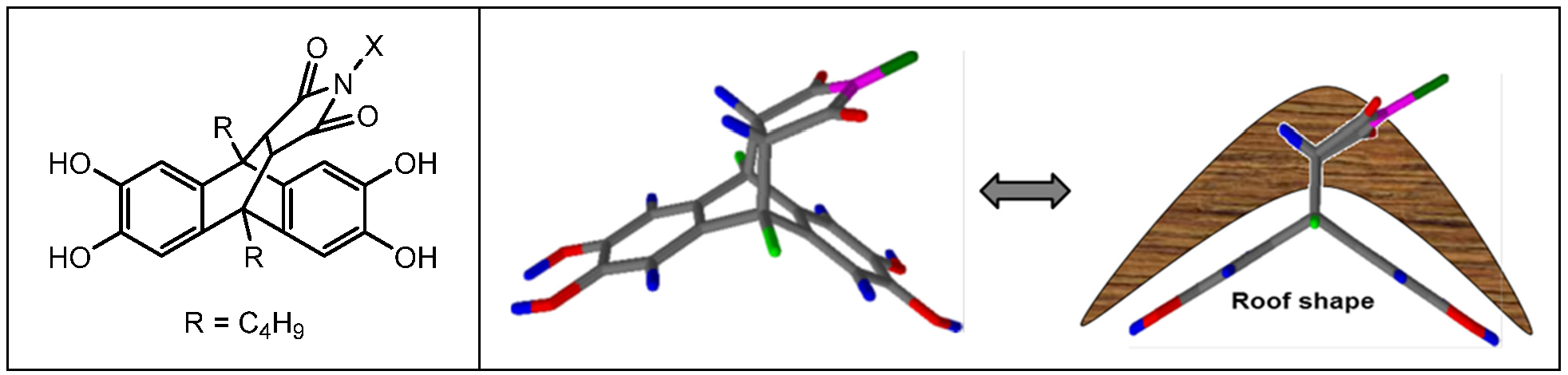
2.1. Materials
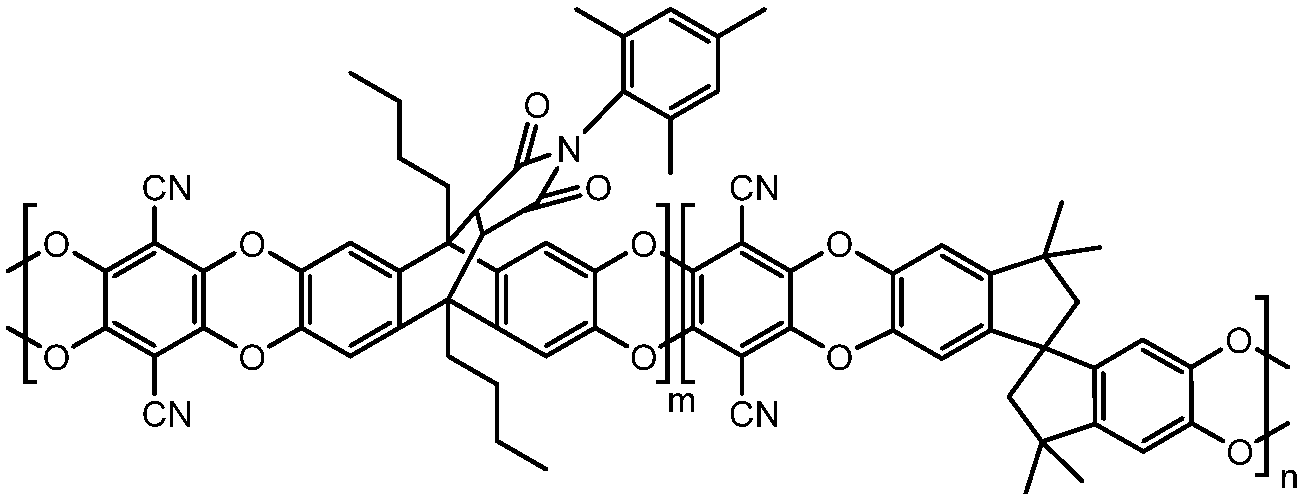
2.2. Synthesis of Polymers
2.2.1. Synthesis of Polymer of Intrinsic Microporosity (PIM-1)
2.2.2. Synthesis of Anthracene Maleimide-Based Homopolymers and Copolymers
2.3. Membrane Preparation
2.4. Gas Permeation Measurement
2.5. Positron Annihilation Lifetime Spectroscopy (PALS)
2.6. Scanning Electron Microscopy
3. Results and Discussion
3.1. Synthesis of Polymers

| Polymers | Comonomer (mol ratio) | TTSBI (mol ratio) | TFTPN (mol ratio) | Mw (g/mol) | PDI | Surface Area (m2/g) |
|---|---|---|---|---|---|---|
| PIM-4bIII-100 | 1 | 0 | 1 | 80200 | 4.2 | 660 |
| PIM-4bIII-75 | 3 | 1 | 4 | 93300 | 3.8 | 790 |
| PIM-4bIII-50 | 1 | 1 | 2 | 147300 | 6.2 | 870 |
| PIM-4bIII-30 | 1 | 2.3 | 3.3 | 118700 | 5.4 | 850 |
| PIM-1 | 0 | 1 | 1 | 221000 | 4.8 | 830 |
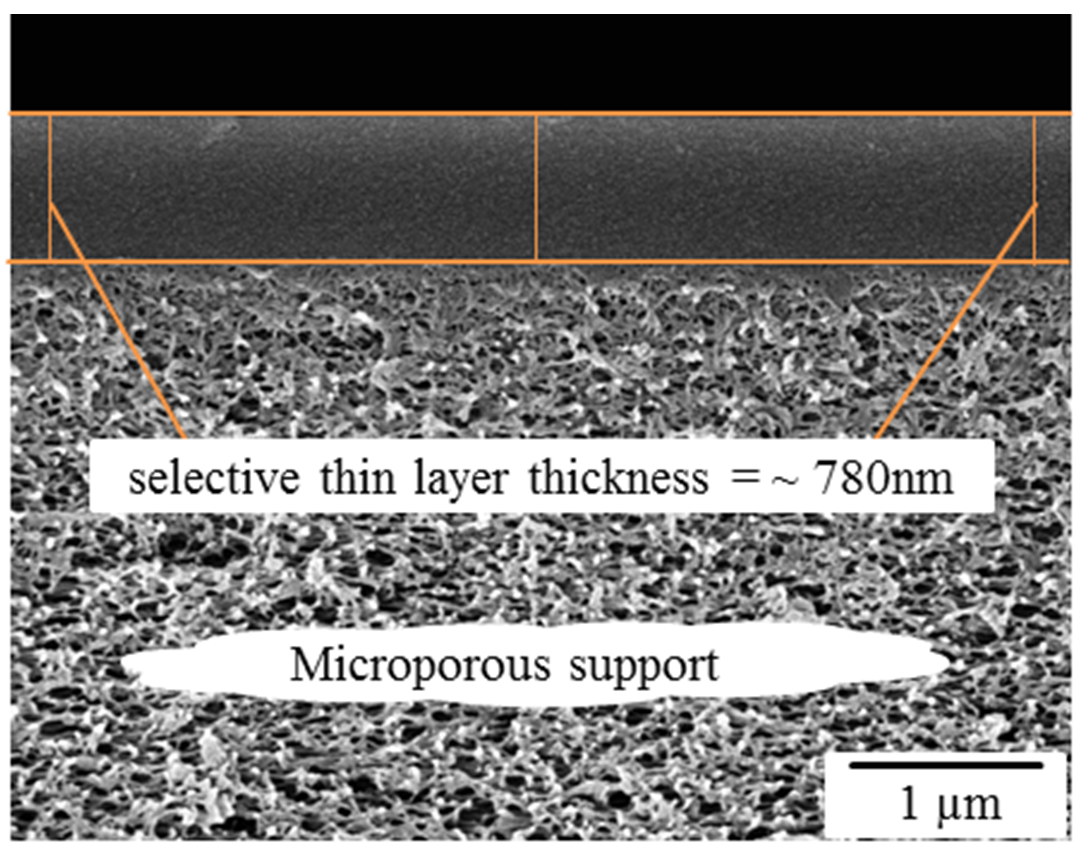
3.2. Cross Section Morphology
3.3. Gas Permeation Performance
| Polymer Samples | Permeance (Nm3/m2 h bar) | ||
|---|---|---|---|
| N2 | O2 | CO2 | |
| PIM-4bIII-75 | 1.74 (±0.15) | 4.48 (±0.14) | 33.41 (±0.26) |
| PIM-4bIII-50 | 1.71 (±0.16) | 4.45 (±0.11) | 33.24 (±0.21) |
| PIM-4bIII-30 | 1.70 (±0.14) | 4.32 (±0.10) | 32.84 (±0.22) |
| PIM-1 | 1.69 (±0.20) | 4.89 (±0.19) | 34.66 (±0.23) |
| Polymer Samples | Permselectivity | |
|---|---|---|
| O2/N2 | CO2/N2 | |
| PIM-1 | 2.9 | 20.5 |
| PIM-4bIII-100 | 2.5 | 18.4 |
| PIM-4bIII-75 | 2.6 | 19.2 |
| PIM-4bIII-50 | 2.6 | 19.4 |
| PIM-4bIII-30 | 2.5 | 19.3 |
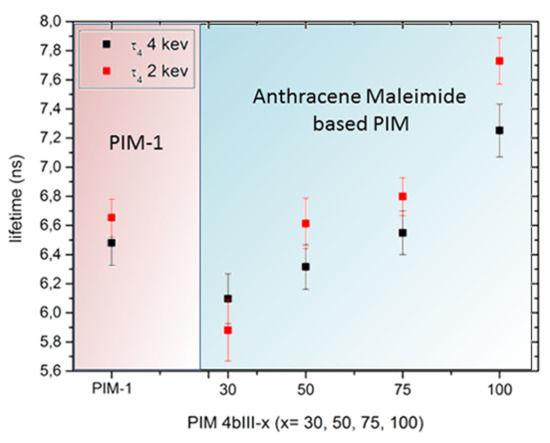
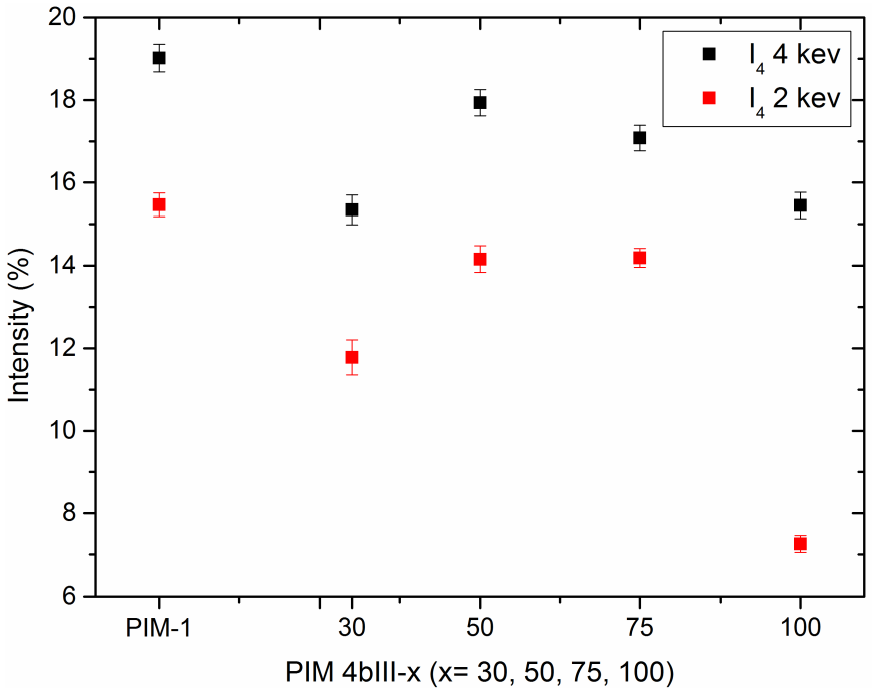
3.4. Positron Annihilation Lifetime Spectroscopy (PALS)

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Pandey, P.; Chauhan, R.S. Membranes for gas separation. Prog. Polym. Sci. 2001, 26, 853–893. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Neumann, S.; Bolmer, S.; Khan, M.M.; Abetz, V. Pebax® with peg functionalized poss as nanocomposite membranes for CO2 separation. J. Membr. Sci. 2013, 437, 286–297. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shishatskiy, S.; Abetz, C.; Georgopanos, P.; Neumann, S.; Khan, M.M.; Filiz, V.; Abetz, V. Influence of temperature upon properties of tailor-made pebax® mh 1657 nanocomposite membranes for post-combustion CO2 capture. J. Membr. Sci. 2014, 469, 344–354. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Khan, M.M.; Gacal, B.N.; Abetz, V. Functionalization of poss nanoparticles and fabrication of block copolymer nanocomposite membranes for CO2 separation. React. Funct. Polym. 2015, 86, 125–133. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Georgopanos, P.; Khan, M.M.; Neumann, S.; Abetz, V. Influence of poly(ethylene glycol) segment length on CO2 permeation and stability of polyactive membranes and their nanocomposites with PEG POSS. ACS Appl. Mater. Interfaces 2015. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Maier, G. Gas separation with polymer membranes. Angew. Chem. Int. Edit. 1998, 37, 2960–2974. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Litwiller, E.; Pinnau, I. Effects of hydroxyl-functionalization and sub-Tg thermal annealing on high pressure pure- and mixed-gas CO2/CH4 separation by polyimide membranes based on 6FDA and triptycene-containing dianhydrides. J. Membr. Sci. 2015, 475, 571–581. [Google Scholar] [CrossRef]
- Yampolskii, Y. Polymeric gas separation membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Hradil, J.; Švec, F. Reactive polymers. 61. Synthesis of strongly basic anion exchange methacrylate resins. React. Polym. 1990, 13, 43–53. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polymers: Basic principle of preparing the new class of polymeric materials. React. Funct. Polym. 2002, 53, 193–203. [Google Scholar] [CrossRef]
- McKeown, N.B.; Gahnem, B.; Msayib, K.J.; Budd, P.M.; Tattershall, C.E.; Mahmood, K.; Tan, S.; Book, D.; Langmi, H.W.; Walton, A. Towards polymer-based hydrogen storage materials: Engineering ultramicroporous cavities within polymers of intrinsic microporosity. Angew. Chem. Int. Ed. 2006, 45, 1804–1807. [Google Scholar] [CrossRef] [PubMed]
- Webster, O.W.; Gentry, F.P.; Farlee, R.D.; Smart, B.E. Hypercrosslinked rigid-rod polymers. Makromol. Chem. Macromol. Symp. 1992, 54–55, 477–482. [Google Scholar] [CrossRef]
- Wood, C.D.; Tan, B.; Trewin, A.; Niu, H.; Bradshaw, D.; Rosseinsky, M.J.; Khimyak, Y.Z.; Campbell, N.L.; Kirk, R.; Stöckel, E.; et al. Hydrogen storage in microporous hypercrosslinked organic polymer networks. Chem. Mater. 2007, 19, 2034–2048. [Google Scholar] [CrossRef]
- Dai, Y.; Guiver, M.D.; Robertson, G.P.; Kang, Y.S. Effect of hexafluoro-2-propanol substituents in polymers on gas permeability and fractional free volume. Macromolecules 2005, 38, 9670–9678. [Google Scholar] [CrossRef]
- Dai, Y.; Guiver, M.D.; Robertson, G.P.; Kang, Y.S.; Lee, K.J.; Jho, J.Y. Preparation and characterization of polysulfones containing both hexafluoroisopropylidene and trimethylsilyl groups as gas separation membrane materials. Macromolecules 2004, 37, 1403–1410. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Pinnau, I.; Thomas, S.; Guiver, M.D. Copolymers of intrinsic microporosity based on 2,2',3,3'-tetrahydroxy-1,1'-dinaphthyl. Macromol. Rapid Commun. 2009, 30, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, B.S.; Msayib, K.J.; McKeown, N.B.; Harris, K.D.M.; Pan, Z.; Budd, P.M.; Butler, A.; Selbie, J.; Book, D.; Walton, A. A triptycene-based polymer of intrinsic microposity that displays enhanced surface area and hydrogen adsorption. Chem. Commun. 2007. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; Elabas, E.S.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E.; Wang, D. Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity. Adv. Mater. 2004, 16, 456–459. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M.; Msayib, K.J.; Ghanem, B.S.; Kingston, H.J.; Tattershall, C.E.; Makhseed, S.; Reynolds, K.J.; Fritsch, D. Polymers of intrinsic microporosity (PIMs): Bridging the void between microporous and polymeric materials. Chem. A Eur. J. 2005, 11, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.; Msayib, K.J.; Budd, P.M.; McKeown, N.B. Novel spirobisindanes for use as precursors to polymers of intrinsic microporosity. Org. Lett. 2008, 10, 2641–2643. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Robertson, G.P.; Dal-Cin, M.M.; Scoles, L.; Guiver, M.D. Polymers of intrinsic microporosity (PIMs) substituted with methyl tetrazole. Polymer 2012, 53, 4367–4372. [Google Scholar] [CrossRef]
- Bezzu, C.G.; Carta, M.; Tonkins, A.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. A spirobifluorene-based polymer of intrinsic microporosity with improved performance for gas separation. Adv. Mater. 2012, 24, 5930–5933. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Dal-Cin, M.M.; Robertson, G.P.; Guiver, M.D. Decarboxylation-induced cross-linking of polymers of intrinsic microporosity (PIMs) for membrane gas separation. Macromolecules 2012, 45, 5134–5139. [Google Scholar] [CrossRef]
- Ma, X.; Salinas, O.; Litwiller, E.; Pinnau, I. Novel spirobifluorene- and dibromospirobifluorene-based polyimides of intrinsic microporosity for gas separation applications. Macromolecules 2013, 46, 9618–9624. [Google Scholar] [CrossRef]
- Rogan, Y.; Starannikova, L.; Ryzhikh, V.; Yampolskii, Y.; Bernardo, P.; Bazzarelli, F.; Jansen, J.C.; McKeown, N.B. Synthesis and gas permeation properties of novel spirobisindane-based polyimides of intrinsic microporosity. Polym. Chem. 2013, 4, 3813–3820. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An efficient polymer molecular sieve for membrane gas separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Del Regno, A.; Gonciaruk, A.; Leay, L.; Carta, M.; Croad, M.; Malpass-Evans, R.; McKeown, N.B.; Siperstein, F.R. Polymers of intrinsic microporosity containing tröger base for CO2 capture. Ind. Eng. Chem. Res. 2013, 52, 16939–16950. [Google Scholar] [CrossRef]
- Shamsipur, H.; Dawood, B.A.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. Thermally rearrangeable PIM-polyimides for gas separation membranes. Macromolecules 2014, 47, 5595–5606. [Google Scholar] [CrossRef]
- Taylor, R.G.D.; Carta, M.; Bezzu, C.G.; Walker, J.; Msayib, K.J.; Kariuki, B.M.; McKeown, N.B. Triptycene-based organic molecules of intrinsic microporosity. Org. Lett. 2014, 16, 1848–1851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, J.; Cooney, R.; Zhang, S. Synthesis of polymers of intrinsic microporosity using an ab-type monomer. Polymer 2015, 57, 45–50. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Lee, M.; Rose, I.; McKeown, N.B. The synthesis of microporous polymers using troger’s base formation. Polym. Chem. 2014, 5, 5267–5272. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Swaidan, R.; Litwiller, E.; Pinnau, I. Ultra-microporous triptycene-based polyimide membranes for high-performance gas separation. Adv. Mater. 2014, 26, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Bengtson, G.; Neumann, S.; Rahman, M.M.; Abetz, V.; Filiz, V. Synthesis, characterization and gas permeation properties of anthracene maleimide-based polymers of intrinsic microporosity. RSC Adv. 2014, 4, 32148–32160. [Google Scholar] [CrossRef]
- Emmler, T.; Heinrich, K.; Fritsch, D.; Budd, P.M.; Chaukura, N.; Ehlers, D.; Rätzke, K.; Faupel, F. Free volume investigation of polymers of intrinsic microporosity (PIMs): PIM-1 and PIM1 copolymers incorporating ethanoanthracene units. Macromolecules 2010, 43, 6075–6084. [Google Scholar] [CrossRef]
- Jean, Y.C.; Mallon, P.E.; Schrader, D.M. Principle and Application of Positron and Positronium Chemistry; World Scientific: River Edge, NJ, USA, 2003. [Google Scholar]
- Koschine, T.; Rätzke, K.; Faupel, F.; Khan, M.M.; Emmler, T.; Filiz, V.; Abetz, V.; Ravelli, L.; Egger, W. Correlation of gas permeation and free volume in new and used high free volume thin film composite membranes. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 213–217. [Google Scholar] [CrossRef]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.M.; Lillepaerg, J.; Abetz, V. Enhanced gas permeability by fabricating mixed matrix membranes of functionalized multiwalled carbon nanotubes and polymers of intrinsic microporosity (PIM). J. Membrane Sci. 2013, 436, 109–120. [Google Scholar] [CrossRef]
- Khan, M.M.; Bengtson, G.; Shishatskiy, S.; Gacal, B.N.; Mushfequr Rahman, M.; Neumann, S.; Filiz, V.; Abetz, V. Cross-linking of polymer of intrinsic microporosity (PIM-1) via nitrene reaction and its effect on gas transport property. Eur. Polym. J. 2013, 49, 4157–4166. [Google Scholar] [CrossRef]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Rahman, M.M.; Shishatskiy, S.; Abetz, V. Functionalized carbon nanotube mixed matrix membranes of polymers of intrinsic microporosity (PIMs) for gas separation. Procedia Eng. 2012, 44, 1899–1901. [Google Scholar] [CrossRef]
- Khan, M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.; Abetz, V. Functionalized carbon nanotubes mixed matrix membranes of polymers of intrinsic microporosity for gas separation. Nanoscale Res. Lett. 2012, 7, 504. [Google Scholar] [CrossRef] [PubMed]
- Egger, W. Positron Sources and Positron Beams, Physics with Many Positrons; IOS Press: Amsterdam, The Netherlands, 2010; Volume 174. [Google Scholar]
- Ohrt, C.; Koschine, T.; Rätzke, K.; Faupel, F.; Willner, L.; Schneider, G.J. Free volume in pep-silica nanocomposites with varying molecular weight. Polymer 2014, 55, 143–149. [Google Scholar] [CrossRef]
- Harms, S.; Rätzke, K.; Faupel, F.; Chaukura, N.; Budd, P.M.; Egger, W.; Ravelli, L. Aging and free volume in a polymer of intrinsic microporosity (PIM-1). J. Adhes. 2012, 88, 608–619. [Google Scholar] [CrossRef]
- Konietzny, R.; Koschine, T.; Rätzke, K.; Staudt, C. Poss-hybrid membranes for the removal of sulfur aromatics by pervaporation. Sep. Purif. Technol. 2014, 123, 175–182. [Google Scholar] [CrossRef]
- Harms, S.; Rätzke, K.; Pakula, C.; Zaporojtchenko, V.; Strunskus, T.; Egger, W.; Sperr, P.; Faupel, F. Free volume changes on optical switching in azobenzene-polymethylmethacrylate blends studied by a pulsed low-energy positron beam. J. Polymer Sci. Part B Polym. Phys. 2011, 49, 404–408. [Google Scholar] [CrossRef]
- Jeazet, H.; Koschine, T.; Staudt, C.; Raetzke, K.; Janiak, C. Correlation of gas permeability in a metal-organic framework MIL-101(Cr)–polysulfone mixed-matrix membrane with free volume measurements by positron annihilation lifetime spectroscopy (PALS). Membranes 2013, 3, 331–353. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, D.; Bengtson, G.; Carta, M.; McKeown, N.B. Synthesis and gas permeation properties of spirobischromane-based polymers of intrinsic microporosity. Macromol. Chem. Phys. 2011, 212, 1137–1146. [Google Scholar] [CrossRef]
- Du, N.; Song, J.; Robertson, G.P.; Pinnau, I.; Guiver, M.D. Linear high molecular weight ladder polymer via fast polycondensation of 5,5',6,6'-tetrahydroxy-3,3,3',3'-tetramethylspirobisindane with 1,4-dicyanotetrafluorobenzene. Macromol. Rapid Commun. 2008, 29, 783–788. [Google Scholar] [CrossRef]
- Fritsch, D.; Bengtson, G.; Carta, M.; McKeown, N.B. Synthesis and gas permeation properties of spirobischromane-based polymers of intrinsic microporosity. Macromol. Chem. Phys. 2011, 212, 1137–1146. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamada, Y. Characterization of 6FDA-based hyperbranched and linear polyimide–silica hybrid membranes by gas permeation and 129Xe NMR measurements. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 291–298. [Google Scholar] [CrossRef]
- Jansen, J.C.; Macchione, M.; Tocci, E.; de Lorenzo, L.; Yampolskii, Y.P.; Sanfirova, O.; Shantarovich, V.P.; Heuchel, M.; Hofmann, D.; Drioli, E. Comparative study of different probing techniques for the analysis of the free volume distribution in amorphous glassy perfluoropolymers. Macromolecules 2009, 42, 7589–7604. [Google Scholar] [CrossRef]
- Schrader, D.R.; Jean, Y.C. Positron and positronium in liquids. In Positron and Positronium Chemistry; Elsevier Sci. Pub.: Amsterdam, The Netherlands, 1988. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.M.; Filiz, V.; Emmler, T.; Abetz, V.; Koschine, T.; Rätzke, K.; Faupel, F.; Egger, W.; Ravelli, L. Free Volume and Gas Permeation in Anthracene Maleimide-Based Polymers of Intrinsic Microporosity. Membranes 2015, 5, 214-227. https://doi.org/10.3390/membranes5020214
Khan MM, Filiz V, Emmler T, Abetz V, Koschine T, Rätzke K, Faupel F, Egger W, Ravelli L. Free Volume and Gas Permeation in Anthracene Maleimide-Based Polymers of Intrinsic Microporosity. Membranes. 2015; 5(2):214-227. https://doi.org/10.3390/membranes5020214
Chicago/Turabian StyleKhan, Muntazim Munir, Volkan Filiz, Thomas Emmler, Volker Abetz, Toenjes Koschine, Klaus Rätzke, Franz Faupel, Werner Egger, and Luca Ravelli. 2015. "Free Volume and Gas Permeation in Anthracene Maleimide-Based Polymers of Intrinsic Microporosity" Membranes 5, no. 2: 214-227. https://doi.org/10.3390/membranes5020214
APA StyleKhan, M. M., Filiz, V., Emmler, T., Abetz, V., Koschine, T., Rätzke, K., Faupel, F., Egger, W., & Ravelli, L. (2015). Free Volume and Gas Permeation in Anthracene Maleimide-Based Polymers of Intrinsic Microporosity. Membranes, 5(2), 214-227. https://doi.org/10.3390/membranes5020214