Lipoprotein Particles as Shuttles for Hydrophilic Cargo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Cells
2.2. Lipoprotein Particle Isolation and Labeling
2.3. Insulin Loading of HDL Particles
2.4. Immobilization of HDL Particles for Imaging via Confocal Microscopy and Force Spectroscopy Imaging
2.5. Atomic Force Microscopy (AFM) Imaging and Particle Analysis
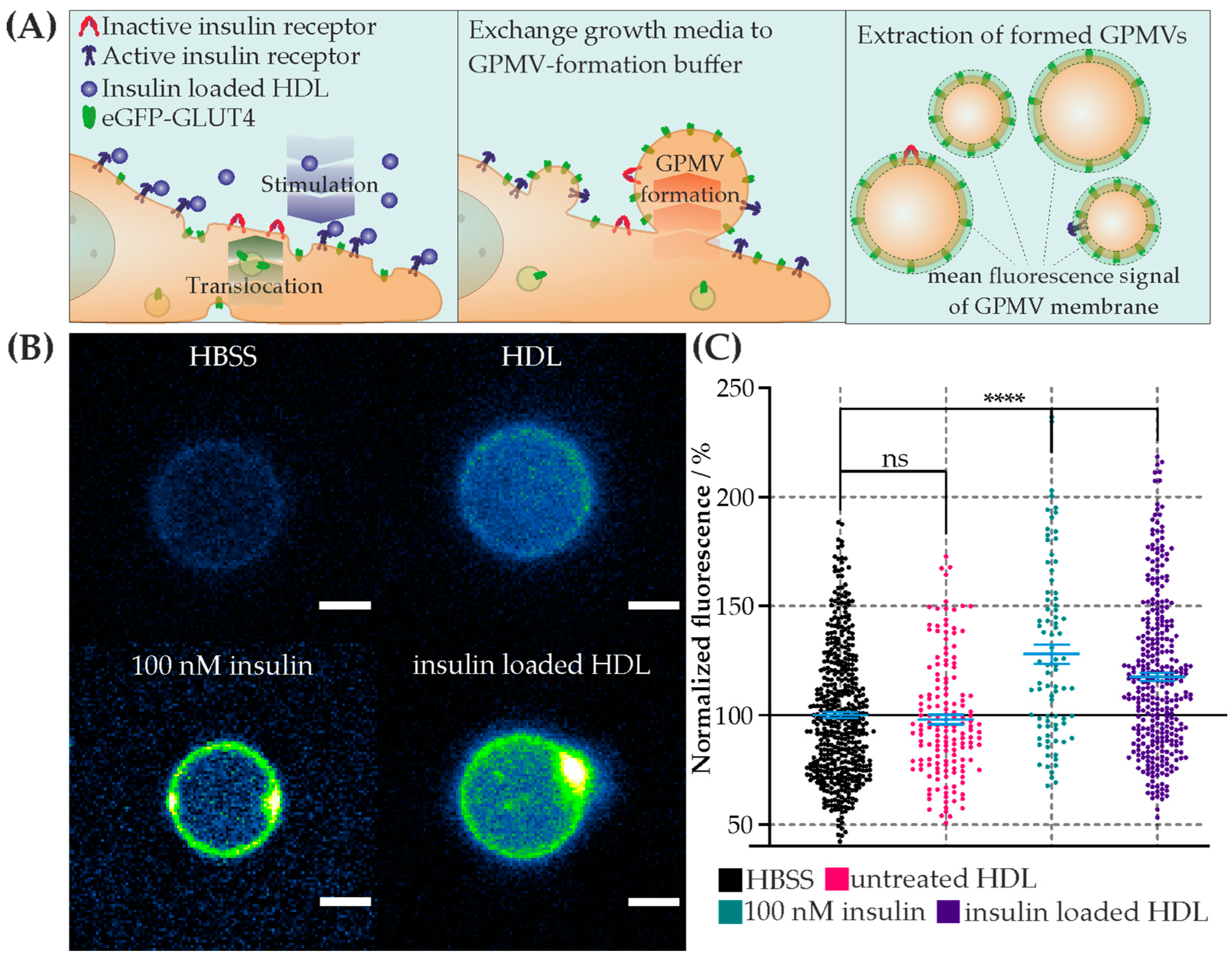
2.6. Cell Stimulation and Giant Plasma Membrane Vesicle (GPMV) Formation
2.7. Confocal Microscopy of GPMVs and Giant Unilamellar Vesicle (GUV)
3. Results
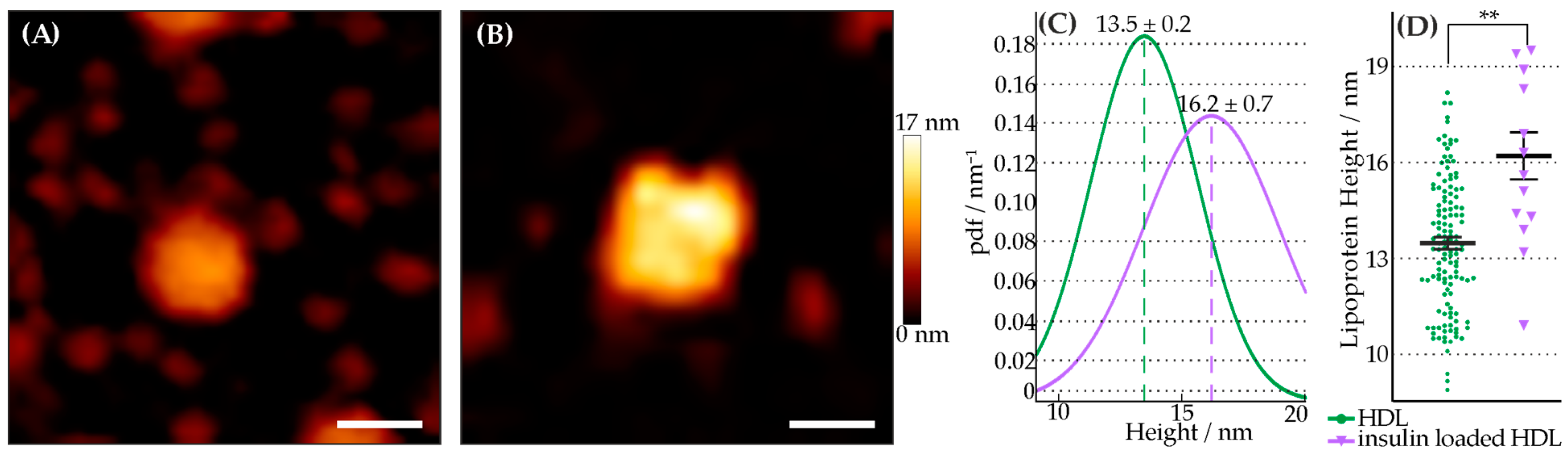
3.1. Characterization of Insulin-Loaded HDL Particles
3.2. Transfer of Lipid-Anchored Insulin from HDL Particles to Artificial Membranes
3.3. Cell Stimulation via Insulin-Loaded HDL Particles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lund-Katz, S.; Phillips, M.C. High Density Lipoprotein Structure-Function and Role in Reverse Cholesterol Transport. In Cholesterol Binding and Cholesterol Transport Proteins: Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2010; pp. 183–227. [Google Scholar] [CrossRef]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.J.; Davidson, W.S. Structure of HDL: Particle Subclasses and Molecular Components. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–51. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement fromthe European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. The LDL Receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Receptor-mediated endocytosis: Insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA 1979, 76, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R. An overview of reverse cholesterol transport. Eur. Heart J. 1998, 19 (Suppl. A), A31–A35. [Google Scholar] [CrossRef]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef]
- Axmann, M.; Strobl, W.M.; Plochberger, B.; Stangl, H. Cholesterol transfer at the plasma membrane. Atherosclerosis 2019, 290, 111–117. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Webb, D. The LDL to HDL Cholesterol Ratio as a Valuable Tool to Evaluate Coronary Heart Disease Risk. J. Am. Coll. Nutr. 2008, 27, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.P.; Van Der Steeg, W.A.; Holme, I.; Gaffney, M.; Cater, N.B.; Barter, P.; Deedwania, P.; Olsson, A.G.; Boekholdt, S.M.; Demicco, D.A.; et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008, 117, 3002–3009. [Google Scholar] [CrossRef]
- Ali, K.M.; Wonnerth, A.; Huber, K.; Wojta, J. Cardiovascular disease risk reduction by raising HDL cholesterol—Current therapies and future opportunities. Br. J. Pharmacol. 2012, 167, 1177–1194. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, M.; Singh, J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: Molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 2018, 17, 121. [Google Scholar] [CrossRef]
- Plochberger, B.; Axmann, M.; Röhrl, C.; Weghuber, J.; Brameshuber, M.; Rossboth, B.K.; Mayr, S.; Ros, R.; Bittman, R.; Stangl, H.; et al. Direct observation of cargo transfer from HDL particles to the plasma membrane. Atherosclerosis 2018, 277, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-J.; Azhar, S.; Kraemer, F.B. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Gillotte-Taylor, K.; Boullier, A.; Witztum, J.L.; Steinberg, D.; Quehenberger, O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 2001, 42, 1474–1482. [Google Scholar] [CrossRef]
- Yvan-charvet, L.; Wang, N.; Tall, A.R. The role of HDL, ABCA1 and ABCG1 transporters in cholesterol. Arter. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, J.L.; Kraemer, F.B.; Cooper, A.D. Transport of beta-very low density lipoproteins and chylomicron remnants by macrophages is mediated by the low density lipoprotein receptor pathway. J. Biol. Chem. 1987, 262, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Mooberry, L.K.; Sabnis, N.A.; Panchoo, M.; Nagarajan, B.; Lacko, A.G. Targeting the SR-B1 Receptor as a Gateway for Cancer Therapy and Imaging. Front. Pharmacol. 2016, 7, 466. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; Zelenko, Z.; Neel, B.A.; Antoniou, I.M.; Rajan, L.; Kase, N.; LeRoith, D. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene 2017, 36, 6462–6471. [Google Scholar] [CrossRef] [PubMed]
- Axmann, M.; Meier, S.; Karner, A.; Strobl, W.; Stangl, H.; Plochberger, B. Serum and Lipoprotein Particle miRNA Profile in Uremia Patients. Genes 2018, 9, 533. [Google Scholar] [CrossRef]
- Almer, G.; Mangge, H.; Zimmer, A.; Prassl, R. Lipoprotein-Related and Apolipoprotein-Mediated Delivery Systems for Drug Targeting and Imaging. Curr. Med. Chem. 2015, 22, 3631–3651. [Google Scholar] [CrossRef]
- Mo, Z.C.; Ren, K.; Liu, X.; Tang, Z.L.; Yi, G.H. A high-density lipoprotein-mediated drug delivery system. Adv. Drug Deliv. Rev. 2016, 106, 132–147. [Google Scholar] [CrossRef]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J.Y. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.T.; Cruz, S.; Narayanaswami, V. Reconfiguring nature’s cholesterol accepting lipoproteins as nanoparticle platforms for transport and delivery of therapeutic and imaging agents. Nanomaterials 2020, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Pownall, H.J.; Rosales, C.; Gillard, B.K.; Ferrari, M. Native and Reconstituted Plasma Lipoproteins in Nanomedicine: Physicochemical Determinants of Nanoparticle Structure, Stability, and Metabolism. Methodist Debakey Cardiovasc. J. 2016, 12, 146–150. [Google Scholar] [CrossRef]
- Huang, H.; Cruz, W.; Chen, J.; Zheng, G. Learning from biology: Synthetic lipoproteins for drug delivery. WIREs Nanomed. Nanobiotechnology 2015, 7, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, M.; Wolska, A.; Gutmann, D.A.P.; Remaley, A.T. Reconstituted Discoidal High-Density Lipoproteins: Bioinspired Nanodiscs with Many Unexpected Applications. Curr. Atheroscler. Rep. 2018, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.A.; Moschetti, A.; Ryan, R.O. Reconstituted HDL as a therapeutic delivery device. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2021, 1866, 159025. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.O.; Blanchette, C.D.; Chromy, B.A.; Kuhn, E.A.; Segelke, B.W.; Corzett, M.; Bench, G.; Mason, P.W.; Hoeprich, P.D. Immobilization of His-Tagged Proteins on Nickel-Chelating Nanolipoprotein Particles. Bioconjug. Chem. 2009, 20, 460–465. [Google Scholar] [CrossRef]
- Herringson, T.P.; Altin, J.G. Convenient targeting of stealth siRNA-lipoplexes to cells with chelator lipid-anchored molecules. J. Control Release 2009, 139, 229–238. [Google Scholar] [CrossRef]
- TUMA, P.L.; HUBBARD, A.L. Transcytosis: Crossing Cellular Barriers. Physiol. Rev. 2003, 83, 871–932. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Neuhauser, C.; Aumiller, T.; Stallinger, A.; Iken, M.; Weghuber, J. Identification of Insulin-Mimetic Plant Extracts: From an In Vitro High-Content Screen to Blood Glucose Reduction in Live Animals. Molecules 2021, 26, 4346. [Google Scholar] [CrossRef] [PubMed]
- Lanzerstorfer, P.; Stadlbauer, V.; Chtcheglova, L.A.; Haselgrübler, R.; Borgmann, D.; Wruss, J.; Hinterdorfer, P.; Schröder, K.; Winkler, S.M.; Höglinger, O.; et al. Identification of novel insulin mimetic drugs by quantitative total internal reflection fluorescence (TIRF) microscopy. Br. J. Pharmacol. 2014, 171, 5237–5251. [Google Scholar] [CrossRef] [PubMed]
- Röhrl, C.; Pagler, T.A.; Strobl, W.; Ellinger, A.; Neumüller, J.; Pavelka, M.; Stangl, H.; Meisslitzer-Ruppitsch, C. Characterization of endocytic compartments after holo-high density lipoprotein particle uptake in HepG2 cells. Histochem. Cell Biol. 2010, 133, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Axmann, M.; Karner, A.; Meier, S.M.; Stangl, H.; Plochberger, B. Enrichment of native lipoprotein particles with microRNA and subsequent determination of their absolute/relative microRNA content and their cellular transfer rate. J. Vis. Exp. 2019, 2019, 59573. [Google Scholar] [CrossRef]
- Kaufmann, T.; Ravoo, B.J. Stamps, inks and substrates: Polymers in microcontact printing. Polym. Chem. 2010, 1, 371–387. [Google Scholar] [CrossRef]
- Tokunaga, M.; Imamoto, N.; Sakata-Sogawa, K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods 2008, 5, 159–161. [Google Scholar] [CrossRef]
- Blanchette, C.D.; Cappuccio, J.A.; Kuhn, E.A.; Segelke, B.W.; Benner, W.H.; Chromy, B.A.; Coleman, M.A.; Bench, G.; Hoeprich, P.D.; Sulchek, T.A. Atomic force microscopy differentiates discrete size distributions between membrane protein containing and empty nanolipoprotein particles. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 724–731. [Google Scholar] [CrossRef]
- Sakurai, T.; Takeda, S.; Takahashi, J.; Takahashi, Y.; Wada, N.; Trirongjitmoah, S.; Namita, T.; Jin, S.; Ikuta, A.; Furumaki, H.; et al. Measurement of single low-density lipoprotein particles by atomic force microscopy. Ann. Clin. Biochem. Int. J. Lab. Med. 2013, 50, 564–570. [Google Scholar] [CrossRef]
- Gan, C.; Ao, M.; Liu, Z.; Chen, Y. Imaging and force measurement of LDL and HDL by AFM in air and liquid. FEBS Open Bio 2015, 5, 276–282. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Luo, C.; Chen, Y. Dynamic AFM detection of the oxidation-induced changes in size, stiffness, and stickiness of low-density lipoprotein. J. Nanobiotechnology 2020, 18, 167. [Google Scholar] [CrossRef]
- Chromy, B.A.; Arroyo, E.; Blanchette, C.D.; Bench, G.; Benner, H.; Cappuccio, J.A.; Coleman, M.A.; Henderson, P.T.; Hinz, A.K.; Kuhn, E.A.; et al. Different Apolipoproteins Impact Nanolipoprotein Particle Formation. J. Am. Chem. Soc. 2007, 129, 14348–14354. [Google Scholar] [CrossRef]
- Stark, R.W.; Drobek, T.; Heckl, W.M. Thermomechanical noise of a free v-shaped cantilever for atomic-force microscopy. Ultramicroscopy 2001, 86, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Plato, J. von A.N. Kolmogorov, Grundbegriffe der wahrscheinlichkeitsrechnung (1933). In Landmark Writings in Western Mathematics 1640–1940; Elsevier: Amsterdam, The Netherlands, 2005; pp. 960–969. ISBN 9780444508713. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Sezgin, E.; Kaiser, H.-J.; Baumgart, T.; Schwille, P.; Simons, K.; Levental, I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 2012, 7, 1042–1051. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Lanzerstorfer, P.; Neuhauser, C.; Weber, F.; Stübl, F.; Weber, P.; Wagner, M.; Plochberger, B.; Wieser, S.; Schneckenburger, H.; et al. Fluorescence Microscopy-Based Quantitation of GLUT4 Translocation: High Throughput or High Content? Int. J. Mol. Sci. 2020, 21, 7964. [Google Scholar] [CrossRef] [PubMed]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80. [Google Scholar] [CrossRef]
- Plochberger, B.; Sych, T.; Weber, F.; Novacek, J.; Axmann, M.; Stangl, H.; Sezgin, E. Lipoprotein Particles Interact with Membranes and Transfer Their Cargo without Receptors. Biochemistry 2020, 59, 4421–4428. [Google Scholar] [CrossRef]
- Shi, S.; Kong, N.; Feng, C.; Shajii, A.; Bejgrowicz, C.; Tao, W.; Farokhzad, O.C. Drug Delivery Strategies for the Treatment of Metabolic Diseases. Adv. Healthc. Mater. 2019, 8, 1801655. [Google Scholar] [CrossRef]
- Plochberger, B.; Röhrl, C.; Preiner, J.; Rankl, C.; Brameshuber, M.; Madl, J.; Bittman, R.; Ros, R.; Sezgin, E.; Eggeling, C.; et al. HDL particles incorporate into lipid bilayers—A combined AFM and single molecule fluorescence microscopy study. Sci. Rep. 2017, 7, 15886. [Google Scholar] [CrossRef]
- Axmann, M.; Sezgin, E.; Karner, A.; Novacek, J.; Brodesser, M.D.; Röhrl, C.; Preiner, J.; Stangl, H.; Plochberger, B. Receptor-Independent Transfer of Low Density Lipoprotein Cargo to Biomembranes. Nano Lett. 2019, 19, 2562–2567. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, F.; Axmann, M.; Horner, A.; Schwarzinger, B.; Weghuber, J.; Plochberger, B. Lipoprotein Particles as Shuttles for Hydrophilic Cargo. Membranes 2023, 13, 471. https://doi.org/10.3390/membranes13050471
Weber F, Axmann M, Horner A, Schwarzinger B, Weghuber J, Plochberger B. Lipoprotein Particles as Shuttles for Hydrophilic Cargo. Membranes. 2023; 13(5):471. https://doi.org/10.3390/membranes13050471
Chicago/Turabian StyleWeber, Florian, Markus Axmann, Andreas Horner, Bettina Schwarzinger, Julian Weghuber, and Birgit Plochberger. 2023. "Lipoprotein Particles as Shuttles for Hydrophilic Cargo" Membranes 13, no. 5: 471. https://doi.org/10.3390/membranes13050471





