On the Use of Permselectivity to Describe the Selective Transfer of Organic Acids in Electrodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Analysis
2.3. Experimental Procedure
2.4. Chemicals
3. Results and Discussion
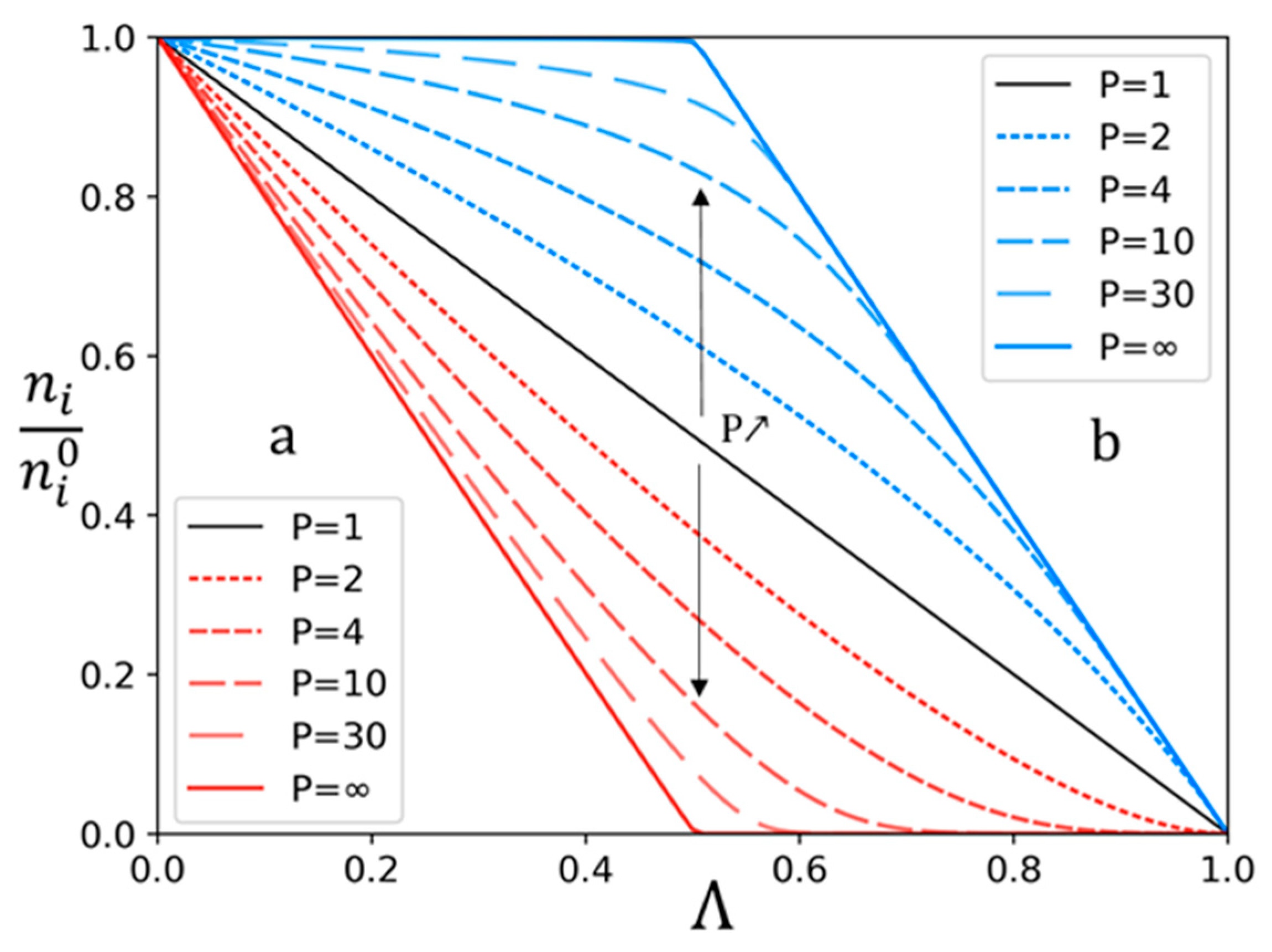
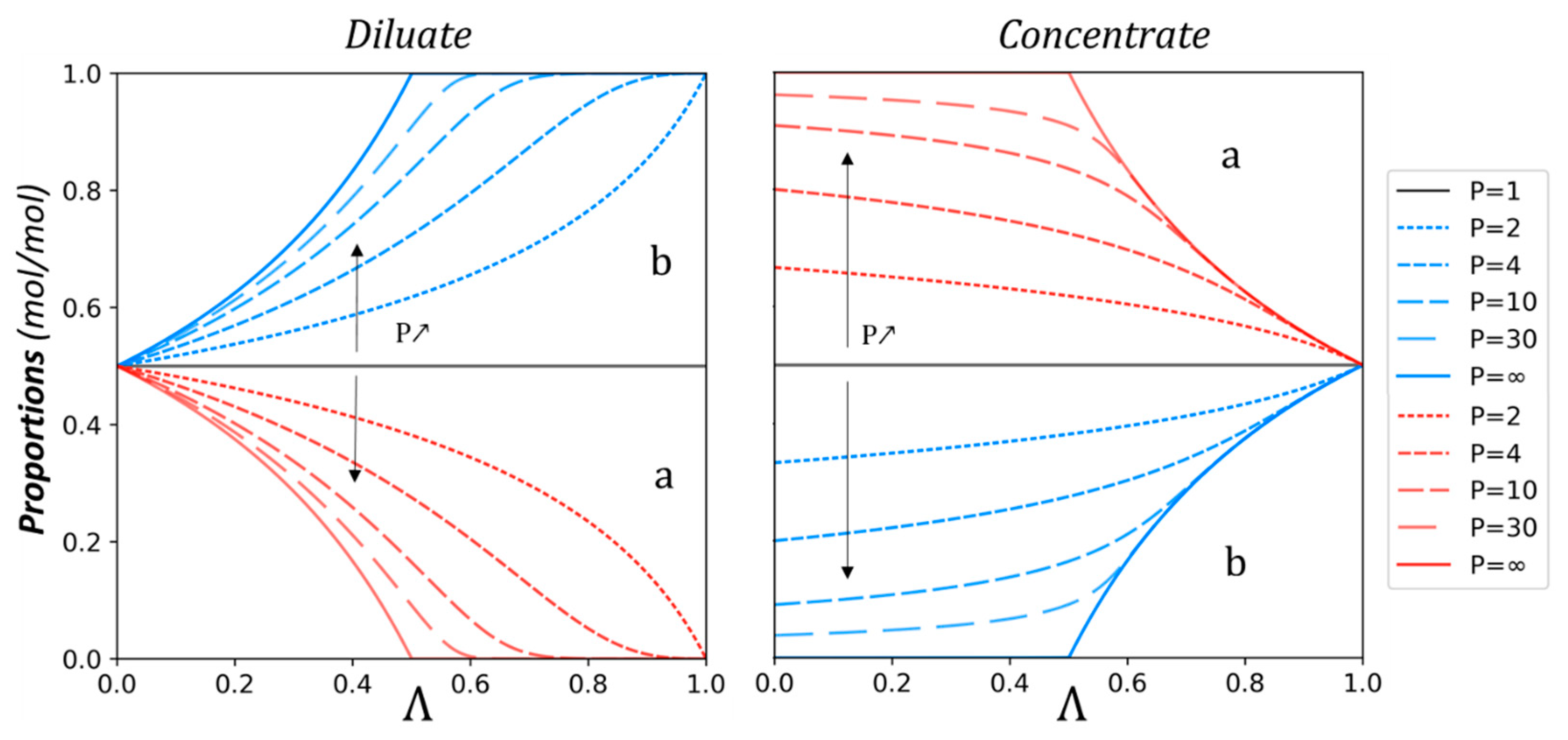
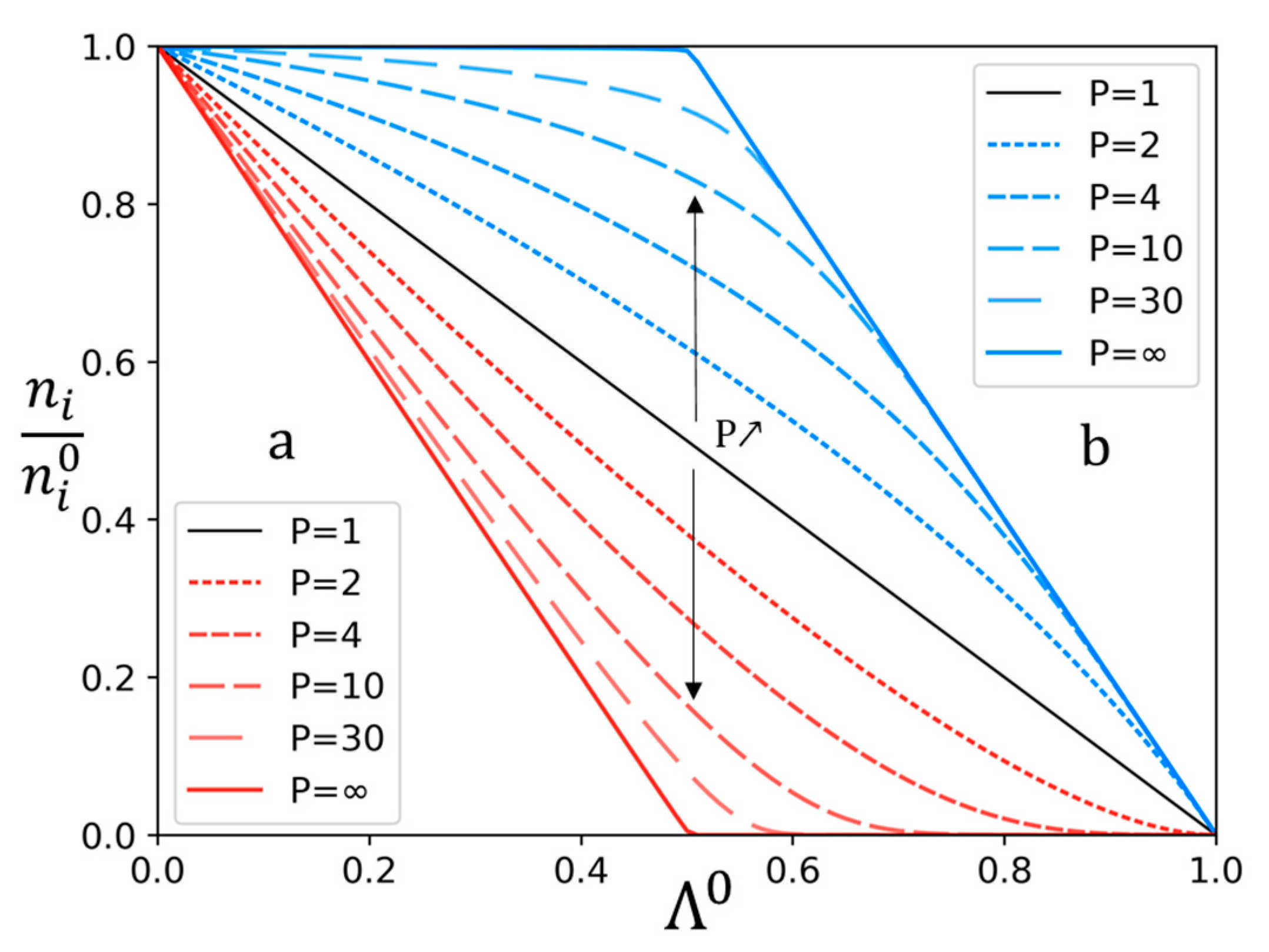
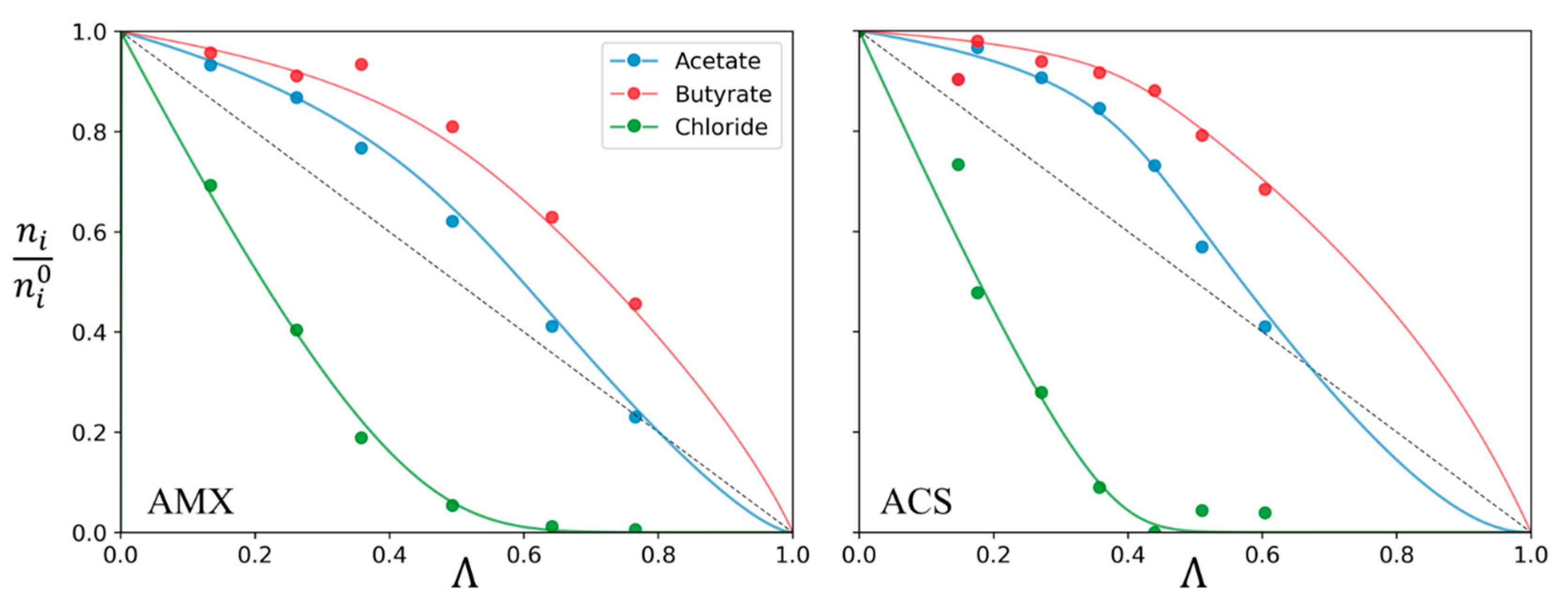
3.1. Theoretical Approach
3.2. Experimental Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| List of symbols | |
| C | Concentration (mol/m3) |
| F | Faraday constant (C/eq) |
| I | Current intensity (A) |
| J | Solute flux (mol.s−1) |
| M | Number of considered counter-ions |
| n | Quantity (mol) |
| N | Number of cell-pairs |
| P | Permselectivity |
| t | Time (s) |
| tm | Transport number in the membrane |
| V | Volume (m3) |
| z | Valence |
| Greek symbols | |
| η | Faradic yield |
| Λ | Demineralization rate |
| Λ0 | individual demineralization rates mean |
| χ | Conductivity (mS/cm) |
| Subscripts and superscript | |
| Ac | Acetate |
| Bu | Butyrate |
| Cl | Chloride |
| i | Ion |
References
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.-P.; Lovitt, R. Complex Effluent Streams as a Potential Source of Volatile Fatty Acids. Waste Biomass-Valorization 2013, 4, 557–581. [Google Scholar] [CrossRef]
- Zacharof, M.-P.; Mandale, S.J.; Williams, P.M.; Lovitt, R.W. Nanofiltration of treated digested agricultural wastewater for recovery of carboxylic acids. J. Clean. Prod. 2016, 112, 4749–4761. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Guwy, A.; Premier, G.C.; Dinsdale, R.M.; Reilly, M. Removal and recovery of inhibitory volatile fatty acids from mixed acid fermentations by conventional electrodialysis. Bioresour. Technol. 2015, 189, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Massanet-Nicolau, J.; Fernandez-Feito, R.; Dinsdale, R.; Guwy, A. Recovery and enhanced yields of volatile fatty acids from a grass fermentation via in-situ solids separation and electrodialysis. J. Clean. Prod. 2021, 296, 126430. [Google Scholar] [CrossRef]
- Pan, X.-R.; Li, W.-W.; Huang, L.; Liu, H.-Q.; Wang, Y.-K.; Geng, Y.-K.; Lam, P.K.-S.; Yu, H.-Q. Recovery of high-concentration volatile fatty acids from wastewater using an acidogenesis-electrodialysis integrated system. Bioresour. Technol. 2018, 260, 61–67. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Bailly, M. Production processes of fermented organic acids targeted around membrane operations: Design of the concentration step by conventional electrodialysis. J. Membr. Sci. 2001, 191, 129–142. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T.; Zhang, Y.; Xue, Y.; Chen, G. Application of electrodialysis to the production of organic acids: State-of-the-art and recent developments. J. Membr. Sci. 2007, 288, 1–12. [Google Scholar] [CrossRef]
- Dai, K.; Wen, J.-L.; Wang, Y.-L.; Wu, Z.-G.; Zhao, P.-J.; Zhang, H.-H.; Wang, J.-J.; Zeng, R.J.; Zhang, F. Impacts of medium composition and applied current on recovery of volatile fatty acids during coupling of electrodialysis with an anaerobic digester. J. Clean. Prod. 2019, 207, 483–489. [Google Scholar] [CrossRef]
- Wei, P.; Xia, A.; Liao, Q.; Sun, C.; Huang, Y.; Fu, Q.; Zhu, X.; Lin, R. Enhancing fermentative hydrogen production with the removal of volatile fatty acids by electrodialysis. Bioresour. Technol. 2018, 263, 437–443. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Zhou, M.; Vassilev, I.; Freguia, S.; Zhang, Y.; Keller, J.; Ledezma, P.; Virdis, B. Selective Extraction of Medium-Chain Carboxylic Acids by Electrodialysis and Phase Separation. ACS Omega 2021, 6, 7841–7850. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, M.; Jeong, H.; Ko, S.; Moon, S.-H.; Chang, I.S. Recycling of minerals with acetate separation in biological syngas fermentation with an electrodialysis system. Chem. Eng. J. 2023, 459, 141555. [Google Scholar] [CrossRef]
- Luiz, A.; McClure, D.D.; Lim, K.; Leslie, G.; Coster, H.G.; Barton, G.W.; Kavanagh, J.M. Potential upgrading of bio-refinery streams by electrodialysis. Desalination 2017, 415, 20–28. [Google Scholar] [CrossRef]
- Luiz, A.; Spencer, E.; McClure, D.D.; Coster, H.G.; Barton, G.W.; Kavanagh, J.M. Membrane selection for the desalination of bio-refinery effluents using electrodialysis. Desalination 2018, 428, 1–11. [Google Scholar] [CrossRef]
- Kelman, S.; Grieves, R.B. Electrodialytic transport of inorganic and of organic anions. J. Appl. Chem. 1968, 18, 20–24. [Google Scholar] [CrossRef]
- Schrodt, J.T.; Yonikas, P.J.; Grieves, R.B. Continuous-flow electrodialysis of acetate, butyrate, and chloride: A correlating equation. Can. J. Chem. Eng. 1969, 47, 398–402. [Google Scholar] [CrossRef]
- Moon, P.J.; Parulekar, S.J.; Tsai, S.-P. Competitive anion transport in desalting of mixtures of organic acids by batch electrodialysis. J. Membr. Sci. 1998, 141, 75–89. [Google Scholar] [CrossRef]
- Shaposhnik, V.; Kesore, K. An early history of electrodialysis with permselective membranes. J. Membr. Sci. 1997, 136, 35–39. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis—Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Membr. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Xu, X.; He, Q.; Ma, G.; Wang, H.; Nirmalakhandan, N.; Xu, P. Selective separation of mono- and di-valent cations in electrodialysis during brackish water desalination: Bench and pilot-scale studies. Desalination 2018, 428, 146–160. [Google Scholar] [CrossRef]
- Matos, C.T.; Fortunato, R.; Velizarov, S.; Reis, M.A.; Crespo, J. Removal of mono-valent oxyanions from water in an ion exchange membrane bioreactor: Influence of membrane permselectivity. Water Res. 2008, 42, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Sata, T.; Yamane, Y.; Matsusaki, K. Relationship of permselectivity between two anions to water content of anion exchange membranes with pyridinium groups. Electrochim. Acta 1997, 42, 2427–2431. [Google Scholar] [CrossRef]
- Luo, T.; Roghmans, F.; Wessling, M. Ion mobility and partition determine the counter-ion selectivity of ion exchange membranes. J. Membr. Sci. 2020, 597, 117645. [Google Scholar] [CrossRef]
- Reig, M.; Farrokhzad, H.; Van der Bruggen, B.; Gibert, O.; Cortina, J.L. Synthesis of a monovalent selective cation exchange membrane to concentrate reverse osmosis brines by electrodialysis. Desalination 2015, 375, 1–9. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Takagi, R.; Matsuyama, H. Surface modification of an anion exchange membrane to improve the selectivity for monovalent anions in electrodialysis—Experimental verification of theoretical predictions. J. Membr. Sci. 2015, 490, 301–310. [Google Scholar] [CrossRef]
- Takagi, R.; Vaselbehagh, M.; Matsuyama, H. Theoretical study of the permselectivity of an anion exchange membrane in electrodialysis. J. Membr. Sci. 2014, 470, 486–493. [Google Scholar] [CrossRef]
- Güler, E.; van Baak, W.; Saakes, M.; Nijmeijer, K. Monovalent-ion-selective membranes for reverse electrodialysis. J. Membr. Sci. 2014, 455, 254–270. [Google Scholar] [CrossRef]
- Indusekhar, V.; Trivedi, G.; Shah, B. Removal of nitrate by electrodialysis. Desalination 1991, 84, 213–221. [Google Scholar] [CrossRef]
- Hell, F.; Lahnsteiner, J.; Frischherz, H.; Baumgartner, G. Experience with full-scale electrodialysis for nitrate and hardness removal. Desalination 1998, 117, 173–180. [Google Scholar] [CrossRef]
- Kesore, K.; Janowski, F.; Shaposhnik, V. Highly effective electrodialysis for selective elimination of nitrates from drinking water. J. Membr. Sci. 1997, 127, 17–24. [Google Scholar] [CrossRef]
- Kikhavani, T.; Ashrafizadeh, S.; Van der Bruggen, B. Nitrate Selectivity and Transport Properties of a Novel Anion Exchange Membrane in Electrodialysis. Electrochim. Acta 2014, 144, 341–351. [Google Scholar] [CrossRef]
- Han, L.; Galier, S.; Balmann, H.R.-D. Ion hydration number and electro-osmosis during electrodialysis of mixed salt solution. Desalination 2015, 373, 38–46. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Fuoco, A.; Galier, S.; Balmann, H.R.-D.; De Luca, G. Correlation between macroscopic sugar transfer and nanoscale interactions in cation exchange membranes. J. Membr. Sci. 2015, 493, 311–320. [Google Scholar] [CrossRef]
- Galier, S.; Savignac, J.; Balmann, H.R.-D. Influence of the ionic composition on the diffusion mass transfer of saccharides through a cation-exchange membrane. Sep. Purif. Technol. 2013, 109, 1–8. [Google Scholar] [CrossRef]
- Han, L.; Galier, S.; Balmann, H.R.-D. A phenomenological model to evaluate the performances of electrodialysis for the desalination of saline water containing organic solutes. Desalination 2017, 422, 17–24. [Google Scholar] [CrossRef]
- Borges, F.; Balmann, H.R.-D.; Guardani, R. Investigation of the mass transfer processes during the desalination of water containing phenol and sodium chloride by electrodialysis. J. Membr. Sci. 2008, 325, 130–138. [Google Scholar] [CrossRef]
- Scoma, A.; Varela-Corredor, F.; Bertin, L.; Gostoli, C.; Bandini, S. Recovery of VFAs from anaerobic digestion of dephenolized Olive Mill Wastewaters by Electrodialysis. Sep. Purif. Technol. 2016, 159, 81–91. [Google Scholar] [CrossRef]
- Dong, T.; Yao, J.; Wang, Y.; Luo, T.; Han, L. On the permselectivity of di- and mono-valent cations: Influence of applied current density and ionic species concentration. Desalination 2020, 488, 114521. [Google Scholar] [CrossRef]
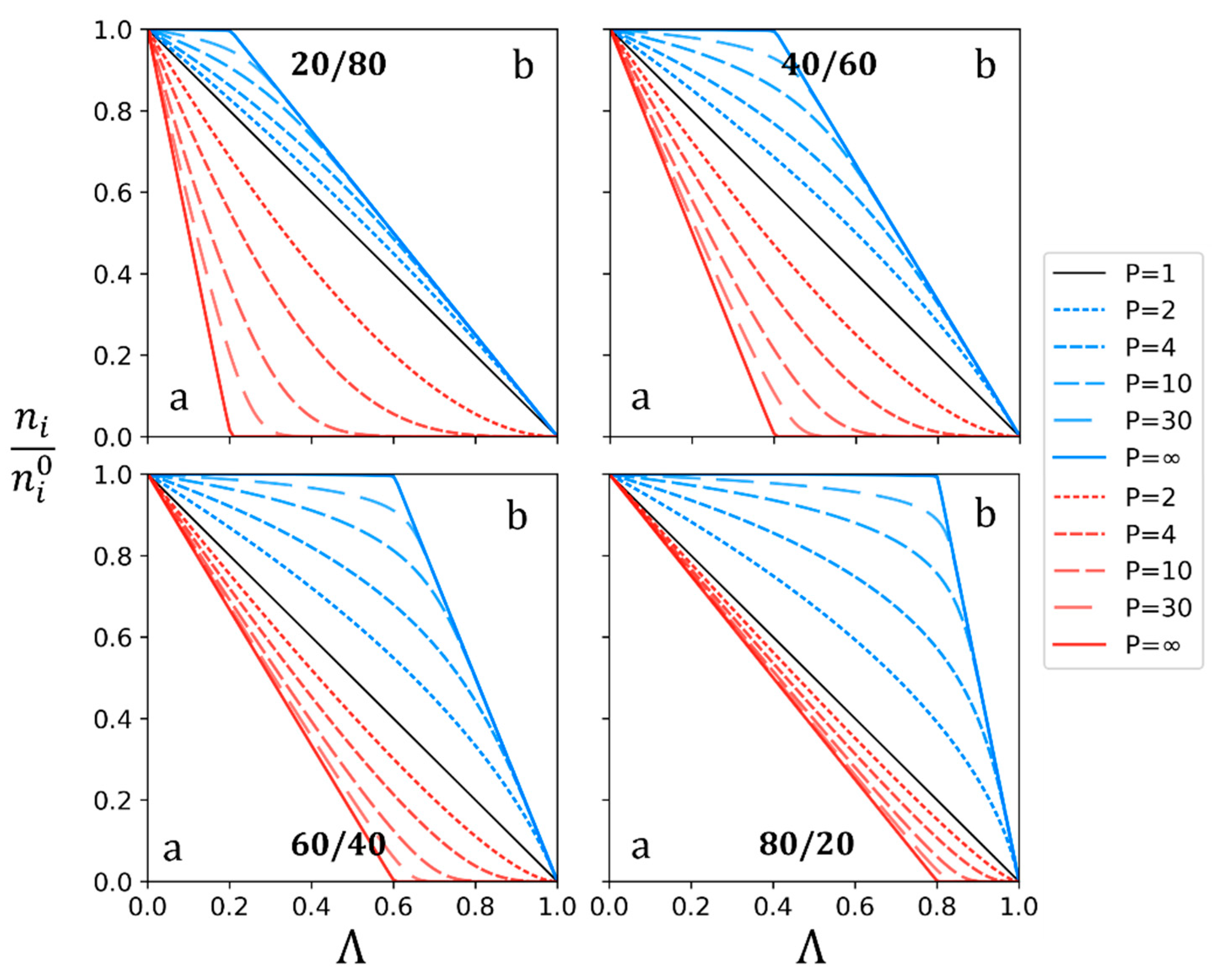
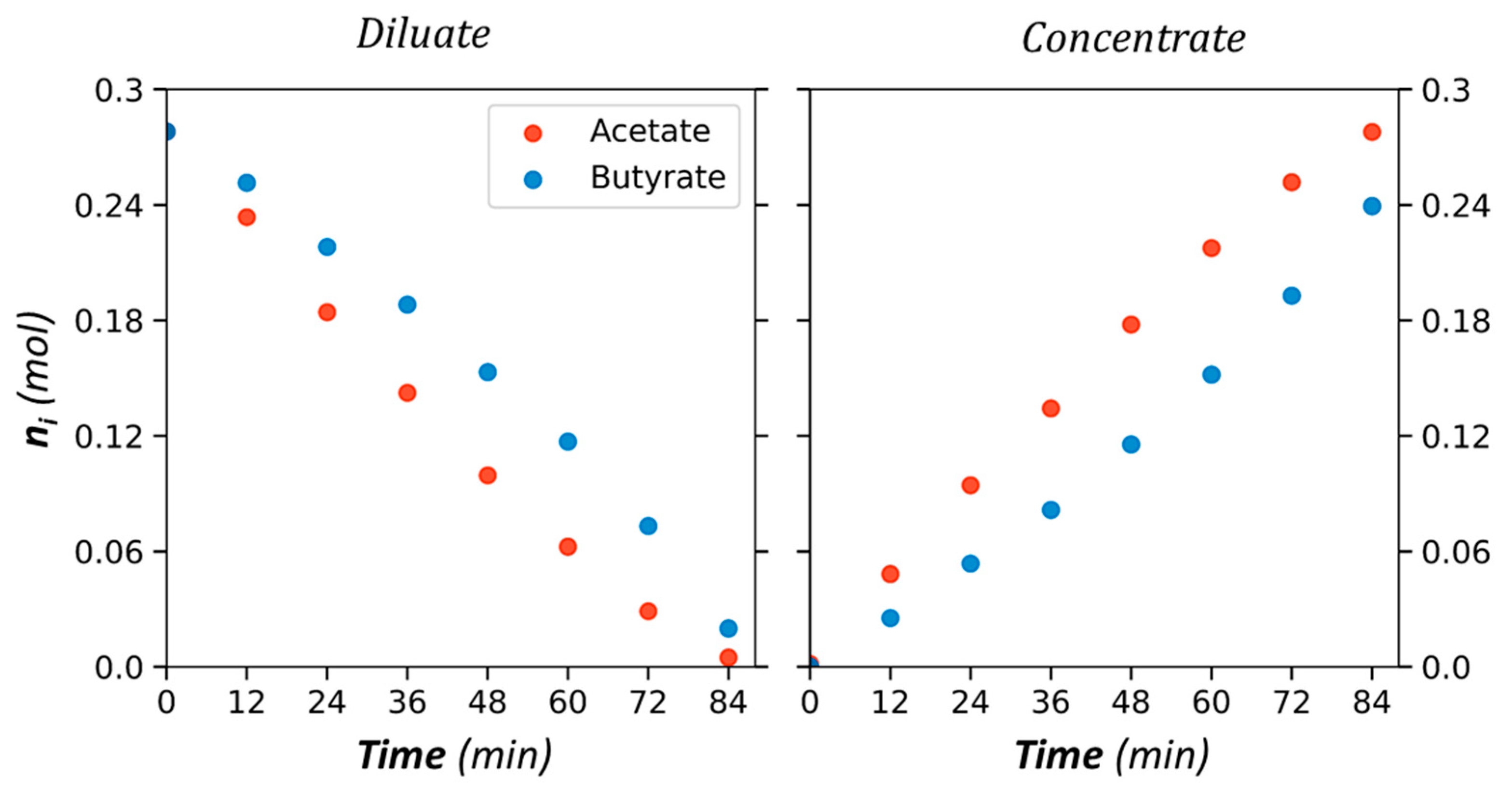


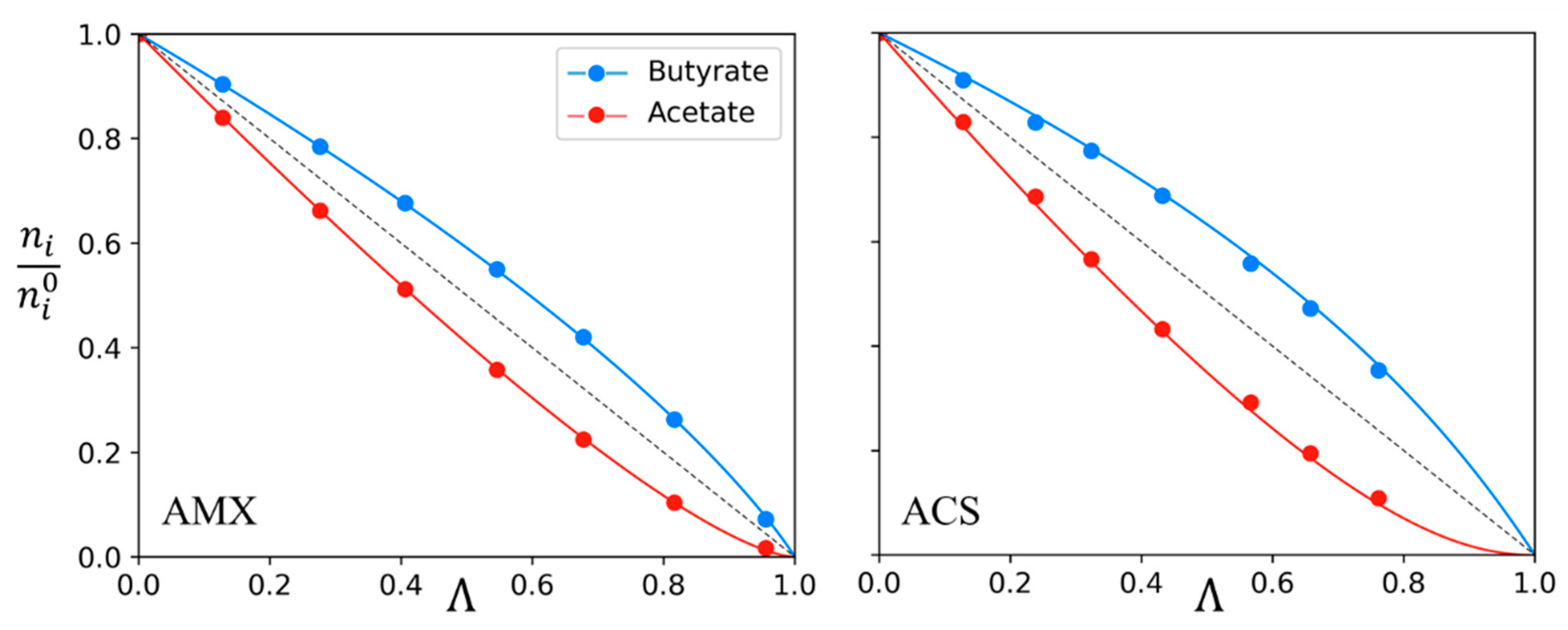
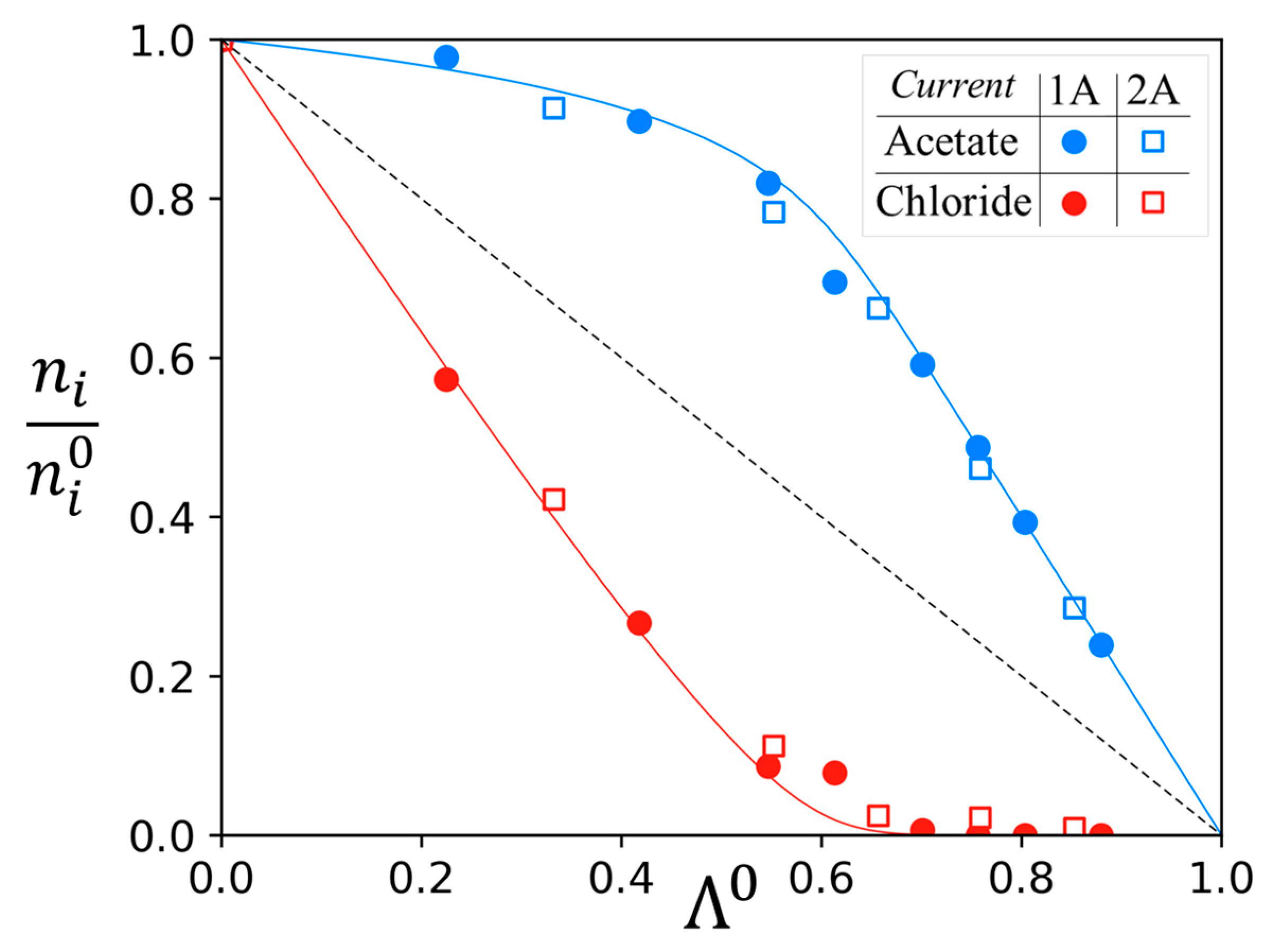
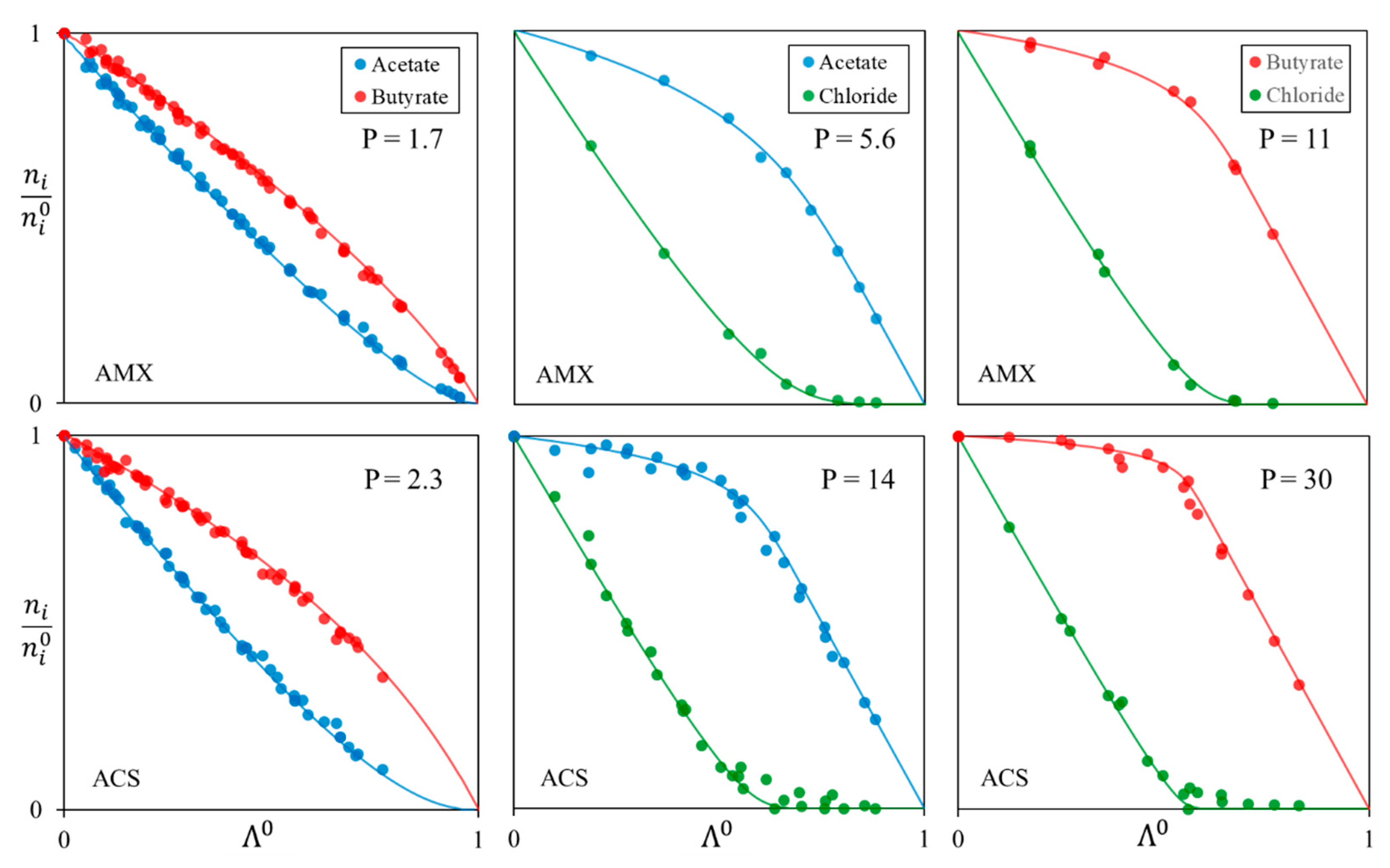
| Anion-Exchange Membranes | Initial Anion–Molar Proportion (%) | Current Intensity (A) | Diluate Initial Total Anion Concentration (g/L) | ||
|---|---|---|---|---|---|
| Acetate | Butyrate | Chloride | |||
| AMX | 93 | 7 | 1; 2; 3 | 20 | |
| 93 | 7 | 1 | 5 | ||
| 93 | 7 | 40 | |||
| 85 | 15 | 20 | |||
| 50 | 50 | ||||
| 33 | 33 | 33 | |||
| 50 | 50 | 2 | |||
| 50 | 50 | 1 | |||
| ACS | 50 | 50 | 1 | 20 | |
| 50 | 50 | ||||
| 50 | 50 | ||||
| 85 | 15 | 1; 2 | 25 | ||
| 85 | 15 | 1.5 | |||
| 33 | 33 | 33 | 1 | 20 | |
| 80 | 20 | ||||
| 90 | 10 | ||||
| Equations | Data | Variables |
|---|---|---|
| na0 Initial quantity of a | na Quantity of solute a | |
| nb0 Initial quantity of b | nb Quantity of solute b | |
| Pa/b Permselectivity | Λ Demineralization rate |
| Permselectivity | ||
|---|---|---|
| Membrane | AMX | ACS |
| Ac/Bu | 1.7 ± 0.1 | 2.3 ± 0.1 |
| Cl/Ac | 5.6 ± 0.6 | 14 ± 3 |
| Cl/Bu | 11 ± 2 | 30 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caveriviere, R.; Galier, S.; Roux-de Balmann, H. On the Use of Permselectivity to Describe the Selective Transfer of Organic Acids in Electrodialysis. Membranes 2023, 13, 545. https://doi.org/10.3390/membranes13060545
Caveriviere R, Galier S, Roux-de Balmann H. On the Use of Permselectivity to Describe the Selective Transfer of Organic Acids in Electrodialysis. Membranes. 2023; 13(6):545. https://doi.org/10.3390/membranes13060545
Chicago/Turabian StyleCaveriviere, Robin, Sylvain Galier, and Hélène Roux-de Balmann. 2023. "On the Use of Permselectivity to Describe the Selective Transfer of Organic Acids in Electrodialysis" Membranes 13, no. 6: 545. https://doi.org/10.3390/membranes13060545







