The Endocytic Receptor Megalin and its Associated Proteins in Proximal Tubule Epithelial Cells
Abstract
:1. Introduction
2. Structure and Function of Megalin
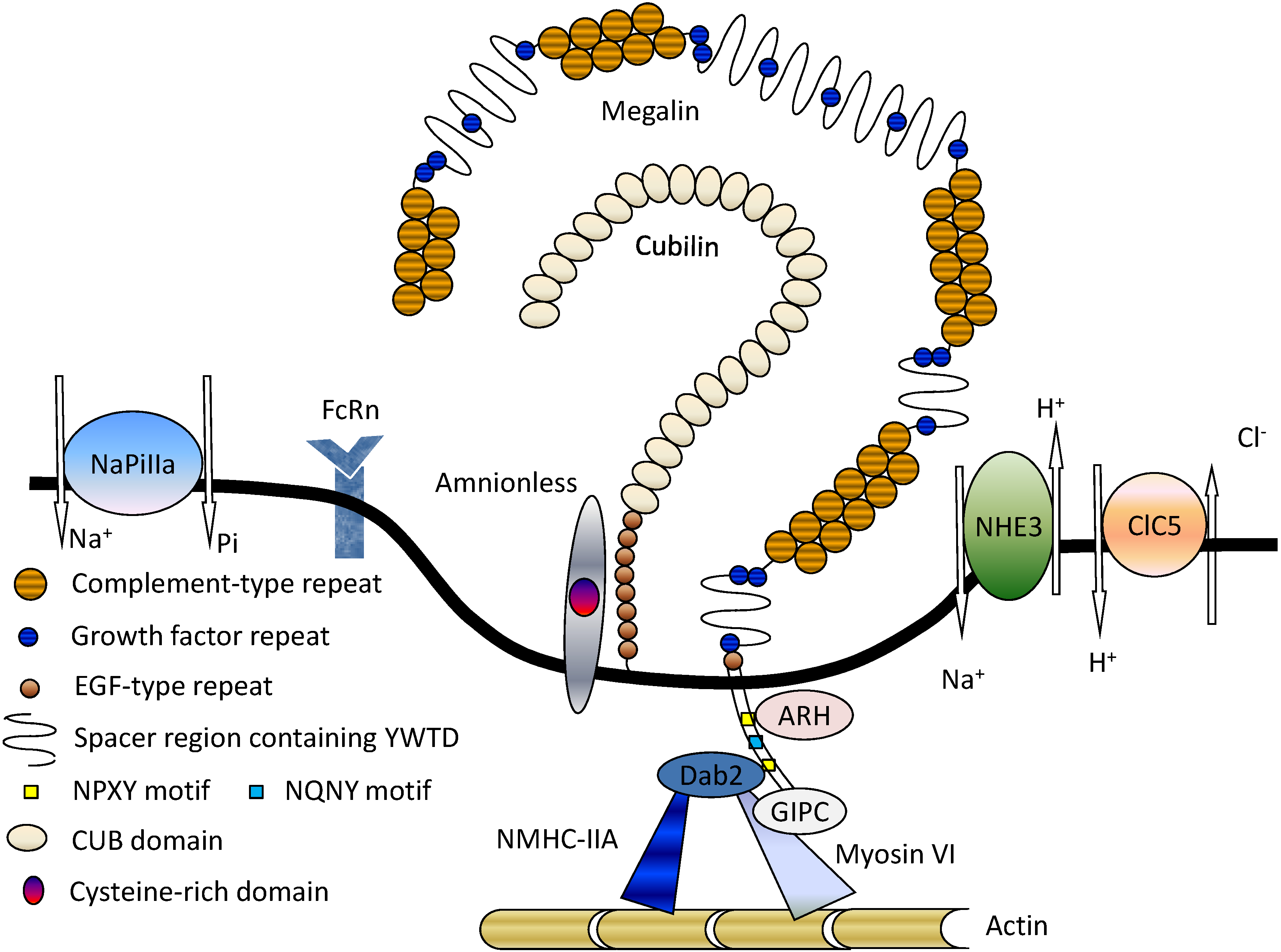
3. Megalin-Associated Molecules
3.1. Cubilin
3.2. Na+/H+ Exchanger Isoform 3 (NHE3)
3.3. 2Cl−/H+-Exchanger (ClC-5)
3.4. Type IIa Sodium Phosphate Co-Transporter (NaPi-IIa)
3.5. FcRn
3.6. Intracellular Adaptor Proteins
4. Megalin Trafficking and Expression in PTECs
4.1. Role of Adaptor Proteins in Megalin Trafficking
4.2. Megalin-Expressing Exosomes
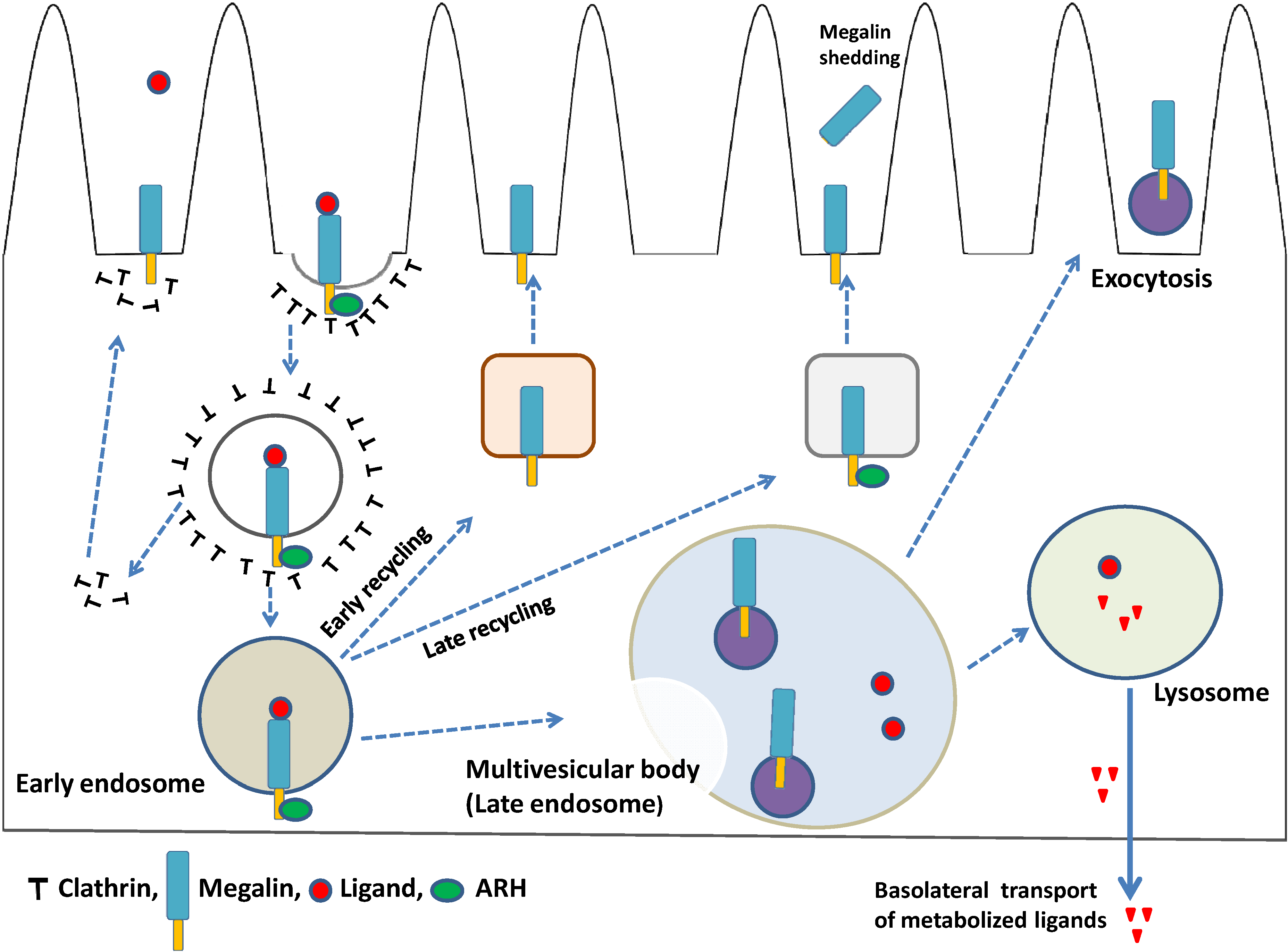
4.3. Megalin Expression and Regulated Intramembrane Proteolysis (RIP)
5. Megalin-Mediated PTEC Injury
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Evans, P.R.; Owen, D.J. Endocytosis and vesicle trafficking. Curr. Opin. Struct. Biol. 2002, 12, 814–821. [Google Scholar] [CrossRef]
- Scita, G.; di Fiore, P.P. The endocytic matrix. Nature 2010, 463, 464–473. [Google Scholar] [CrossRef]
- Taylor, M.J.; Perrais, D.; Merrifield, C.J. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011, 9, e1000604:1–e1000604:23. [Google Scholar]
- Maldonado-Báez, L.; Wendland, B. Endocytic adaptors: Recruiters, coordinators and regulators. Trends Cell Biol. 2006, 16, 505–513. [Google Scholar] [CrossRef]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell. Sci. 2000, 113, 3365–3374. [Google Scholar]
- Kerjaschki, D.; Farquhar, M.G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc. Natl. Acad. Sci. USA 1982, 79, 5557–5561. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Farquhar, M.G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J. Exp. Med. 1983, 157, 667–686. [Google Scholar] [CrossRef]
- Saito, A.; Pietromonaco, S.; Loo, A.K.; Farquhar, M.G. Complete cloning and sequencing of rat gp330/‘megalin’ a distinctive member of the low density lipoprotein receptor gene family. Proc. Natl. Acad. Sci. USA 1994, 91, 9725–9729. [Google Scholar] [CrossRef]
- Hjälm, G.; Murray, E.; Crumley, G.; Harazim, W.; Lundgren, S.; Onyango, I.; Ek, B.; Larsson, M.; Juhlin, C.; Hellman, P.; et al. Cloning and sequencing of human gp330, a Ca2+-binding receptor with potential intracellular signaling properties. Eur. J. Biochem. 1996, 239, 132–137. [Google Scholar]
- Xia, Y.R.; Bachinsky, D.R.; Smith, J.A.; McCluskey, R.T.; Warden, C.H.; Lusis, A.J. Mapping of the glycoprotein 330 (Gp330) gene to mouse chromosome 2. Genomics 1993, 17, 780–781. [Google Scholar] [CrossRef]
- Raychowdhury, R.; Niles, J.L.; McCluskey, R.T.; Smith, J.A. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science 1989, 244, 1163–1165. [Google Scholar]
- Davis, C.G.; Goldstein, J.L.; Sudhof, T.C.; Anderson, R.G.; Russell, D.W.; Brown, M.S. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature 1987, 326, 760–765. [Google Scholar] [CrossRef]
- Takeda, T.; Yamazaki, H.; Farquhar, M.G. Identification of an apical sorting determinant in the cytoplasmic tail of megalin. Am. J. Physiol. Cell. Physiol. 2003, 284, C1105–C1113. [Google Scholar] [CrossRef]
- Saito, A.; Sato, H.; Iino, N.; Takeda, T. Physiology and Pathophysiology of Megalin-Mediated Endocytosis in Renal Proximal Tubule Cells. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; Volume 14, pp. 169–184. [Google Scholar]
- Verroust, P.J.; Kozyraki, R.; Hammond, T.G.; Moestrup, S.K.; Christensen, E.I. Physiopathologic role of cubilin and megalin. Adv. Nephrol. Necker. Hosp. 2000, 30, 127–145. [Google Scholar]
- Christensen, E.I.; Verroust, P.J.; Nielsen, R. Receptor mediated endocytosis in renal proximal tubule. Pflugers. Arch. 2009, 458, 1039–1048. [Google Scholar] [CrossRef]
- Prabakaran, T.; Nielsen, R.; Larsen, J.V.; Sørensen, S.S.; Feldt-Rasmussen, U.; Saleem, M.A.; Petersen, C.M.; Verroust, P.J.; Christensen, E.I. Receptor-mediated endocytosis of α-galactosidase A in human podocytes in Fabry disease. Nephrol. Dial. Transplant. 2012, 27, 3156–3159. [Google Scholar] [CrossRef]
- Chun, J.T.; Wang, L.; Pasinetti, G.M.; Finch, C.E.; Zlokovic, B.V. Glycoprotein 330/megalin (LRP-2) has low prevalence as mRNA and protein in brain microvessels and choroid plexus. Exp. Neurol. 1999, 157, 194–201. [Google Scholar] [CrossRef]
- Wicher, G.; Larsson, M.; Svenningsen, A.F.; Gyllencreutz, E.; Rask, L.; Aldskogius, H. Low density lipoprotein receptor-related protein-2/megalin is expressed in oligodendrocytes in the mouse spinal cord white matter. J. Neurosci. Res. 2006, 83, 864–873. [Google Scholar] [CrossRef]
- Bento-Abreu, A.; Velasco, A.; Polo-Hernández, E.; Pérez-Reyes, P.L.; Tabernero, A.; Medina, J.M. Megalin is a receptor for albumin in astrocytes and is required for the synthesis of the neurotrophic factor oleic acid. J. Neurochem. 2008, 106, 1149–1159. [Google Scholar] [CrossRef]
- Chung, R.S.; Penkowa, M.; Dittmann, J.; King, C.E.; Bartlett, C.; Asmussen, J.W.; Hidalgo, J.; Carrasco, J.; Leung, Y.K.; Walker, A.K.; et al. Redefining the role of metallothionein within the injured brain: Extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. J. Biol. Chem. 2008, 283, 15349–15358. [Google Scholar] [CrossRef]
- Yammani, R.R.; Seetharam, S.; Seetharam, B. Cubilin and megalin expression and their interaction in the rat intestine: Effect of thyroidectomy. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E900–E907. [Google Scholar]
- Erranz, B.; Miquel, J.F.; Argraves, W.S.; Barth, J.L.; Pimentel, F.; Marzolo, M.P. Megalin and cubilin expression in gallbladder epithelium and regulation by bile acids. J. Lipid Res. 2004, 45, 2185–2198. [Google Scholar] [CrossRef]
- Lundgren, S.; Hjälm, G.; Hellman, P.; Ek, B.; Juhlin, C.; Rastad, J.; Klareskog, L.; Åkerström, G.; Rask, L. A protein involved in calcium sensing of the human parathyroid and placental cytotrophoblast cells belonging to the LDL-receptor superfamily. Exp. Cell Res. 1994, 212, 344–350. [Google Scholar] [CrossRef]
- Lundgren, S.; Carling, T.; Hjälm, G.; Juhlin, C.; Rastad, J.; Pihlgren, U.; Rask, L.; Akerström, G.; Hellman, P. Tissue distribution of human gp330/megalin, a putative Ca2+-sensing protein. J. Histochem. Cytochem. 1997, 45, 383–392. [Google Scholar] [CrossRef]
- Argraves, W.S.; Morales, C.R. Immunolocalization of cubilin, megalin, apolipoprotein J, and apolipoprotein A-I in the uterus and oviduct. Mol. Reprod. Dev. 2004, 69, 419–427. [Google Scholar] [CrossRef]
- Leheste, J.R.; Rolinski, B.; Vorum, H.; Hilpert, J.; Nykjaer, A.; Jacobsen, C.; Aucouturier, P.; Moskaug, J.O.; Otto, A.; Christensen, E.I.; et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol. 1999, 155, 1361–1370. [Google Scholar] [CrossRef]
- Kantarci, S.; Al-Gazali, L.; Hill, R.S.; Donnai, D.; Black, G.C.; Bieth, E.; Chassaing, N.; Lacombe, D.; Devriendt, K.; et al. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat. Genet. 2007, 39, 957–959. [Google Scholar] [CrossRef]
- Pober, B.R.; Longoni, M.; Noonan, K.M. A Review of Donnai-Barrow and Facio-oculo-acousticorenal (DB/FOAR) Syndrome: Clinical features and differential diagnosis. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 76–81. [Google Scholar] [CrossRef]
- McCarthy, R.A.; Barth, J.L.; Chintalapudi, M.R.; Knaak, C.; Argraves, W.S. Megalin functions as an endocytic sonic hedgehog receptor. J. Biol. Chem. 2002, 277, 25660–25667. [Google Scholar]
- Orlando, R.A.; Rader, K.; Authier, F.; Yamazaki, H.; Posner, B.I.; Bergeron, J.J.; Farquhar, M.G. Megalin is an endocytic receptor for insulin. J. Am. Soc. Nephrol. 1998, 9, 1759–1766. [Google Scholar]
- Cui, S.; Verroust, P.J.; Moestrup, S.K.; Christensen, E.I. Megalin/gp330 mediates uptake of albumin in renal proximal tuble. Am. J. Physiol. Renal. Physiol. 1996, 271, F900–F907. [Google Scholar]
- Gburek, J.; Verroust, P.J.; Willnow, T.E.; Fyfe, J.C.; Nowacki, W.; Jacobsen, C.; Moestrup, S.K.; Christensen, E.I. Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J. Am. Soc. Nephrol. 2002, 13, 423–430. [Google Scholar]
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96, 507–515. [Google Scholar]
- Christensen, E.I.; Moskaug, J.O.; Vorum, H.; Jacobsen, C.; Gundersen, T.E.; Nykjaer, A.; Blomhoff, R.; Willnow, T.E.; Moestrup, S.K. Evidence for an essential role of megalin in transepithelial transport of retinol. J. Am. Soc. Nephrol. 1999, 10, 685–695. [Google Scholar]
- Saito, A.; Kazama, J.J.; Iino, N.; Cho, K.; Sato, N.; Yamazaki, H.; Oyama, Y.; Takeda, T.; Orlando, R.A.; Shimizu, F. Bioengineered implantation of megalin-expressing cells: A potential intracorporeal therapeutic model for uremic toxin protein clearance in renal failure. J. Am. Soc. Nephrol. 2003, 14, 2025–2032. [Google Scholar] [CrossRef]
- Saito, A.; Nagai, R.; Tanuma, A.; Hama, H.; Cho, K.; Takeda, T.; Yoshida, Y.; Toda, T.; Shimizu, F.; Horiuchi, S.; Gejyo, F. Role of megalin in endocytosis of advanced glycation end products: Implications for a novel protein binding to both megalin and advanced glycation end products. J. Am. Soc. Nephrol. 2003, 14, 1123–1131. [Google Scholar] [CrossRef]
- Batuman, V.; Guan, S. Receptor-mediated endocytosis of immunoglobulin light chains by renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 1997, 272, F521–F530. [Google Scholar]
- Klassen, R.B.; Allen, P.L.; Batuman, V.; Crenshaw, K.; Hammond, T.G. Light chains are a ligand for megalin. J. Appl. Physiol. 2005, 98, 257–263. [Google Scholar]
- Sengul, S.; Erturk, S.; Khan, A.M.; Batuman, V. Receptor-Associated Protein blocks internalization and cytotoxicity of Myeloma Light Chain in cultured human proximal tubular cells. PLoS One 2013, 8, e70276:1–e70276:8. [Google Scholar]
- Moestrup, S.K.; Cui, S.; Vorum, H.; Bregengård, C.; Bjørn, S.E.; Norris, K.; Gliemann, J.; Christensen, E.I. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J. Clin. Invest. 1995, 96, 1404–1413. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H.; Storm, T.; Weyer, K.; Nielsen, R. Endocytic receptors in the renal proximal tubule. Physiology 2012, 27, 223–236. [Google Scholar] [CrossRef]
- Seetharam, B.; Levine, J.S.; Ramsey, M.; Alpers, D.H. Purification, properties, and immunochemical localization of a receptor for intrinsic factor-cobalamin complex in the rat kidney. J. Biol. Chem. 1988, 263, 4443–4449. [Google Scholar]
- Seetharam, B.; Christensen, E.I.; Moestrup, S.K.; Hammond, T.G.; Verroust, P.J. Identification of rat yolk sac target protein of teratogenic antibodies, gp280, as intrinsic factor-cobalamin receptor. J. Clin. Invest. 1997, 99, 2317–2322. [Google Scholar] [CrossRef]
- Aminoff, M.; Carter, J.E.; Chadwick, R.B.; Johnson, C.; Gräsbeck, R.; Abdelaal, M.A.; Broch, H.; Jenner, L.B.; Verroust, P.J.; Moestrup, S.K.; et al. Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nat. Genet. 1999, 21, 309–313. [Google Scholar] [CrossRef]
- Prabakaran, T.; Christensen, E.I.; Nielsen, R.; Verroust, P.J. Cubilin is expressed in rat and human glomerular podocytes. PLoS One 2011, 6, e25065:1–e25065:11. [Google Scholar]
- Kristiansen, M.; Kozyraki, R.; Jacobsen, C.; Nexø, E.; Verroust, P.J.; Moestrup, S.K. Molecular dissection of the intrinsic factor-vitamin B12 receptor, cubilin, discloses regions important for membrane association and ligand binding. J. Biol. Chem. 1999, 274, 20540–20544. [Google Scholar]
- Kozyraki, R.; Kristiansen, M.; Silahtaroglu, A.; Hansen, C.; Jacobsen, C.; Tommerup, N.; Verroust, P.J.; Moestrup, S.K. The human intrinsic factor-vitamin B12 receptor, cubilin: Molecular characterization and chromosomal mapping of the gene to 10p within the autosomal recessive megaloblastic anemia (MGA1) region. Blood 1998, 91, 3593–3600. [Google Scholar]
- Birn, H.; Fyfe, J.C.; Jacobsen, C.; Mounier, F.; Verroust, P.J.; Orskov, H.; Willnow, T.E.; Moestrup, S.K.; Christensen, E.I. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J. Clin. Invest. 2000, 105, 1353–1361. [Google Scholar] [CrossRef]
- Kozyraki, R.; Fyfe, J.; Verroust, P.J.; Jacobsen, C.; Dautry-Varsat, A.; Gburek, J.; Willnow, T.E.; Christensen, E.I.; Moestrup, S.K. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc. Natl. Acad. Sci. USA 2001, 98, 12491–12496. [Google Scholar] [CrossRef]
- Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.R.; Jacobsen, C.; Nielsen, M.S.; Verroust, P.J.; Aminoff, M.; de la Chapelle, A.; Moestrup, S.K.; et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc. Natl. Acad. Sci. USA 2001, 98, 13895–13900. [Google Scholar] [CrossRef]
- Yammani, R.R.; Seetharam, S.; Seetharam, B. Identification and characterization of two distinct ligand binding regions of cubilin. J. Biol. Chem. 2001, 276, 44777–44784. [Google Scholar] [CrossRef]
- Fyfe, J.C.; Madsen, M.; Højrup, P.; Christensen, E.I.; Tanner, S.M.; de la Chapelle, A.; He, Q.; Moestrup, S.K. The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood 2004, 103, 1573–1579. [Google Scholar] [CrossRef]
- Coudroy, G.; Gburek, J.; Kozyraki, R.; Madsen, M.; Trugnan, G.; Moestrup, S.K.; Verroust, P.J.; Maurice, M. Contribution of cubilin and amnionless to processing and membrane targeting of cubilin-amnionless complex. J. Am. Soc. Nephrol. 2005, 16, 2330–2337. [Google Scholar] [CrossRef]
- Kalantry, S.; Manning, S.; Haub, O.; Tomihara-Newberger, C.; Lee, H.G.; Fangman, J.; Disteche, C.M.; Manova, K.; Lacy, E. The amnionless gene, essential for mouse gastrulation, encodes a visceral endoderm-specific protein with an extracellular cysteine-rich domain. Nat. Genet. 2001, 27, 412–416. [Google Scholar] [CrossRef]
- He, Q.; Madsen, M.; Kilkenney, A.; Gregory, B.; Christensen, E.I.; Vorum, H.; Højrup, P.; Schäffer, A.A.; Kirkness, E.F.; et al. Amnionless function is required for cubilin brush-border expression and intrinsic factor-cobalamin (vitamin B12) absorption in vivo. Blood 2005, 106, 1447–1453. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Chao, Y.; Muir, K.; Han, Z. Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J. Am. Soc. Nephrol. 2013, 24, 209–216. [Google Scholar] [CrossRef]
- Preisig, P.A; Rector, F.C., Jr. Role of Na+-H+ antiport in rat proximal tubule NaCl absorption. Am. J. Physiol. Renal Physiol. 1988, 255, F461–F465. [Google Scholar]
- Preisig, P.A.; Ives, H.E.; Cragoe, E.J., Jr.; Alpern, R.J.; Rector, F.C., Jr. Role of the Na+/H+ antiporter in rat proximal tubule bicarbonate absorption. J. Clin. Invest. 1987, 80, 970–978. [Google Scholar] [CrossRef]
- Nagami, G.T. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J. Clin. Invest. 1988, 81, 159–164. [Google Scholar] [CrossRef]
- Bobulescu, I.A.; Moe, O.W. Luminal Na+/H+ exchange in the proximal tubule. Pflugers. Arch. 2009, 458, 5–21. [Google Scholar] [CrossRef]
- Pan, W.; Borovac, J.; Spicer, Z.; Hoenderop, J.G.; Bindels, R.J.; Shull, G.E.; Doschak, M.R.; Cordat, E.; Alexander, R.T. The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re)absorption. Am. J. Physiol. Renal Physiol. 2012, 302, F943–F956. [Google Scholar] [CrossRef]
- LaPointe, MS.; Sodhi, C.; Sahai, A.; Batlle, D. Na+/H+ exchange activity and NHE-3 expression in renal tubules from the spontaneously hypertensive rat. Kidney Int. 2002, 62, 157–165. [Google Scholar]
- Biemesderfer, D.; Nagy, T.; DeGray, B.; Aronson, P.S. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J. Biol. Chem. 1999, 274, 17518–17524. [Google Scholar] [CrossRef]
- Biemesderfer, D.; DeGray, B.; Aronson, P.S. Active (9.6 s) and inactive (21 s) oligomers of NHE3 in microdomains of the renal brush border. J. Biol. Chem. 2001, 276, 10161–10167. [Google Scholar] [CrossRef]
- Schultheis, P.J.; Clarke, L.L.; Meneton, P.; Miller, M.L.; Soleimani, M.; Gawenis, L.R.; Riddle, T.M.; Duffy, J.J.; Doetschman, T.; Wang, T.; et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat. Genet. 1998, 19, 282–285. [Google Scholar]
- Besse-Eschmann, V.; Klisic, J.; Nief, V.; Le Hir, M.; Kaissling, B.; Ambühl, P.M. Regulation of the proximal tubular sodium/proton exchanger NHE3 in rats with Puromycin Aminonucleoside (PAN)-induced nephrotic syndrome. J. Am. Soc. Nephrol. 2002, 13, 2199–2206. [Google Scholar] [CrossRef]
- Fuster, D.G.; Bobulescu, I.A.; Zhang, J.; Wade, J.; Moe, O.W. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am. J. Physiol. Renal Physiol. 2007, 292, F577–F585. [Google Scholar]
- Wesson, D.E. Endothelin role in kidney Acidification. Semin. Nephrol. 2006, 26, 393–398. [Google Scholar] [CrossRef]
- Uchida, S. In vivo role of CLC chloride channels in the kidney. Am. J. Physiol. Renal Physiol. 2000, 279, F802–F808. [Google Scholar]
- Scheel, O.; Zdebik, A.A.; Lourdel, S.; Jentsch, T.J. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 2005, 436, 424–427. [Google Scholar] [CrossRef]
- Jentsch, T.J. Chloride transport in the kidney: Lessons from human disease and knockout mice. J. Am. Soc. Nephrol. 2005, 16, 1549–1561. [Google Scholar] [CrossRef]
- Ceol, M.; Tiralongo, E.; Baelde, H.J.; Vianello, D.; Betto, G.; Marangelli, A.; Bonfante, L.; Valente, M.; Della Barbera, M.; D’Angelo, A.; et al. Involvement of the tubular ClC-type exchanger ClC-5 in glomeruli of human proteinuric nephropathies. PLoS One 2012, 7, e45605:1–e45605:7. [Google Scholar]
- Günther, W.; Lüchow, A.; Cluzeaud, F.; Vandewalle, A.; Jentsch, T.J. ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc. Natl. Acad. Sci. USA 1998, 95, 8075–8080. [Google Scholar]
- Devuyst, O.; Christie, P.T.; Courtoy, P.J.; Beauwens, R.; Thakker, R.V. Intra-renal and subcellular distribution of the human chloride channel, CLC-5, reveals a pathophysiological basis for Dent's disease. Hum. Mol. Genet. 1999, 8, 247–257. [Google Scholar] [CrossRef]
- Piwon, N.; Günther, W.; Schwake, M.; Bösl, M.R.; Jentsch, T.J. ClC-5 Cl–channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 2000, 408, 369–373. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Wang, Y.; Guggino, S.E.; Guggino, W.B.; Verkman, A.S. Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem. Biophys. Res. Commun. 2005, 329, 941–946. [Google Scholar] [CrossRef]
- Rickheit, G.; Wartosch, L.; Schaffer, S.; Stobrawa, S.M.; Novarino, G.; Weinert, S.; Jentsch, T.J. Role of ClC-5 in renal endocytosis is unique among ClC exchangers and does not require PY-motif-dependent ubiquitylation. J. Biol. Chem. 2010, 285, 17595–17603. [Google Scholar]
- Reed, A.A.; Loh, N.Y.; Terryn, S.; Lippiat, J.D.; Partridge, C.; Galvanovskis, J.; Williams, S.E.; Jouret, F.; Wu, F.T.; Courtoy, P.J. CLC-5 and KIF3B interact to facilitate CLC-5 plasma membrane expression, endocytosis, and microtubular transport: Relevance to pathophysiology of Dent’s disease. Am. J. Physiol. Renal Physiol. 2010, 298, F365–F380. [Google Scholar] [CrossRef]
- Hryciw, D.H.; Jenkin, K.A.; Simcocks, A.C.; Grinfeld, E.; McAinch, A.J.; Poronnik, P. The interaction between megalin and ClC-5 is scaffolded by the Na+–H+ exchanger regulatory factor 2 (NHERF2) in proximal tubule cells. Int. J. Biochem. Cell Biol. 2012, 44, 815–823. [Google Scholar] [CrossRef]
- Christensen, E.I.; Devuyst, O.; Dom, G.; Nielsen, R.; Van der Smissen, P.; Verroust, P.; Leruth, M.; Guggino, W.B.; Courtoy, P.J. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc. Natl. Acad. Sci. USA 2003, 100, 8472–8477. [Google Scholar] [CrossRef]
- Maritzen, T.; Lisi, S.; Botta, R.; Pinchera, A.; Fanelli, G.; Viacava, P.; Marcocci, C.; Marinò, M. ClC-5 does not affect megalin expression and function in the thyroid. Thyroid 2006, 16, 725–730. [Google Scholar]
- Wrong, O.M.; Norden, A.G.; Feest, T.G. Dent’s disease; a familial proximal renal tubular syndrome with low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. Q.J.M. 1994, 87, 473–493. [Google Scholar]
- Lloyd, S.E.; Pearce, S.H.; Fisher, S.E.; Steinmeyer, K.; Schwappach, B.; Scheinman, S.J.; Harding, B.; Bolino, A.; Devoto, M.; Goodyer, P.; et al. A common molecular basis for three inherited kidney stone diseases. Nature 1996, 379, 445–449. [Google Scholar]
- Wang, S.S.; Devuyst, O.; Courtoy, P.J.; Wang, X.T.; Wang, H.; Wang, Y.; Thakker, R.V.; Guggino, S.; Guggino, W.B. Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum. Mol. Genet. 2000, 9, 2937–2945. [Google Scholar]
- Devuyst, O.; Jouret, F.; Auzanneau, C.; Courtoy, P.J. Chloride channels and endocytosis: New insights from Dent’s disease and ClC-5 knockout mice. Nephron Physiol. 2005, 99, 69–73. [Google Scholar]
- Tanuma, A.; Sato, H.; Takeda, T.; Hosojima, M.; Obayashi, H.; Hama, H.; Iino, N.; Hosaka, K.; Kaseda, R.; Imai, N.; et al. Functional characterization of a novel missense CLCN5 mutation causing alterations in proximal tubular endocytic machinery in Dent’s disease. Nephron Physiol. 2007, 107, 87–97. [Google Scholar] [CrossRef]
- Devuyst, O.; Thakker, R.V. Dent’s disease. Orphanet. J. Rare Dis. 2010, 5, 28:1–28:8. [Google Scholar]
- Addis, M.; Meloni, C.; Tosetto, E.; Ceol, M.; Cristofaro, R.; Melis, M.A.; Vercelloni, P.; Del Prete, D.; Marra, G.; Anglani, F. An atypical Dent’s disease phenotype caused by co-inheritance of mutations at CLCN5 and OCRL genes. Eur. J. Hum. Genet. 2013, 21, 687–690. [Google Scholar] [CrossRef]
- Miyamoto, K.; Tatsumi, S.; Segawa, H.; Ohkido, I.; Takeda, E. Identification and functional analysis of three isoforms for the Na+-dependent phosphate co-transporter (NaPi-2) in rat kidney. Nephrol. Dial. Transplant. 2000, 15, 31–33. [Google Scholar]
- Beck, L.; Karaplis, A.C.; Amizuka, N.; Hewson, A.S.; Ozawa, H.; Tenenhouse, H.S. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl. Acad. Sci. USA 1998, 95, 5372–5377. [Google Scholar]
- Marks, J.; Debnam, E.S.; Unwin, R.J. Phosphate homeostasis and the renal-gastrointestinal axis. Am. J. Physiol. Renal Physiol. 2010, 299, F285–F296. [Google Scholar] [CrossRef]
- Hernando, N.; Gisler, S.M.; Pribanic, S.; Deliot, N.; Capuano, P.; Wagner, C.A.; Moe, O.W.; Biber, J.; Murer, H. NaPi-IIa and interacting partners. J. Physiol. 2005, 567, 21–26. [Google Scholar]
- Bachmann, S.; Schlichting, U.; Geist, B.; Mutig, K.; Petsch, T.; Bacic, D.; Wagner, C.A.; Kaissling, B.; Biber, J.; Murer, H.; et al. Kidney-specific inactivation of the megalin gene impairs trafficking of renal inorganic sodium phosphate cotransporter (NaPi-IIa). J. Am. Soc. Nephrol. 2004, 15, 892–900. [Google Scholar] [CrossRef]
- Riquier, A.D.; Lee, D.H.; McDonough, A.A. Renal NHE3 and NaPi2 partition into distinct membrane domains. Am. J. Physiol. Cell Physiol. 2009, 296, C900–C910. [Google Scholar] [CrossRef]
- Tanimura, A.; Yamada, F.; Saito, A.; Ito, M.; Kimura, T.; Anzai, N.; Horie, D.; Yamamoto, H.; Miyamoto, K.; Taketani, Y.; et al. Analysis of different complexes of type IIa sodium dependent phosphate transporter in rat renal cortex using blue-native polyacrylamide gel electrophoresis. J. Med. Invest. 2011, 58, 140–147. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Caldas, Y.A.; Breusegem, S.Y.; Andrés-Hernando, A.; Cicerchi, C.; Levi, M.; Sorribas, V. Inorganic phosphate modulates the expression of the NaPi-2a transporter in the trans-golgi network and the interaction with PIST in the proximal tubule. Biomed. Res. Int. 2013, 2013, 513932:1–513932:9. [Google Scholar]
- Gattineni, J.; Bates, C.; Twombley, K.; Dwarakanath, V.; Robinson, M.L.; Goetz, R.; Mohammadi, M.; Baum, M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am. J. Physiol. Renal Physiol. 2009, 297, F282–F291. [Google Scholar]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Simister, N.E.; Mostov, K.E. An Fc receptor structurally related to MHC class I antigens. Nature 1989, 337, 184–187. [Google Scholar]
- Burmeister, W.P.; Huber, A.H.; Bjorkman, P.J. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature 1994, 372, 379–383. [Google Scholar] [CrossRef]
- Chaudhury, C.; Mehnaz, S.; Robinson, J.M.; Hayton, W.L.; Pearl, D.K.; Roopenian, D.C.; Anderson, C.L. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 2003, 197, 315–322. [Google Scholar] [CrossRef]
- Chaudhury, C.; Brooks, C.L.; Carter, D.C.; Robinson, J.M.; Anderson, C.L. Albumin binding to FcRn: Distinct from the FcRn-IgG interaction. Biochemistry 2006, 45, 4983–4990. [Google Scholar]
- Nagai, J.; Sato, K.; Yumoto, R.; Takano, M. Megalin/cubilin-mediated uptake of FITC-labeled IgG by OK kidney epithelial cells. Drug Metab. Pharmacokinet. 2011, 26, 474–485. [Google Scholar] [CrossRef]
- Haymann, J.P.; Levraud, J.P.; Bouet, S.; Kappes, V.; Hagege, J.; Nguyen, G.; Xu, Y.; Rondeau, E.; Sraer, J.D. Characterization and localization of the neonatal Fc receptor in adult human kidney. J. Am. Soc. Nephrol. 2000, 11, 632–639. [Google Scholar]
- Akilesh, S.; Huber, T.B.; Wu, H.; Wang, G.; Hartleben, B.; Kopp, J.B.; Miner, J.H.; Roopenian, D.C.; Unanue, E.R.; Shaw, A.S. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 967–972. [Google Scholar]
- Sarav, M.; Wang, Y.; Hack, B.K.; Chang, A.; Jensen, M.; Bao, L.; Quigg, R.J. Renal FcRn reclaims albumin but facilitates elimination of IgG. J. Am. Soc. Nephrol. 2009, 20, 1941–1952. [Google Scholar] [CrossRef]
- Tenten, V.; Menzel, S.; Kunter, U.; Sicking, E.M.; van Roeyen, C.R.; Sanden, S.K.; Kaldenbach, M.; Boor, P.; Fuss, A.; Uhlig, S.; et al. Albumin is recycled from the primary urine by tubular transcytosis. J. Am. Soc. Nephrol. 2013, 24, 1966–1980. [Google Scholar]
- Christensen, E.I.; Birn, H. Tubular handling of albumin—degradation or salvation? Nat. Rev. Nephrol. 2013, 9, 700–702. [Google Scholar] [CrossRef]
- Gotthardt, M.; Trommsdorff, M.; Nevitt, M.F.; Shelton, J.; Richardson, J.A.; Stockinger, W.; Nimpf, J.; Herz, J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem. 2000, 275, 25616–25624. [Google Scholar]
- Larsson, M.; Hjalm, G.; Sakwe, A.M.; Engstrom, A.; Hoglund, A.S.; Larsson, E.; Robinson, R.C.; Sundberg, C.; Rask, L. Selective interaction of megalin with postsynaptic density-95 (PSD-95)-like membrane-associated guanylate kinase (MAGUK) proteins. Biochem. J. 2003, 373, 381–391. [Google Scholar] [CrossRef]
- Lou, X.; McQuistan, T.; Orlando, R.A.; Farquhar, M.G. GAIP, GIPC and Galphai3 are concentrated in endocytic compartments of proximal tubule cells: Putative role in regulating megalin’s function. J. Am. Soc. Nephrol. 2002, 13, 918–927. [Google Scholar]
- Petersen, H.H.; Hilpert, J.; Militz, D.; Zandler, V.; Jacobsen, C.; Roebroek, A.J.; Willnow, T.E. Functional interaction of megalin with the megalin binding protein (MegBP), a novel tetratrico peptide repeat-containing adaptor molecule. J. Cell Sci. 2003, 116, 453–461. [Google Scholar]
- Rader, K.; Orlando, R.A.; Lou, X.; Farquhar, M.G. Characterization of ANKRA, a novel ankyrin repeat protein that interacts with the cytoplasmic domain of megalin. J. Am. Soc. Nephrol. 2000, 11, 2167–2178. [Google Scholar]
- Chen, W.J.; Goldstein, J.L.; Brown, M.S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem. 1990, 265, 3116–3123. [Google Scholar]
- Nagai, M.; Meerloo, T.; Takeda, T.; Farquhar, M.G. The adaptor protein ARH escorts megalin to and through endosomes. Mol. Biol. Cell. 2003, 14, 4984–4996. [Google Scholar]
- Oleinikov, A.V.; Zhao, J.; Makker, S.P. Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem. J. 2000, 347, 613–621. [Google Scholar] [CrossRef]
- Traub, L.M. Tickets to ride: Selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 2009, 10, 583–596. [Google Scholar] [CrossRef]
- Nagai, J.; Christensen, E.I.; Morris, S.M.; Willnow, T.E.; Cooper, J.A.; Nielsen, R. Mutually-dependent localization of megalin and Dab2 in the renal proximal tubule. Am. J. Physiol. Renal Physiol. 2005, 289, F569–F576. [Google Scholar]
- Maurer, M.E.; Cooper, J.A. Endocytosis of megalin by visceral endoderm cells requires the Dab2 adaptor protein. J. Cell Sci. 2005, 118, 5345–5355. [Google Scholar]
- Maurer, M.E.; Cooper, J.A. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J. Cell Sci. 2006, 119, 4235–4246. [Google Scholar]
- Hasson, T. Myosin VI: Two distinct roles in endocytosis. J. Cell Sci. 2003, 116, 3453–3461. [Google Scholar] [CrossRef]
- Avraham, K.B.; Hasson, T.; Steel, K.P.; Kingsley, D.M.; Russell, L.B.; Mooseker, M.S.; Copeland, N.G.; Jenkins, N.A. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat. Genet. 1995, 11, 369–375. [Google Scholar] [CrossRef]
- Hosaka, K.; Takeda, T.; Iino, N.; Hosojima, M.; Sato, H.; Kaseda, R.; Yamamoto, K.; Kobayashi, A.; Gejyo, F.; Saito, A. Megalin and nonmuscle myosin heavy chain IIA interact with the adaptor protein Disabled-2 in proximal tubule cells. Kidney Int. 2009, 75, 1308–1315. [Google Scholar]
- Kelley, M.J.; Jawien, W.; Ortel, T.L.; Korczak, J.F. Mutation of MYH9, encoding non-muscle myosin heavy chain A, in May-Hegglin anomaly. Nat. Genet. 2000, 26, 106–108. [Google Scholar]
- Seri, M.; Cusano, R.; Gangarossa, S.; Caridi, G.; Bordo, D.; Lo Nigro, C.; Ghiggeri, G.M.; Ravazzolo, R.; Savino, M.; Del Vecchio, M.; et al. Mutations in MYH9 result in the May-Hegglin anomaly, and Fechtner and Sebastian syndromes. The May-Heggllin/Fechtner Syndrome Consortium. Nat. Genet. 2000, 26, 103–105. [Google Scholar]
- Seri, M.; Savino, M.; Bordo, D.; Cusano, R.; Rocca, B.; Meloni, I.; Di, B.; Koivisto, P.A.; Bolognesi, M.; Ghiggeri, G.M.; et al. Epstein syndrome: Another renal disorder with mutations in the nonmuscle myosin heavy chain 9 gene. Hum. Genet. 2002, 110, 182–186. [Google Scholar] [CrossRef]
- Kao, W.H.; Klag, M.J.; Meoni, L.A.; Reich, D.; Berthier-Schaad, Y.; Li, M.; Coresh, J.; Patterson, N.; Tandon, A.; Powe, N.R.; et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat. Genet. 2008, 40, 1185–1192. [Google Scholar] [CrossRef]
- Kopp, J.B.; Smith, M.W.; Nelson, G.W.; Johnson, R.C.; Freedman, B.I.; Bowden, D.W.; Oleksyk, T.; McKenzie, L.M.; Kajiyama, H.; Ahuja, T.S. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat. Genet. 2008, 40, 1175–1184. [Google Scholar]
- Koral, K.; Erkan, E. PKB/Akt partners with Dab2 in albumin Endocytosis. Am. J. Physiol. Renal Physiol. 2012, 302, F1013–F1024. [Google Scholar] [CrossRef]
- Garcia, C.K.; Wilund, K.; Arca, M.; Zuliani, G.; Fellin, R.; Maioli, M.; Calandra, S.; Bertolini, S.; Cossu, F.; Grishin, N.; et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 2001, 292, 1394–1398. [Google Scholar] [CrossRef]
- Mishra, S.K.; Watkins, S.C.; Traub, L.M. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc. Natl. Acad. Sci. USA 2002, 99, 16099–16104. [Google Scholar]
- He, G.; Gupta, S.; Michaely, P.; Hobbs, H.H.; Cohen, J.C. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin and AP-2. J. Biol. Chem. 2002, 277, 44044–44049. [Google Scholar]
- Lehtonen, S.; Shah, M.; Nielsen, R.; Iino, N.; Ryan, J.J.; Zhou, H.; Farquhar, M.G. The endocytic adaptor protein ARH associates with motor and centrosomal proteins and is involved in centrosome assembly and cytokinesis. Mol. Biol. Cell. 2008, 19, 2949–2961. [Google Scholar] [CrossRef]
- Shah, M.; Baterina, O.Y., Jr.; Taupin, V.; Farquhar, M.G. ARH directs megalin to the endocytic recycling compartment to regulate its proteolysis and gene expression. J. Cell. Biol. 2013, 202, 113–127. [Google Scholar] [CrossRef]
- Kang, R.S.; Fölsch, H. ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J. Cell Biol. 2011, 193, 51–60. [Google Scholar] [CrossRef]
- Pedersen, G.A.; Chakraborty, S.; Steinhauser, A.L.; Traub, L.M.; Madsen, M. AMN directs endocytosis of the intrinsic factor-vitamin B12 receptor cubam by engaging ARH or Dab2. Traffic 2010, 11, 706–720. [Google Scholar]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artifacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar]
- van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A common pathway for a specialized function. J. Biochem. 2006, 140, 13–21. [Google Scholar]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Lv, L.L.; Cao, Y.; Liu, D.; Xu, M.; Liu, H.; Tang, R.N.; Ma, K.L.; Liu, B.C. Isolation and quantification of microRNAs from urinary exosomes/microvesicles for biomarker discovery. Int. J. Biol. Sci. 2013, 9, 1021–1031. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert. Rev. Proteomics 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef]
- Raimondo, F.; Corbetta, S.; Morosi, L.; Chinello, C.; Gianazza, E.; Castoldi, G.; Di Gioia, C.; Bombardi, C.; Stella, A.; Battaglia, C. Urinary exosomes and diabetic nephropathy: A proteomic approach. Mol. Biosyst. 2013, 9, 1139–1146. [Google Scholar] [CrossRef]
- Russo, L.M.; del Re, E.; Brown, D.; Lin, H.Y. Evidence for a role of transforming growth factor (TGF)-beta1 in the induction of postglomerular albuminuria in diabetic nephropathy: Amelioration by soluble TGF-beta type II receptor. Diabetes 2007, 56, 380–388. [Google Scholar]
- Hosojima, M.; Sato, H.; Yamamoto, K.; Kaseda, R.; Soma, T.; Kobayashi, A.; Kabasawa, H.; Takeyama, A.; Ikuyama, K.; et al. Regulation of megalin expression in cultured proximal tubule cells by angiotensin II type 1A receptor- and insulin-mediated signaling cross talk. Endocrinology 2009, 150, 871–878. [Google Scholar]
- Takeyama, A.; Sato, H.; Soma-Nagae, T.; Kabasawa, H.; Suzuki, A.; Yamamoto-Kabasawa, K.; Hosojima, M.; Kaneko, R.; Higuchi, F.; Kaseda, R.; et al. Megalin is downregulated via LPS-TNF-α-ERK1/2 signaling pathway in proximal tubule cells. Biochem. Biophys. Res. Commun. 2011, 407, 108–112. [Google Scholar] [CrossRef]
- Cabezas, F.; Lagos, J.; Céspedes, C.; Vio, C.P.; Bronfman, M.; Marzolo, M.P. Megalin/LRP2 expression is induced by peroxisome proliferator-activated receptor -alpha and -gamma: Implications for PPARs’ roles in renal function. PLoS One 2011, 6, e16794:1–e16794:17. [Google Scholar]
- Reisman, S.A.; Chertow, G.M.; Hebbar, S.; Vaziri, N.D.; Ward, K.W.; Meyer, C.J. Bardoxolone methyl decreases megalin and activates nrf2 in the kidney. J. Am. Soc. Nephrol. 2012, 23, 1663–1673. [Google Scholar] [CrossRef]
- Zou, Z.; Chung, B.; Nguyen, T.; Mentone, S.; Thomson, B.; Biemesderfer, D. Linking receptor-mediated endocytosis and cell signaling: Evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J. Biol. Chem. 2004, 279, 34302–34310. [Google Scholar]
- Li, Y.; Cong, R.; Biemesderfer, D. The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. Am. J. Physiol. Cell Physiol. 2008, 295, C529–C537. [Google Scholar] [CrossRef]
- Christ, A.; Terryn, S.; Schmidt, V.; Christensen, E.I.; Huska, M.R.; Andrade-Navarro, M.A.; Hubner, N.; Devuyst, O.; Hammes, A.; Willnow, T.E. The soluble intracellular domain of megalin does not affect renal proximal tubular function in vivo. Kidney Int. 2010, 78, 473–477. [Google Scholar]
- Motoyoshi, Y.; Matsusaka, T.; Saito, A.; Pastan, I.; Willnow, T.E.; Mizutani, S.; Ichikawa, I. Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney Int. 2008, 74, 1262–1269. [Google Scholar] [CrossRef]
- Shalamanova, L.; McArdle, F.; Amara, A.B.; Jackson, M.J.; Rustom, R. Albumin overload induces adaptive responses in human proximal tubular cells through oxidative stress but not via angiotensin II type 1 receptor. Am. J. Physiol. Renal Physiol. 2007, 292, F1846–1857. [Google Scholar] [CrossRef]
- Morigi, M.; Macconi, D.; Zoja, C.; Donadelli, R.; Buelli, S.; Zanchi, C.; Ghilardi, M.; Remuzzi, G. Protein overload-induced NF-kappaB activation in proximal tubular cells requires H2O2 through a PKC-dependent pathway. J. Am. Soc. Nephrol. 2002, 13, 1179–1189. [Google Scholar]
- Burns, W.C.; Kantharidis, P.; Thomas, M.C. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs 2007, 185, 222–231. [Google Scholar] [CrossRef]
- Strutz, F.M. EMT and proteinuria as progression factors. Kidney Int. 2009, 75, 475–481. [Google Scholar]
- Saito, A.; Takeda, T.; Sato, K.; Hama, H.; Tanuma, A.; Kaseda, R.; Suzuki, Y.; Gejyo, F. Significance of proximal tubular metabolism of advanced glycation end products in kidney diseases. Ann. N. Y. Acad. Sci. 2005, 1043, 637–643. [Google Scholar] [CrossRef]
- Verbeke, P.; Perichon, M.; Friguet, B.; Bakala, H. Inhibition of nitric oxide synthase activity by early and advanced glycation end products in cultured rabbit proximal tubular epithelial cells. Biochim. Biophys. Acta 2000, 1502, 481–494. [Google Scholar]
- Obeid, R.; Shannan, B.; Herrmann, W. Advanced glycation end products overload might explain intracellular cobalamin deficiency in renal dysfunction, diabetes and aging. Med. Hypotheses 2011, 77, 884–848. [Google Scholar]
- Sengul, S.; Zwizinski, C.; Batuman, V. Role of MAPK pathways in light chain-induced cytokine production in human proximal tubule cells. Am. J. Physiol. Renal Physiol. 2003, 284, F1245–F1254. [Google Scholar]
- Li, M.; Hering-Smith, K.S.; Simon, E.E.; Batuman, V. Myeloma light chains induce epithelial-mesenchymal transition in human renal proximal tubule epithelial cells. Nephrol. Dial. Transplant. 2008, 23, 860–870. [Google Scholar]
- Hutchison, C.A.; Batuman, V.; Behrens, J.; Bridoux, F.; Sirac, C.; Dispenzieri, A.; Herrera, G.A.; Lachmann, H.; Sanders, P.W. International Kidney and Monoclonal Gammopathy Research Group. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat. Rev. Nephrol. 2011, 8, 43–51. [Google Scholar] [CrossRef]
- Ogasawara, S.; Hosojima, M.; Kaseda, R.; Kabasawa, H.; Yamamoto-Kabasawa, K.; Kurosawa, H.; Sato, H.; Iino, N.; Takeda, T.; Suzuki, Y.; et al. Significance of urinary full-length and ectodomain forms of megalin in patients with type 2 diabetes. Diabetes Care 2012, 35, 1112–1118. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De, S.; Kuwahara, S.; Saito, A. The Endocytic Receptor Megalin and its Associated Proteins in Proximal Tubule Epithelial Cells. Membranes 2014, 4, 333-355. https://doi.org/10.3390/membranes4030333
De S, Kuwahara S, Saito A. The Endocytic Receptor Megalin and its Associated Proteins in Proximal Tubule Epithelial Cells. Membranes. 2014; 4(3):333-355. https://doi.org/10.3390/membranes4030333
Chicago/Turabian StyleDe, Shankhajit, Shoji Kuwahara, and Akihiko Saito. 2014. "The Endocytic Receptor Megalin and its Associated Proteins in Proximal Tubule Epithelial Cells" Membranes 4, no. 3: 333-355. https://doi.org/10.3390/membranes4030333
APA StyleDe, S., Kuwahara, S., & Saito, A. (2014). The Endocytic Receptor Megalin and its Associated Proteins in Proximal Tubule Epithelial Cells. Membranes, 4(3), 333-355. https://doi.org/10.3390/membranes4030333



