Membrane-Based Hybrid Method for Purifying PEGylated Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein PEGylation
2.3. Stirred Cell Filtration
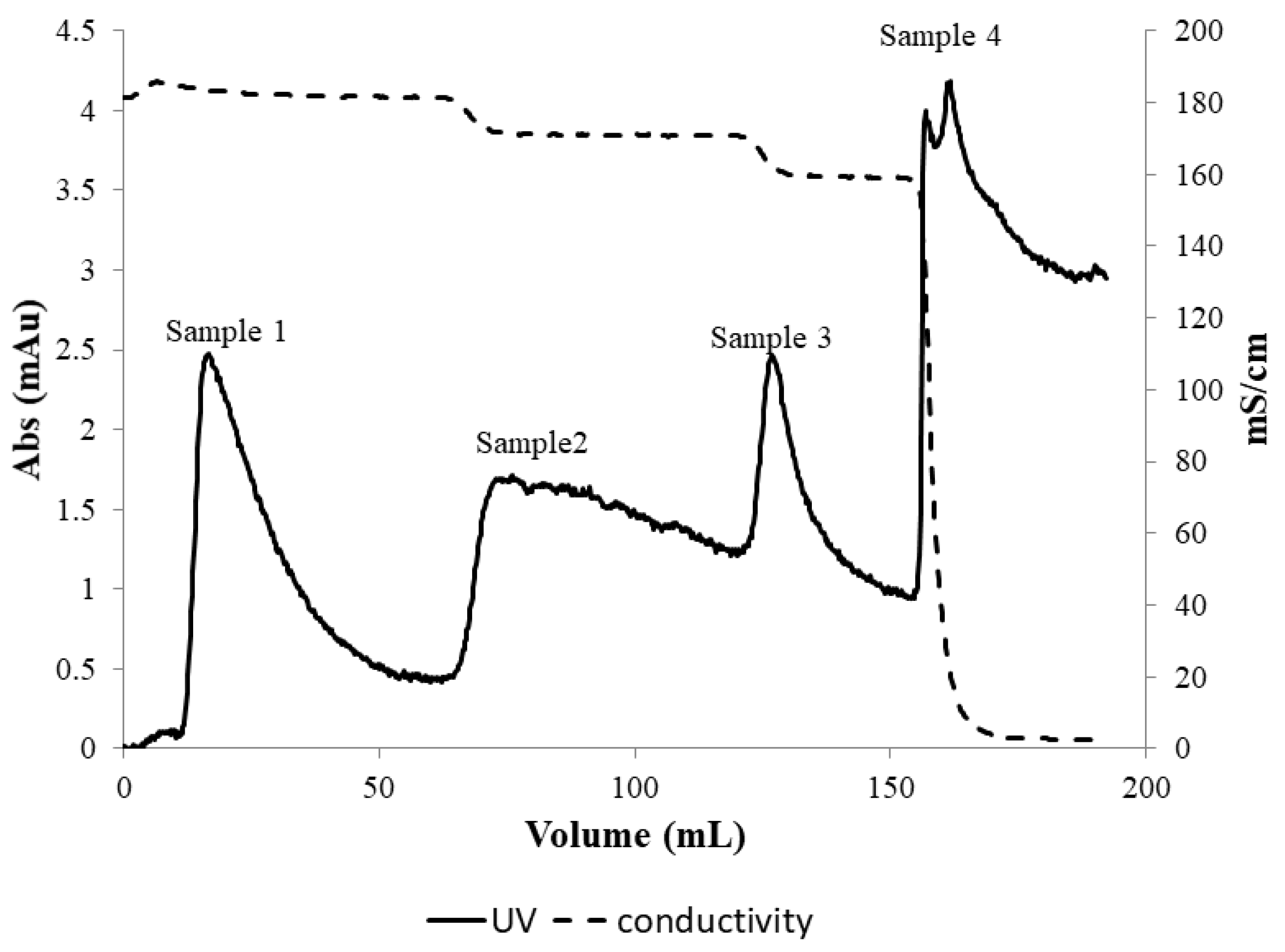
2.4. Tangential Flow Filtration
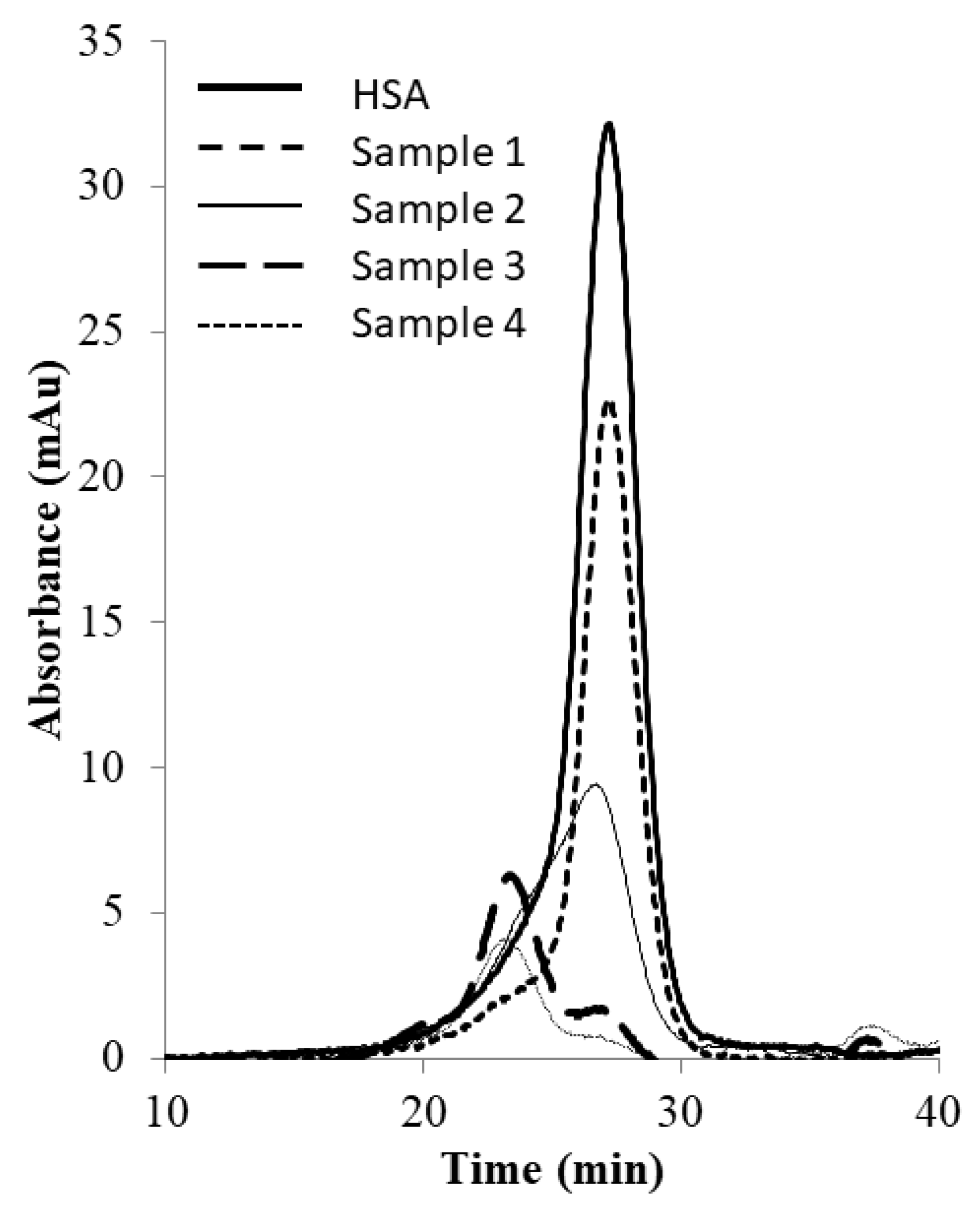
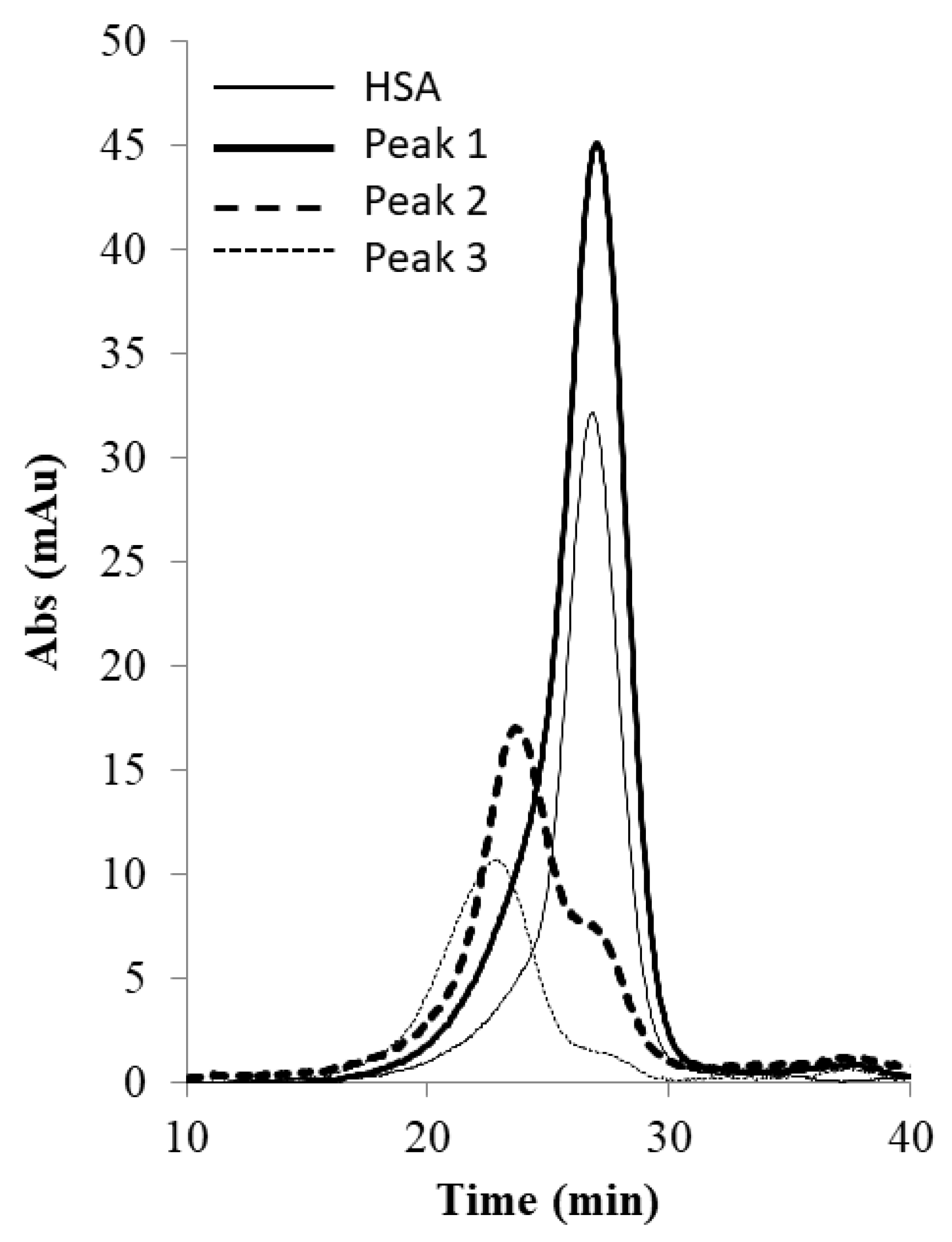
2.5. SEC Analysis
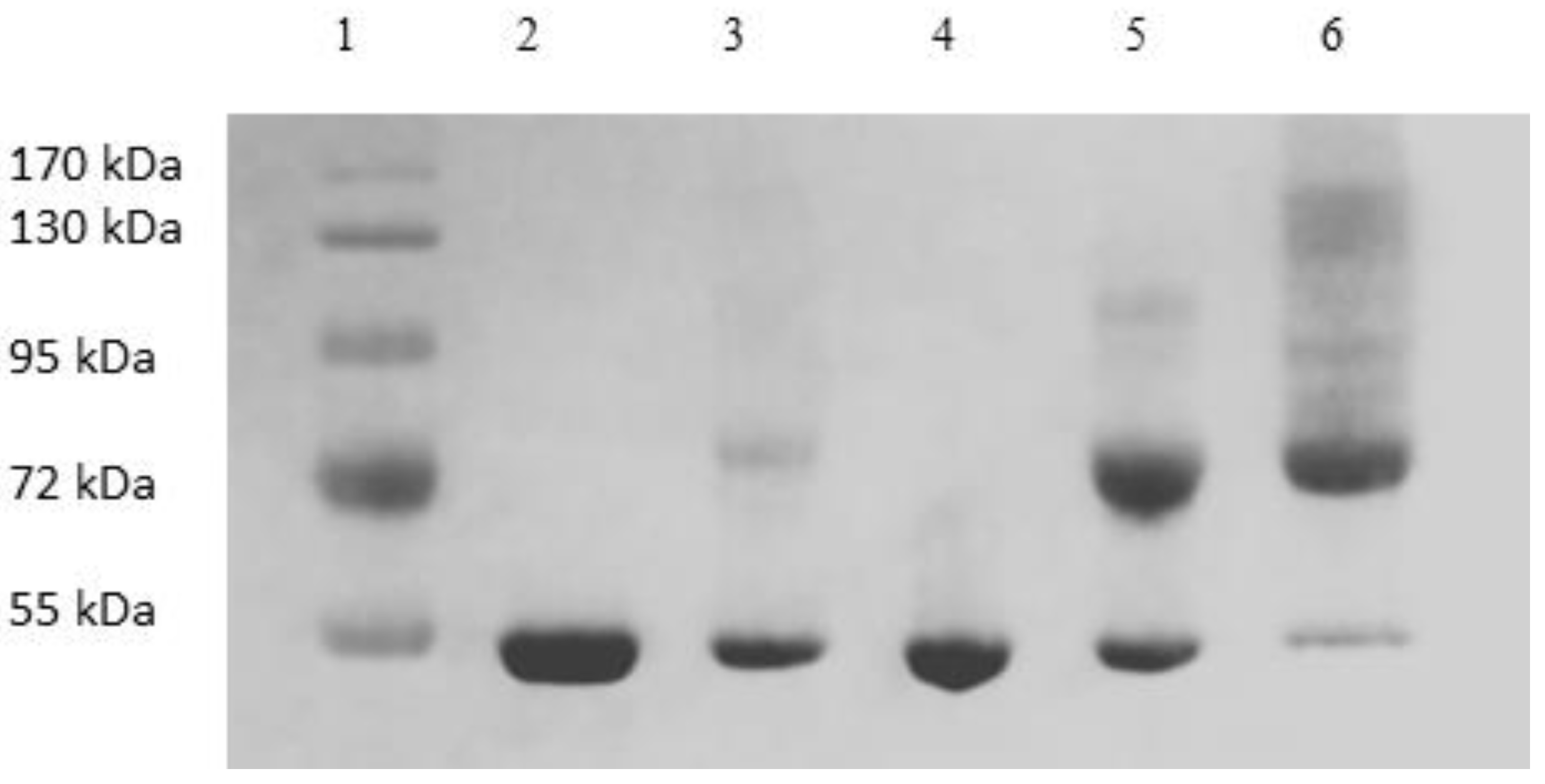
2.6. SDS-PAGE
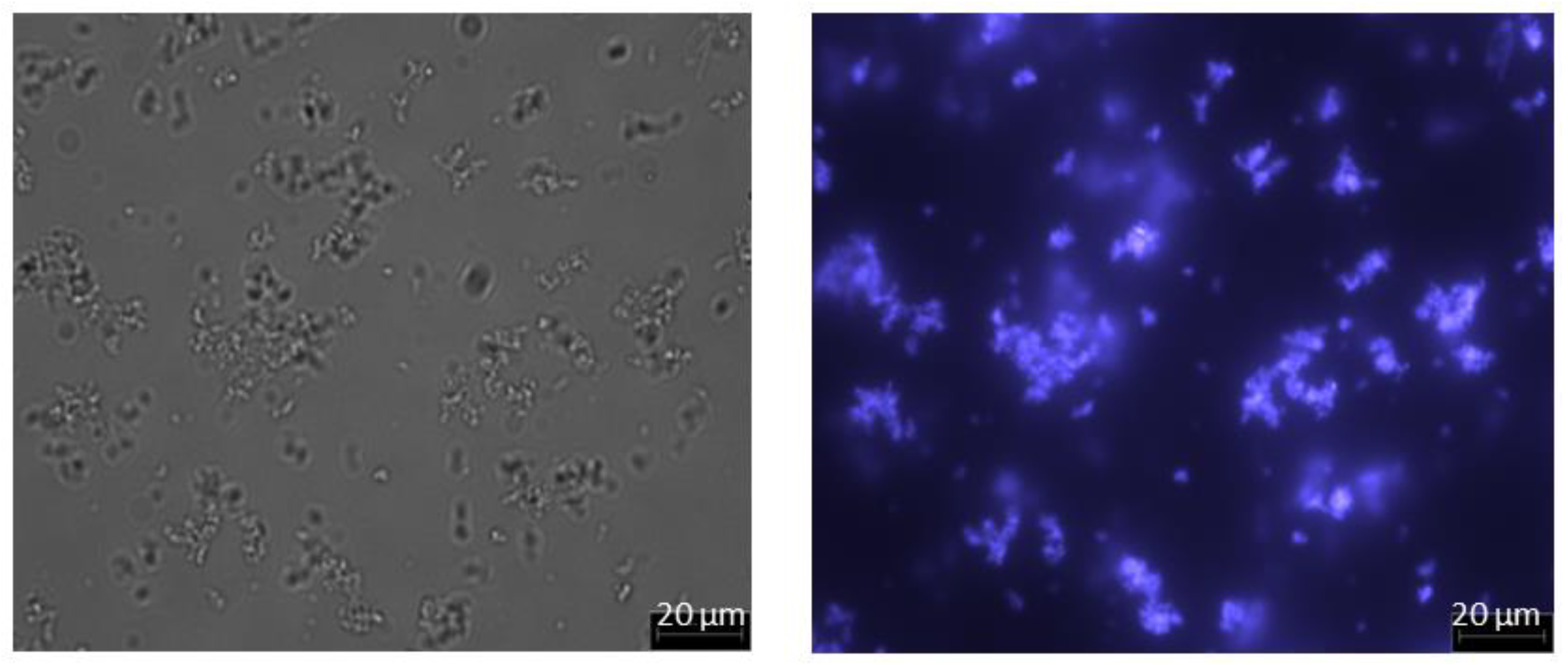
2.7. Microscopy
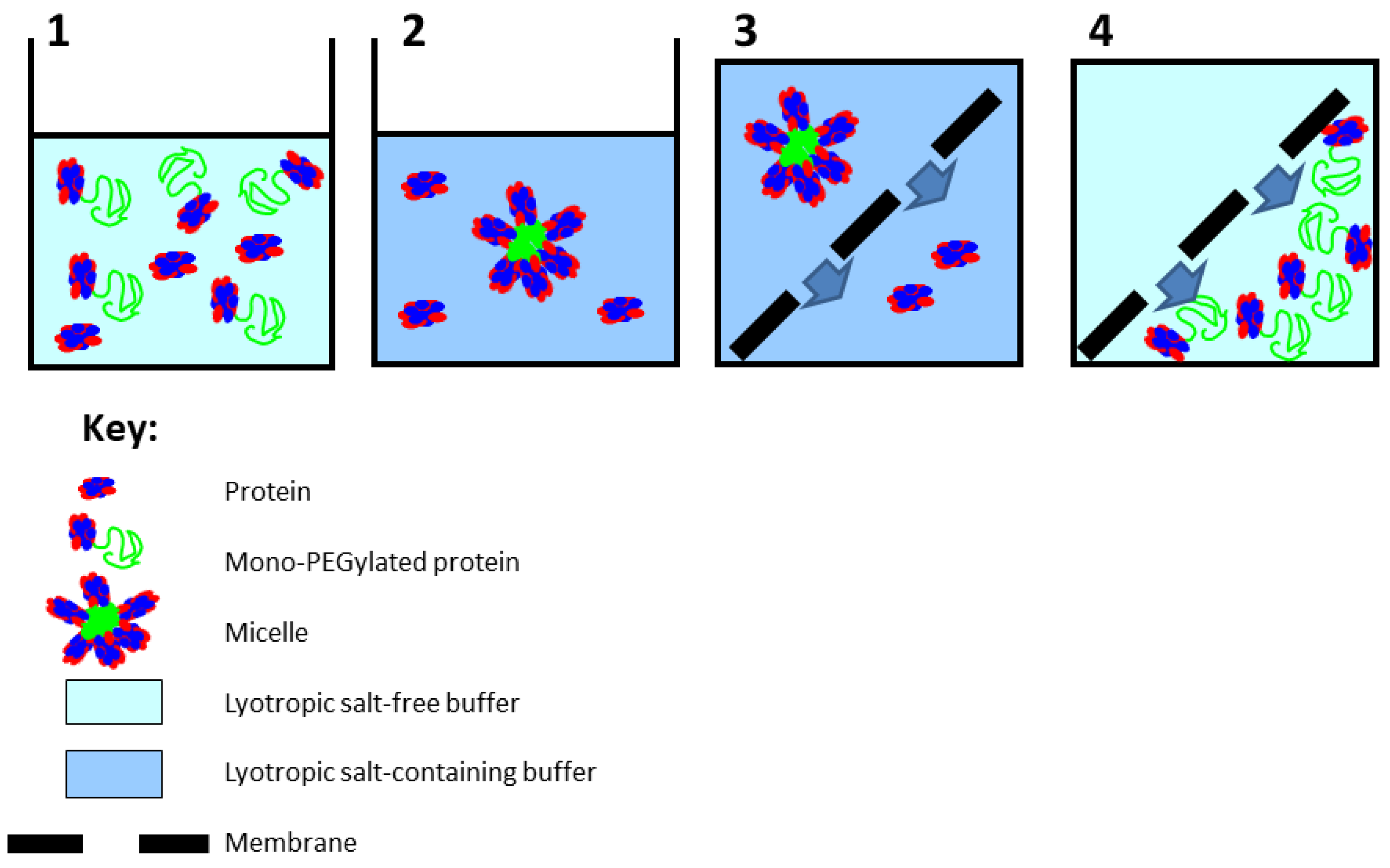
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abuchowski, A.; Vanes, T.; Palczuk, N.C.; Davis, F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977, 252, 3578–3581. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Veronese, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in anti-cancer therapy: An overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef]
- Swierczewska, M.; Lee, K.C.; Lee, S. What is the future of PEGylated therapies? Expert Opin. Emerg. Drugs 2015, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of biopharmaceuticals: A review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Dozier, J.K.; Distefano, M.D. Site-specific PEGylation of therapeutic proteins. Int. J. Mol. Sci. 2015, 16, 25831–25864. [Google Scholar] [CrossRef]
- Fee, C.J.; Van Alstine, J.M. PEG-proteins: Reaction engineering and separation issues. Chem. Eng. Sci. 2006, 61, 924–939. [Google Scholar] [CrossRef]
- Fee, C.J. Size-exclusion reaction chromatography (SERC): A new technique for protein PEGylation. Biotechnol. Bioeng. 2003, 82, 200–206. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Isakari, Y.; Itoh, D.; Yamamoto, S. PEG chain length impacts yield of solid-phase protein PEGylation and efficiency of PEGylated protein separation by ion-exchange chromatography: Insights of mechanistic models. Biotechnol. J. 2013, 8, 801–810. [Google Scholar] [CrossRef]
- Pabst, T.M.; Buckley, J.J.; Ramasubramanyan, N.; Hunter, A.K. Comparison of strong anion-exchangers for the purification of a PEGylated protein. J. Chromatogr. A 2007, 1147, 172–182. [Google Scholar] [CrossRef]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2012, 64, 116–127. [Google Scholar] [CrossRef]
- Chen, G.; Butani, N.; Ghosh, R. Fast and high-resolution fractionation of positional isomers of a PEGylated protein using membrane chromatography. J. Chromatogr. B 2022, 1203, 123292. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Pagano, J.; Yu, D.; Ghose, S.; Li, Z.; Ghosh, R. Fast and high-resolution purification of a PEGylated protein using a z2 laterally-fed membrane chromatography device. J. Chromatogr. A 2021, 1652, 462375. [Google Scholar] [CrossRef]
- Yu, D.; Shang, X.; Ghosh, R. Fractionation of different PEGylated forms of a protein by chromatography using environment-responsive membranes. J. Chromatogr. A 2010, 1217, 5595–5601. [Google Scholar] [CrossRef] [PubMed]
- Mayolo-Deloisa, K.; González-Valdez, J.; Rito-Palomares, M. PEGylated protein separation using different hydrophobic interaction supports: Conventional and monolithic supports. Biotechnol. Prog. 2016, 32, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Manzano, L.A.; Lienqueo, M.E.; Escalante-Vázquez, E.J.; Rito-Palomares, M.; Asenjo, J.A. Optimized purification of mono-PEGylated lysozyme by heparin affinity chromatography using response surface methodology. J. Chem. Technol. Biotechnol. 2017, 92, 2554–2562. [Google Scholar] [CrossRef]
- Mejía-Manzano, L.A.; Campos-García, V.R.; Perdomo-Abúndez, F.C.; Medina-Rivero, E.; González-Valdez, J. Mono-PEGylated lysozyme purification with increased productivity and isomer differentiation through heparin monolith chromatography. J. Chromatogr. B 2022, 1204, 123323. [Google Scholar] [CrossRef]
- Przybycien, T.M.; Pujar, N.S.; Steele, L.M. Alternative bioseparation operations: Life beyond packed-bed chromatography. Curr. Opin. Biotechnol. 2004, 15, 469–478. [Google Scholar] [CrossRef]
- Molek, J.R.; Zydney, A.L. Ultrafiltration characteristics of pegylated proteins. Biotechnol. Bioeng. 2006, 95, 474–482. [Google Scholar] [CrossRef]
- Molek, J.R.; Zydney, A.L. Separation of PEGylated α-Lactalbumin from Unreacted Precursors and Byproducts Using Ultrafiltration. Biotechnol. Prog. 2007, 23, 1417–1424. [Google Scholar] [CrossRef]
- Kwon, B.; Molek, J.; Zydney, A.L. Ultrafiltration of PEGylated proteins: Fouling and concentration polarization effects. J. Membr. Sci. 2008, 319, 206–213. [Google Scholar] [CrossRef]
- Molek, J.; Ruanjaikaen, K.; Zydney, A.L. Effect of electrostatic interactions on transmission of PEGylated proteins through charged ultrafiltration membranes. J. Membr. Sci. 2010, 353, 60–69. [Google Scholar] [CrossRef]
- Santos, J.H.P.M.; Mendonça, C.M.N.; Silva, A.R.P.; Oliveira, R.P.S.; Pessoa, A.; Coutinho, J.A.O.; Ventura, S.P.M.; Rangel-Yagui, C.O. An integrated process combining the reaction and purification of PEGylated proteins. Green Chem. 2019, 21, 6407–6418. [Google Scholar] [CrossRef]
- Santos, J.H.; Carretero, G.; Coutinho, J.A.; Rangel-Yagui, C.O.; Ventura, S.P. Multistep purification of cytochrome c PEGylated forms using polymer-based aqueous biphasic systems. Green Chem. 2017, 19, 5800–5808. [Google Scholar] [CrossRef]
- Pfister, D.; Morbidelli, M. Integrated process for high conversion and high yield protein PEGylation. Biotechnol. Bioeng. 2016, 113, 1711–1718. [Google Scholar] [CrossRef]
- Kateja, N.; Dureja, S.; Rathore, A.S. Development of an integrated continuous PEGylation and purification process for granulocyte colony stimulating factor. J. Biotechnol. 2020, 322, 79–89. [Google Scholar] [CrossRef]
- Shang, X.; Yu, D.; Ghosh, R. Integrated solid-phase synthesis and purification of PEGylated protein. Biomacromolecules 2011, 12, 2772–2779. [Google Scholar] [CrossRef]
- Shang, X.; Ghosh, R. Membrane reactor for continuous and selective protein mono-PEGylation. J. Membr. Sci. 2014, 451, 177–184. [Google Scholar] [CrossRef]
- Saeki, S.; Kuwahara, N.; Nakata, M.; Kaneko, M. Phase Separation of Poly (ethylene glycol)-Water-Salt Systems. Polymer 1977, 18, 1027–1031. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Goddard, E.D. Aqueous Biphase Formation in Polyethylene Oxide-Inorganic Salt Systems. Langmuir 1987, 3, 25–31. [Google Scholar] [CrossRef]
- Wang, L.; Kanani, D.M.; Ghosh, R. Purification of humanized monoclonal antibodies by membrane-based hybrid bioseparation technique. J. Immunol. Methods 2006, 314, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gu, Q.; Coffman, J.L.; Przybycien, T.; Zydney, A.L. Continuous precipitation for monoclonal antibody capture using countercurrent washing by microfiltration. Biotechnol. Prog. 2019, 35, e2886. [Google Scholar] [CrossRef]
- Wang, L.; Mah, K.Z.; Ghosh, R. Purification of human IgG using membrane based hybrid bioseparation technique and its variants: A comparative study. Sep. Purif. Technol. 2009, 66, 242–247. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Ghosh, R. Purification of equine IgG using membrane based enhanced hybrid bioseparation technique: A potential method for manufacturing hyperimmune antibody. Biotechnol. Bioeng. 2008, 99, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, S.F.; Shang, X.; Ghosh, R. Membrane-Based Hybrid Method for Purifying PEGylated Proteins. Membranes 2023, 13, 182. https://doi.org/10.3390/membranes13020182
Lam SF, Shang X, Ghosh R. Membrane-Based Hybrid Method for Purifying PEGylated Proteins. Membranes. 2023; 13(2):182. https://doi.org/10.3390/membranes13020182
Chicago/Turabian StyleLam, Shing Fung, Xiaojiao Shang, and Raja Ghosh. 2023. "Membrane-Based Hybrid Method for Purifying PEGylated Proteins" Membranes 13, no. 2: 182. https://doi.org/10.3390/membranes13020182
APA StyleLam, S. F., Shang, X., & Ghosh, R. (2023). Membrane-Based Hybrid Method for Purifying PEGylated Proteins. Membranes, 13(2), 182. https://doi.org/10.3390/membranes13020182










