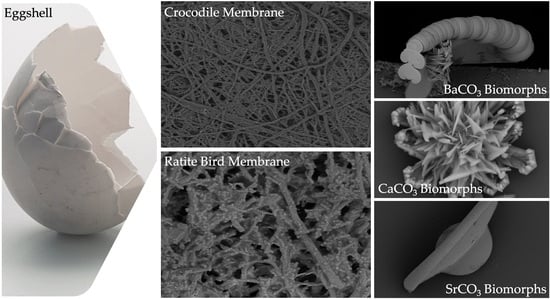
Influence of Intramineral Proteins on the Growth of Carbonate Crystals Using as a Scaffold Membranes of Ratite Birds and Crocodiles Eggshells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eggshells Cleaning and Membranes Separation
2.2. Intramineral Proteins Isolation
2.3. Purification and Characterization of Intramineral Proteins
2.4. Determination of Molecular Weight of Isolated Intramineral Proteins
2.5. Synthesis of Biomorphs
2.6. Morphological Characterization by Scanning Electron Microscopy (SEM)
2.7. Vibrational Spectrocopy Characterization of Biomorphs
2.8. Elemental Analysis of Membranes by Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy (SEM-EDS)
2.9. Biocalcification and Biosilicification of Membranes
2.10. Production of Biomorphs Using Eggshell Membranes as Scaffolds
3. Results and Discussions
3.1. Separation of the Organic Membrane Present in the Eggshells
3.2. Extraction and Characterization of the Intramineral Proteins
3.3. Formation and Determination of the Molecules Present in the Synthethized Biomorphs
3.4. Elemental Analysis and Biomorphs Growth on Eggshell Membranes
3.5. Biocalcification and Biosilicification of Membranes and Calcium Biomorphs Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiner, S.; Dove, P.M. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Weiner, S. Biomineralization: A structural perspective. J. Struct. Biol. 2008, 163, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Steele, J.A.M.; Fratzl, P.; Stevens, M.M. A materials science vision of extracellular matrix mineralization. Nat. Rev. Mater. 2016, 1, 16041. [Google Scholar] [CrossRef]
- Mann, S. Mineralization in Biological Systems. In Inorganic Elements in Biochemistry: Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 1983; Volume 54, pp. 125–174. [Google Scholar]
- Gower, L. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef]
- Guatron, J.; Stapane, L.; Le Roy, N. Avian eggshell biomineralization: An update on its structure, mineralogy, and protein tool kit. BMC Mol. Cell. Biol. 2021, 22, 11. [Google Scholar] [CrossRef]
- Suzuki, M.; Nahasawa, H. Mollusk shell structures and their formation mechanism. Can. J. Zool. 2013, 91, 349–366. [Google Scholar] [CrossRef]
- Mikhailov, K.E. Fossil and Recent Eggshell in Amniotic Vertebrates: Fine Structure, Comparative; Chapter 5; Indiana University Press: Bloomington, IN, USA, 1999. [Google Scholar]
- Yang, W.; Lopez, P.J.; Rosengarten, G. Diatoms: Self-assembled silica nanostructures, and templates for bio/chemical sensors and biomimetic membranes. Analyst 2011, 136, 42–53. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, W.; Jiang, X. Biomimetic collagen nanofibrous materials for bone tissue engineering. Adv. Eng. Mater. 2010, 12, B451–B466. [Google Scholar] [CrossRef]
- Dauphin, Y.; Luquet, G.; Perez-Huerta, A.; Salomé, M. Biomineralization in modern avian calcified eggshells: Similarity versus diversity. Connect Tissue Res. 2018, 58, 67–73. [Google Scholar] [CrossRef]
- Dauphin, Y.; Cuif, J.P.; Salomé, M.; Susini, J.; Williams, C.T. Microstructure, and chemical composition of giant avian eggshells. Anal. Bioanalytical. Chem. 2006, 386, 1761–1771. [Google Scholar] [CrossRef]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The eggshell: Structure, composition, and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Gautron, J.; Murayama, E.; Vignal, A.; Morisson, M.; McKee, M.; Rehault, S.; Hincke, T.M. Cloning of ovocalyxin-36, a novel chicken eggshell protein related to lipopolysaccharide-binding proteins, bactericidal permeability-increasing proteins, and plink family proteins. J. Biol. Chem. 2007, 282, 5273–5286. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.L.; Harding, J.H.; Quigely, D.; Rodger, P.M. Simulations of Ovocleidin-17 binding to calcite surfaces and its implications for eggshells formation. J. Phys. Chem. C 2011, 115, 8175–8183. [Google Scholar] [CrossRef]
- Reyes-Grajeda, J.P.; Juaregui-Zuniga, D.; Rodriguez-Romero, A.; Hernandez-Santoyo, A.; Bolano-Garcia, V.M.; Moreno, A. Crystallization, and preliminary X-ray analysis of ovocleidin-17 a major protein of the Gallus gallus eggshell calcified layer. Prot. Pep. Lett. 2002, 9, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Siedler, F. The amino sequence of ovocledin-17, a major protein fo the avian eggshell calcified layer. IUBMB Life 2008, 47, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, R.; Kini, R.M.; Valiyaveettil, S. Investigation of the role of ansocalcin in the biomineralization in goose eggshell matrix. Proc. Natl. Acad. Sci. USA 2002, 99, 5155–5159. [Google Scholar] [CrossRef]
- Mann, K.; Siedler, F. Amino acids sequence and phosphorylation sites of emu and rhea eggshell c-type lectin-like proteins. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 143, 160–170. [Google Scholar] [CrossRef]
- Reyes-Grajeda, J.P.; Marín-Garcaí, L.; Stojanoff, V.; Moreno, A. Purification, crystallization, and preliminary X-ray analysis of struthiocalcin-1 from ostrich (Struthio camelus) eggshell. Acta Cryst. 2007, F63, 987–989. [Google Scholar] [CrossRef]
- Eiblmeier, J.; Dankesreiter, S.; Ptitzner, A.; Schmalz, G.; Kellermeier, M. Crystallization of mixed alkaline-earth carbonates in silica solution at high pH. Cryst. Growth Des. 2014, 14, 6177–6188. [Google Scholar] [CrossRef]
- Kellermeier, M.; Melero-García, E.; Glaab, F.; Eblmeier, J.; Kienle, L.; Rachel, R.; Kunz, W.; García-Ruiz, J.M. Growth behavior and kinetics of self-assembled silica-carbonate biomorphs. Chem. Eur. J. 2012, 18, 2272–2282. [Google Scholar] [CrossRef]
- Virgen-Ortiz, J.J.; Ibarra-Junquera, V.; Osuna-Casto, J.A.; Escalante-Minakata, P.; Mancilla-Margalli, N.A.; Ornelas-Paz, J.J. Methos to concentrate protein solutions based on dialysis-freezing centrifugation: Enzyme applications. Anal. Biochem. 2012, 426, 4–12. [Google Scholar] [CrossRef]
- Noorduin, W.L.; Grinthal, A.; Mahadevan, L.; Aizenberg, J. Rationally designed complex, hierarchical microarchitectures. Science 2013, 340, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Niu, L.N.; Qi, Y.P.; Yiu, C.K.Y.; Ryou, H.; Arola, D.D.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Subtleties of biomineralization by manipulation of the eggshell membrane. Biomaterials 2011, 32, 8743–8752. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arellano, R.R.; Moreno, A. Obtainment of spherical-shaped calcite crystals induced of intramineral proteins isolated from eggshells of ostrich and emu. Cryst. Growth Des. 2014, 14, 5137–5143. [Google Scholar] [CrossRef]
- Mann, K. Identification of the major proteins of the organic matrix of emu (Dromaius novaehollandiae) and rhea (Rhea americana) eggshell calcified layer. Br. Poult. Sci. 2004, 45, 483–490. [Google Scholar] [CrossRef]
- Cho, Y.T.; Su, H.; Huang, T.L.; Chen, H.C.; Wu, W.K.; Wu, P.C.; Wu, D.C.; Shiea, J. Matrix-assisted lase desorption ionization/time-of-flight mass spectrometry for clinical diagnosis. Clin. Chim. Acta 2013, 415, 266–275. [Google Scholar] [CrossRef]
- Schiller, J.; Arnhold, J.; Benard, S.; Müller, M.; Reichi, S.; Arnold, K. Lipid analysis by matrix-assisted lase desorption and ionization mass spectrometry: A methodological approach. Anal. Biochem. 1999, 267, 46–56. [Google Scholar] [CrossRef]
- Legorreta-Flores, A.; Davila, A.; Velásquez-Gonzáles, O.; Ortega, E.; Ponce, A.; Castillo-Michel, H.; Reyes-Grajeda, J.P.; Hernández-Rivera, R.; Cuéllar-Cruz, M.; Moreno, A. Calcium carbonate crystals shapes mediated by intramineral proteins from eggshells of ratite birds and crocodiles. Implications to the eggshell’s formation of a dinosaur of 70 million years old. Cryst. Growth Des. 2018, 18, 5663–5673. [Google Scholar] [CrossRef]
- Schopf, J.W. Microfossils of the early archean apex chert: New evidence of the antiquity of life. Science 1993, 260, 640–646. [Google Scholar] [CrossRef]
- Panheleux, M.; Nys, Y.; Williams, J.; Gautron, J.; Boldicke, T.; Hincke, M.T. Extraction and quantification by ELISA of eggshell organic matrix proteins (ovocleidin-17, ovalbumin, ovotranferrin) in shell from young and old hens. Poult. Sci. 2000, 79, 580–588. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.; Kalin, O.; Nys, Y.; García-Ruiz, J.M. Influence of the microstructure on the shell strength of eggs laid by hens of different ages. Br. Poult. Sci. 2002, 43, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Carteret, C.; Dandeu, A.; Moussaoui, S.; Muhr, H.; Humbert, B.; Plasari, E. Polymorphism studied by lattice phonon Raman spectroscopy and statistical mixture analysis method. Application to calcium carbonate polymorphs during batch crystallization. Cryst. Growth Des. 2009, 9, 807–812. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Moreno, A. The role of calcium and strontium as the most dominant elements during combinations of different alkaline earth metals in the synthesis of crystalline silica-carbonate biomorphs. Crystals 2019, 9, 381. [Google Scholar] [CrossRef]
- Opel, J.; Wimmer, F.P.; Kellermeier, M.; Cölfen, H. Functionalisation of silica-carbonate biomorphs. Nanoscale Horiz. 2016, 1, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Rolleri, C.H.; Lavalle, M.C.; Mengascini, A.; Rodríguez, M. Sistemática de los helechos maratiáceos (Marattiales-Marattiaceae). Revista del Museo de la Plata, Universidad Nacional de la Plata, Facultad de Ciencias Naturales y Museo. Botánica 2003, 16, 1–21. [Google Scholar]
- Sims, P.A.; Mann, D.G.; Medlin, L.K. Evolution of the diatoms: Insights from fossil, biological and molecular data. Phycologia 2006, 45, 361–402. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms. In Biology & Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; pp. 4–28. [Google Scholar]
- Rouillard, J.; García-Ruiz, J.M.; Gong, J.; Van Zuilen, M.A. A morphogram for silica-whiterite biomorphs and its application to mircofossil identication in the early earth rock record. Giobiology 2018, 16, 279–296. [Google Scholar] [CrossRef]
- Dixit, S.S.; Smol, J.P.; Kingston, J.C. Diatoms: Powerful indicators of environmental change. Environ. Sci. Technol. 1992, 26, 1. [Google Scholar] [CrossRef]
- Quinn, P. The occurrence and research potential of microfossils in inorganic archeological materials. Geoarchaeology 2008, 23, 275–291. [Google Scholar] [CrossRef]
- DeOliveira, D.B.; Laursen, R.A. Control of calcite crystal morphology by a peptide designed to bind to a specific surface. J. Am. Chem. Soc. 1997, 119, 10627–10631. [Google Scholar] [CrossRef]
- Williams, R.J.P.; Fraústo da Silva, J.J.R. Bringing Chemistry to Life: From Matter to Man; Oxford University Press: New York, NY, USA, 1999; pp. 293–298. [Google Scholar]
- Fraústo da Silva, J.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed.; Oxford University Press: New York, NY, USA, 1991; pp. 7–10. [Google Scholar]
- Turner, D.R.; Whitfield, M.; Dickson, A.G. The Equilibrium Speciation of Dissolved Components in Fresh-Water and Seawater at 25 °C and 1 Atm Pressure. Geochim. Cosmochim. Acta 1981, 45, 855–881. [Google Scholar] [CrossRef]
- Arias, J.L.; Fink, D.J.; Xiao, S.Q.; Heuer, A.H.; Caplan, A.I. Biomineralization and eggshells: Cell-mediated acellular compartments of mineralized extracellular matrix. Int. Rev. Cytol. 1993, 145, 217–250. [Google Scholar] [CrossRef] [PubMed]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Elejalde-Cadena, N.R.; Estevez-Espinoza, J.O.; Torres-Costa, V.; Ynsa, M.D.; García-López, G.; Moreno, A. Molecular analysis and examination of posible intramineral proteins of dinosaur eggshells collected in El Rosario, Baja California. ACS Earth Space Chem. 2021, 5, 1552–1563. [Google Scholar] [CrossRef]
- De Meutter, J.; Goormaghtigh, E. FTIR imaging of protein microarrays for high throughput secondary structure determination. Anal. Chem. 2021, 93, 3733–3741. [Google Scholar] [CrossRef]
- El-Fattah, M.A.; El-Saeed, A.M.; El-Ghazawy, R.A. Chemical interaction of different sized fumed silica with epoxy via ultrasonication for improved coating. Prog. Org. Coat. 2019, 129, 1–9. [Google Scholar] [CrossRef]
- Mendes, L.C.; Ribeiro, G.L.; Marques, R.C. In situ hydroxyapatite synthesis: Influence of collagen on its structural and morphological characteristic. Mater. Sci. Appl. 2012, 3, 580–586. [Google Scholar] [CrossRef]
- El Khouri, A.; Zegzouti, A.; Elaatmani, M.; Capitelli, F. Bismuth-substituted hdroxyapatite ceramics synthesis: Morphological, structural, vibrational, and dielectric properties. Inorg. Chem. Commun. 2019, 110, 107568. [Google Scholar] [CrossRef]
- Cancelliere, R.; Rea, G.; Micheli, L.; Mantegazza, P.; Bauer, E.M.; El Khouri, A.; Tempesta, E.; Altomare, A.; Capelli, D.; Capitelli, F. Electrochemical and structural characterization of lanthanum-doped hydroxyapatite: A promising material for sensing applications. Materials 2023, 16, 4522. [Google Scholar] [CrossRef]




















| Control | SCA-1 | SCA-2 | DCA-1 | DCA-2 | CCA-7 | CCA-14 | CCM-1 | CCM-3 |
|---|---|---|---|---|---|---|---|---|
| -- | 94 | -- | 108 | 115 | 113 | -- | 108 | -- |
| 161 | 154 | 156 | 158 | 153 | 159 | 156 | 154 | 153 |
| 279 | 282 | 281 | 283 | 279 | 282 | 282 | 280 | 279 |
| 713 | 714 | 713 | 713 | 713 | 713 | 712 | 711 | 711 |
| 1081 | 1081 | 1081 | 1089 | 1090 | 1081 | 1089 | 1081 | 1081 |
| 1436 | 1437 | 1437 | 1443 | -- | -- | 1436 | 1430 | 1436 |
| 1748 | 1750 | 1749 | 1752 | -- | -- | 1745 | 1755 | 1745 |
| Control | SCA-1 | SCA-2 | DCA-1 | DCA-2 | CCA-7 | CCA-14 | CCM-1 | CCM-3 |
|---|---|---|---|---|---|---|---|---|
| 89 | 94 | 94 | 88 | 93 | 90 | 93 | 91 | 91 |
| 135 | 140 | 140 | 134 | 135 | 138 | 138 | 136 | 136 |
| 153 | 151 | 149 | 150 | 150 | 153 | 156 | 155 | 155 |
| 224 | 222 | 225 | 214 | 224 | 220 | 225 | 224 | 222 |
| 691 | 690 | 690 | 689 | 688 | 689 | 690 | 691 | 690 |
| 1060 | 1060 | 1060 | 1057 | 1056 | 1060 | 1060 | 1060 | 1060 |
| 1347 | -- | -- | -- | -- | 1354 | 1356 | 1354 | 1351 |
| 1421 | 1430 | 1425 | 1428 | 1436 | 1436 | 1422 | 1424 | 1433 |
| -- | -- | -- | -- | -- | 1502 | 1503 | -- | -- |
| -- | -- | -- | -- | -- | 1605 | -- | -- | -- |
| -- | 2909 | 2915 | 2848 | 2841 | 2858 | 2857 | 2852 | 2850 |
| 2932 | 2957 | 2946 | 2947 | 2947 | 2973 | 2971 | 2944 | 2942 |
| Control | SCA-1 | SCA-2 | DCA-1 | DCA-2 | CCA-7 | CCA-14 | CCM-1 | CCM-3 |
|---|---|---|---|---|---|---|---|---|
| -- | 115 | 112 | 111 | 114 | 112 | 116 | 119 | 115 |
| 148 | 149 | 151 | 145 | 144 | 148 | 151 | 151 | 148 |
| 182 | 180 | 179 | 177 | 177 | 181 | 182 | 182 | 180 |
| 243 | 245 | 238 | 234 | 246 | 245 | 250 | 243 | 246 |
| 701 | 700 | 700 | 697 | 696 | 679 | 699 | 699 | 698 |
| 1069 | 1075 | 1070 | 1067 | 1067 | 1069 | 1072 | 1070 | 1075 |
| -- | -- | -- | -- | -- | 1304 | 1305 | 1297 | -- |
| 1362 | -- | -- | 1354 | -- | 1360 | 1364 | -- | -- |
| 1450 | 1443 | 1428 | 1443 | -- | 1447 | 1445 | 1440 | 1446 |
| -- | -- | -- | -- | 1605 | 1601 | 1602 | 1458 | -- |
| -- | 2894 | 2865 | 2847 | -- | 2858 | 2848 | 2853 | 2848 |
| 2932 | 2968 | 2968 | 2943 | 2932 | 2955 | 2964 | 2945 | 2967 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elejalde-Cadena, N.R.; Hernández, D.; Capitelli, F.; Islas, S.R.; Rosales-Hoz, M.J.; Zema, M.; Tarantino, S.C.; Siliqi, D.; Moreno, A. Influence of Intramineral Proteins on the Growth of Carbonate Crystals Using as a Scaffold Membranes of Ratite Birds and Crocodiles Eggshells. Membranes 2023, 13, 869. https://doi.org/10.3390/membranes13110869
Elejalde-Cadena NR, Hernández D, Capitelli F, Islas SR, Rosales-Hoz MJ, Zema M, Tarantino SC, Siliqi D, Moreno A. Influence of Intramineral Proteins on the Growth of Carbonate Crystals Using as a Scaffold Membranes of Ratite Birds and Crocodiles Eggshells. Membranes. 2023; 13(11):869. https://doi.org/10.3390/membranes13110869
Chicago/Turabian StyleElejalde-Cadena, Nerith R., Denisse Hernández, Francesco Capitelli, Selene R. Islas, Maria J. Rosales-Hoz, Michele Zema, Serena C. Tarantino, Dritan Siliqi, and Abel Moreno. 2023. "Influence of Intramineral Proteins on the Growth of Carbonate Crystals Using as a Scaffold Membranes of Ratite Birds and Crocodiles Eggshells" Membranes 13, no. 11: 869. https://doi.org/10.3390/membranes13110869