Fabrication of Loose Nanofiltration Membranes with High Rejection Selectivity between Natural Organic Matter and Salts for Drinking Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of LNF Membranes
2.2. Test of Membrane Performance
3. Results and Discussion
3.1. Effect of PES Concentration
3.2. Effect of PIP Concentration
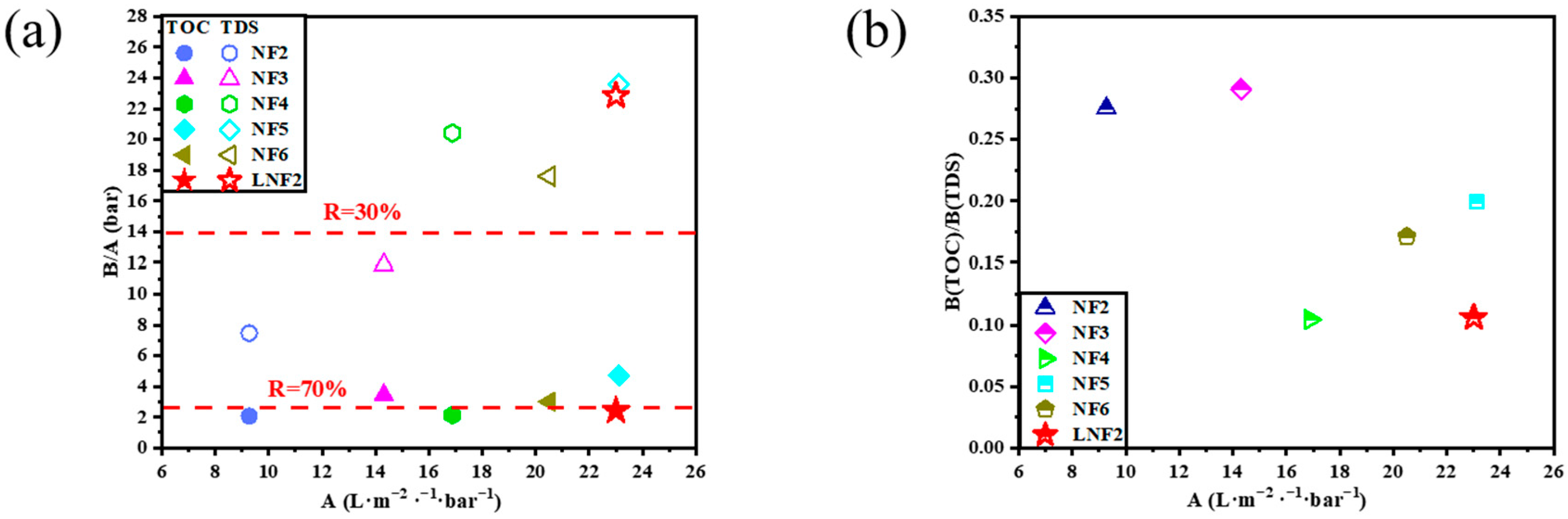
3.3. Rejection Selectivity of the Prepared Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Li, X.H.; Yang, W.L.; Yao, Z.K.; Mei, Y.; Peng, L.E.; Yang, Z.; Shao, S.L.; Tang, C.Y. Nanofiltration for Drinking Water Treatment: A Review. Front. Chem. Sci. Eng. 2022, 16, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Tong, T.Z.; Wang, X.M.; Lin, S.H.; Reid, E.M.; Chen, Y.S. Differentiating Solutes with Precise Nanofiltration for Next Generation Environmental Separations: A Review. Environ. Sci. Technol. 2021, 55, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, P.F.; Li, X.H.; Gan, B.W.; Wang, L.; Song, X.X.; Park, H.D.; Tang, C.Y. A Critical Review on Thin-Film Nanocomposite Membranes with Interlayered Structure: Mechanisms, Recent Developments, and Environmental Applications. Environ. Sci. Technol. 2020, 54, 15563–15583. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Y.; Chang, H.L.; Gao, S.S.; Zhang, R.J. How to Fabricate a Negatively Charged NF Membrane for Heavy Metal Removal via the Interfacial Polymerization between PIP and TMC? Desalination 2020, 491, 114499. [Google Scholar] [CrossRef]
- Shemer, H.; Hasson, D.; Semiat, R. State-of-the-art Review on Post-treatment Technologies. Desalination 2015, 356, 285–293. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Z.; Wang, K.; Wang, X. A Review of the Influence Factors on Flavor of Drinking Water. Water Wastewater Eng. 2022, 48, 143–151. [Google Scholar] [CrossRef]
- World Health Organization. Calcium and Magnesium in Drinking-Water Public Health Significance; World Health Organization: Geneva, Switzerland, 2009.
- Gao, Y.W.; Wang, K.P.; Wang, X.M.; Huang, X. Exploitation of Amine Groups Cooped up in Polyamide Nanofiltration Membranes to Achieve High Rejection of Micropollutants and High Permeance of Divalent Cations. Environ. Sci. Technol. 2022, 56, 10954–10962. [Google Scholar] [CrossRef]
- Feng, X.Q.; Peng, D.L.; Zhu, J.Y.; Wang, Y.; Zhang, Y.T. Recent Advances of Loose Nanofiltration Membranes for Dye/Salt Separation. Sep. Purif. Technol. 2022, 285, 120228. [Google Scholar] [CrossRef]
- Zhang, T.; He, Z.H.; Wang, K.P.; Wang, X.M.; Xie, Y.F.F.; Hou, L. Loose Nanofiltration Membranes for Selective Rejection of Natural Organic Matter and Mineral Salts in Drinking Water Treatment. J. Membr. Sci. 2022, 662, 120970. [Google Scholar] [CrossRef]
- Winter, J.; Barbeau, B.; Berube, P. Nanofiltration and Tight Ultrafiltration Membranes for Natural Organic Matter Removal-Contribution of Fouling and Concentration Polarization to Filtration Resistance. Membranes 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Zhao, Y.Y.; Wang, X.M.; Wen, X.H.; Huang, X.; Xie, Y.F.F. Effect of Varying Piperazine Concentration and Post-modification on Prepared Nanofiltration Membranes in Selectively Rejecting Organic Micropollutants and Salts. J. Membr. Sci. 2019, 582, 274–283. [Google Scholar] [CrossRef]
- Liu, S.; Fang, X.F.; Lou, M.M.; Qi, Y.H.; Li, R.; Chen, G.; Li, Y.L.; Liu, Y.B.; Li, F. Construction of Loose Positively Charged NF Membrane by Layer-by-Layer Grafting of Polyphenol and Polyethyleneimine on the PES/Fe Substrate for Dye/Salt Separation. Membranes 2021, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Epsztein, R.; DuChanois, R.M.; Ritt, C.L.; Noy, A.; Elimelech, M. Towards Single-species Selectivity of Membranes with Subnanometre Pores. Nat. Nanotechnol. 2020, 15, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.F.; Zhao, R.; Uliana, A.A.; Liu, Y.Y.; Zhang, D.M.; Zhang, X.; Xu, D.L.; Gao, Q.Y.; Jin, P.R.; Liu, Y.L.; et al. Separation of Textile Wastewater Using a Highly Permeable Resveratrol-based Loose Nanofiltration Membrane with Excellent Anti-fouling Performance. Chem. Eng. J. 2022, 434, 134705. [Google Scholar] [CrossRef]
- Wang, K.P.; Wang, X.M.; Januszewski, B.; Liu, Y.L.; Li, D.Y.; Fu, R.Y.; Elimelech, M.; Huang, X. Tailored Design of Nanofiltration Membranes for Water Treatment Based on Synthesis-property-performance Relationships. Chem. Soc. Rev. 2022, 51, 672–719. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dai, R.; Wen, Y.; Wang, L.; Wang, Z.; Tang, C. Recent Progress of Nanofiltration Membrane in Water Treantment and Water Reuse. Environ. Eng. 2021, 39, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhu, J.Y.; Zheng, J.F.; Gao, X.Q.; Wang, J.; Wang, X.M.; Xie, Y.F.; Huang, X.; Van der Bruggen, B. A Facile and Scalable Fabrication Procedure for Thin-Film Composite Membranes: Integration of Phase Inversion and Interfacial Polymerization. Environ. Sci. Technol. 2020, 54, 1946–1954. [Google Scholar] [CrossRef]
- Xiao, K.; Sun, J.Y.; Shen, Y.X.; Liang, S.; Liang, P.; Wang, X.M.; Huang, X. Fluorescence Properties of Dissolved Organic Matter as a Function of Hydrophobicity and Molecular Weight: Case Studies from Two Membrane Bioreactors and an Oxidation Ditch. RSC Adv. 2016, 6, 24050–24059. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation—Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Jun, B.M.; Cho, J.; Jang, A.; Chon, K.; Westerhoff, P.; Yoon, Y.; Rho, H. Charge Characteristics (surface charge vs. zeta potential) of Membrane Surfaces to Assess the Salt Rejection Behavior of Nanofiltration Membranes. Sep. Purif. Technol. 2020, 247, 117026. [Google Scholar] [CrossRef]
- Gao, X.Q.; Yu, K.C.; Wang, X.M. Rejection Behaviors and Separation Selectivity of Loose Nanofiltration Membranes for Mineral Ions in Drinking Water. Acta Sci. Circumstantiae 2020, 40, 2700–2707. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wei, W.; Wang, X.M.; Yang, H.W.; Xie, Y.F. Relating the Rejections of Oligomeric Ethylene Glycols and Saccharides by Nanofiltration: Implication for Membrane Pore Size Determination. Sep. Purif. Technol. 2018, 205, 151–158. [Google Scholar] [CrossRef]
- Kiso, Y.; Muroshige, K.; Oguchi, T.; Yamada, T.; Hhirose, M.; Ohara, T.; Shintani, T. Effect of Molecular Shape on Rejection of Uncharged Organic Compounds by Nanofiltration Membranes and on Calculated Pore Radii. J. Membr. Sci. 2010, 358, 101–113. [Google Scholar] [CrossRef]
- Zheng, F.C.; Zhang, Z.Q.; Li, C.X.; Yuan, Q.P. A Comparative Study of Suitability on Different Molecular Size Descriptors with the Consideration of Molecular Geometry in Nanofiltration. J. Membr. Sci. 2009, 332, 13–23. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.F.; Peng, X.S.; Zhang, L.; Gao, C.J. Polyamide Membranes with Nanoscale Turing Structures for Water Purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef]
- Shang, W.; Sun, F.; Jia, W.; Guo, J.; Yin, S.; Wong, P.W.; An, A.K. High-performance Nanofiltration Membrane Structured with Enhanced Stripe Nano-morphology. J. Membr. Sci. 2020, 600, 117852. [Google Scholar] [CrossRef]
- Kucera, J. Biofouling of Polyamide Membranes: Fouling Mechanisms, Current Mitigation and Cleaning Strategies, and Future Prospects. Membranes 2019, 9, 111. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Zhu, Y.Z.; Liu, C.; Lee, K.R.; Hung, W.S.; Wang, Z.Y.; Li, Y.Y.; Elimelech, M.; Jin, J.; Lin, S.H. Polyamide Nanofiltration Membrane with Highly Uniform Sub-nanometre Pores for Sub-1 Angstrom Precision Separation. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Guo, S.W.; Wan, Y.H.; Chen, X.R.; Luo, J.Q. Loose Nanofiltration Membrane Custom-tailored for Resource Recovery. Chem. Eng. J. 2021, 409, 127376. [Google Scholar] [CrossRef]
- Kim, D.; Salazar, O.R.; Nunes, S.P. Membrane Manufacture for Peptide Separation. Green Chem. 2016, 18, 5151–5159. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, J.; Dong, G.; Zhang, Y.; Guo, N.; Liu, J. Sulfonated Halloysite Nanotubes/polyethersulfone Nanocomposite Membrane for Efficient Dye Purification. Sep. Purif. Technol. 2015, 150, 243–251. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Hsub, Y.-Z.; Ruaan, R.-C.; Chuang, C.-J.; Tung, K.-L. Nanofiltration Membranes Synthesized from Hyperbranched Polyethyleneimine. J. Membr. Sci. 2009, 326, 19–26. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Y.; Sun, C.; Yu, S.; Liu, M.; Gao, C. Thin-film Composite Membranes Formed by Interfacial Polymerization with Natural Material Sericin and Trimesoyl Chloride for Nanofiltration. J. Membr. Sci. 2014, 471, 381–391. [Google Scholar] [CrossRef]
- Gu, K.; Wang, S.; Li, Y.; Zhao, X.; Zhou, Y.; Gao, C. A Facile Preparation of Positively Charged Composite Nanofiltration Membrane with High Selectivity and Permeability. J. Membr. Sci. 2019, 581, 214–223. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, M.; Chen, W.; Lü, Z.; Yu, S.; Liu, M.; Gao, C. Efficient Removal of Anionic Dye by Constructing Thin-film Composite Membrane with High Perm-selectivity and Improved Anti-dye-deposition Property. Desalination 2020, 476, 114228. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Liu, Y.; Guo, Z.; Shao, L. Nanofiltration Membrane Achieving Dual Resistance to Fouling and Chlorine for “Green” Separation of Antibiotics. J. Membr. Sci. 2015, 493, 156–166. [Google Scholar] [CrossRef]
- Dong, T.-T.; Chen, G.-H.; Gao, C.-J. Preparation of Chitin Xanthate/polyacrylonitrile NF Composite Membrane with Cross-linking Agent Hydrogen Peroxide and Its Characterization. J. Membr. Sci. 2007, 304, 33–39. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Wang, Z.X.; Zhang, Y.; Zhang, Y.; Ma, J.; Shao, L. Bio-inspired Loose Nanofiltration Membranes with Optimized Separation performance for antibiotics removals. J. Membr. Sci. 2018, 554, 385–394. [Google Scholar] [CrossRef]
- Zhu, J.; Tsehaye, M.T.; Wang, J.; Uliana, A.; Tian, M.; Yuan, S.; Li, J.; Zhang, Y.; Volodin, A.; Van der Bruggen, B. A Rapid Deposition of Polydopamine Coatings Induced by Iron (III) Chloride/hydrogen Peroxide for Loose Nanofiltration. J. Colloid Interface Sci. 2018, 523, 86–97. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Parvulescu, V.; Anastasescu, M.; Petrescu, S.; Albu, C.; Nechifor, G.; Radu, G.L. New Hybrid Nanofiltration Membranes with Enhanced Flux and Separation Performances Based on Polyphenylene Ether-Ether-Sulfone/Polyacrylonitrile/SBA-15. Membranes 2022, 12, 689. [Google Scholar] [CrossRef]
- Xu, R.; Qin, W.; Tian, Z.S.; He, Y.; Wang, X.M.; Wen, X.H. Enhanced Micropollutants Removal by Nanofiltration and Their Environmental Risks in Wastewater Reclamation: A Pilot-scale Study. Sci. Total Environ. 2020, 744, 488–496. [Google Scholar] [CrossRef] [PubMed]

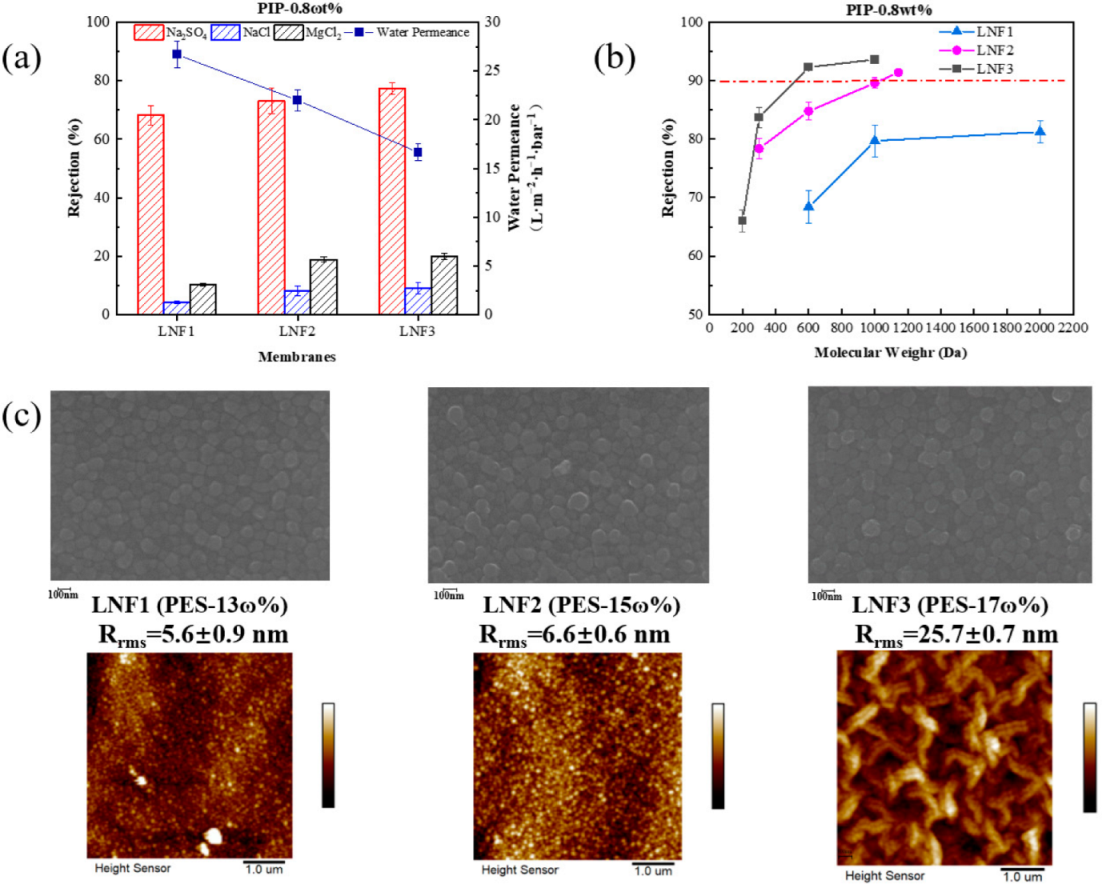
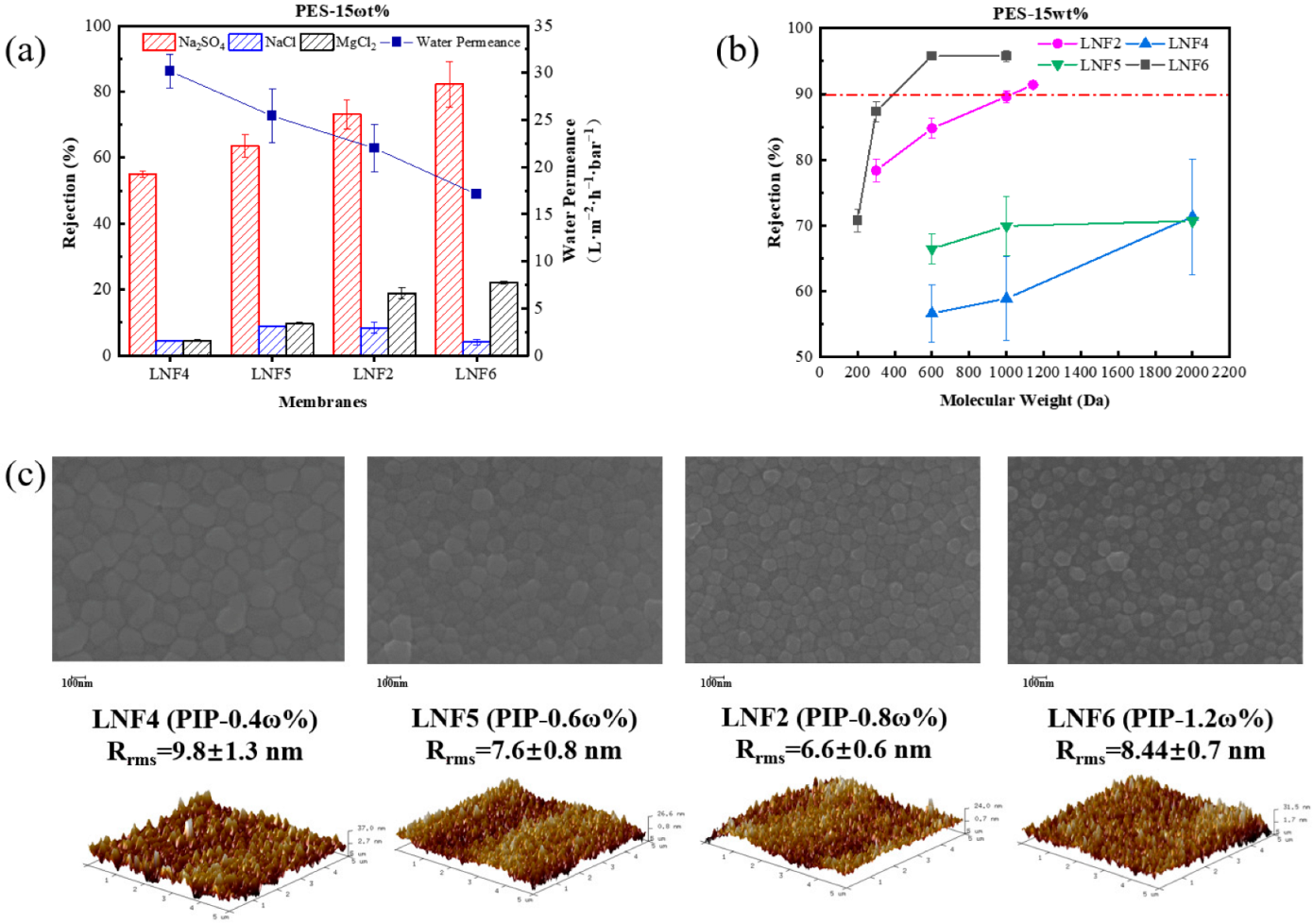
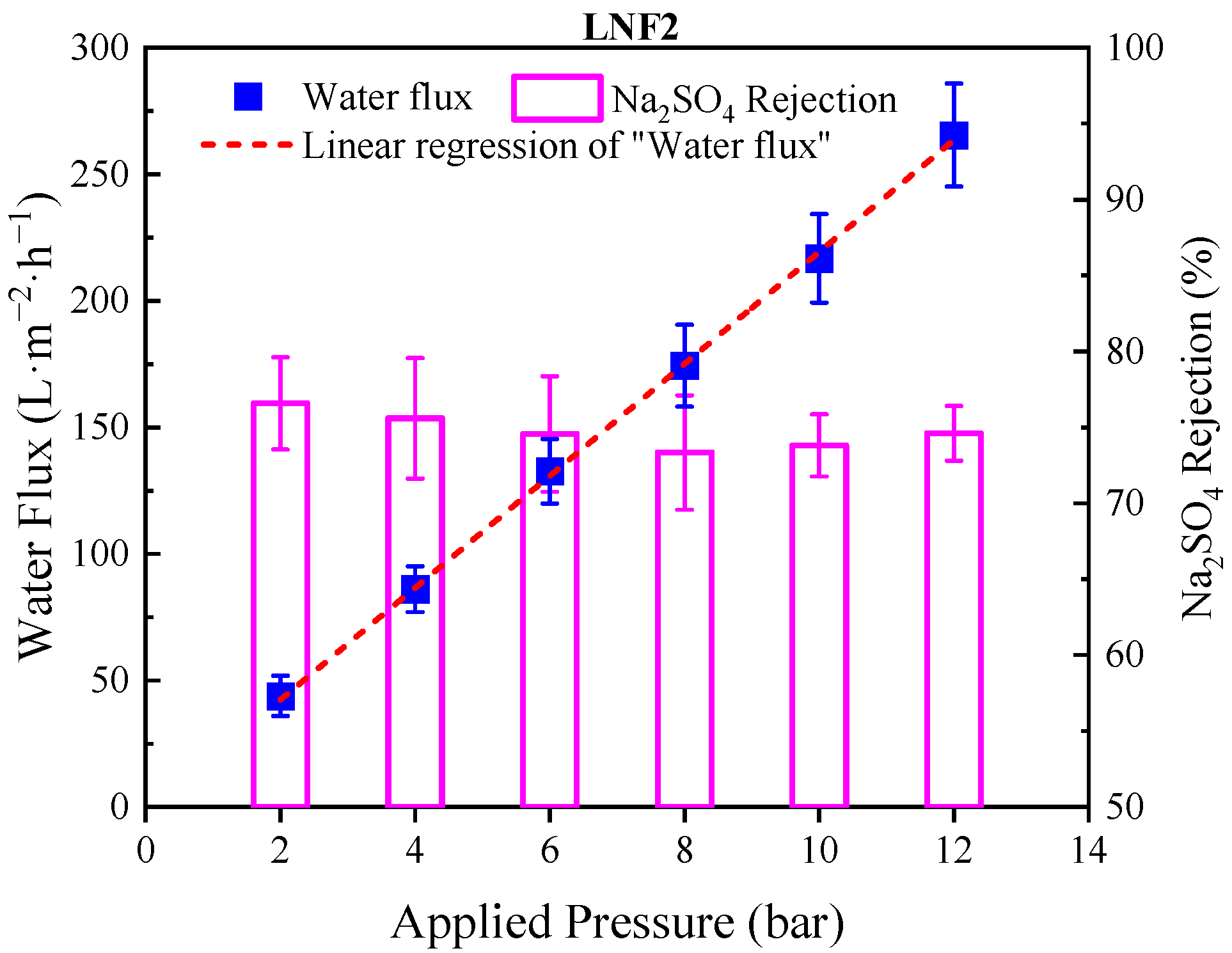
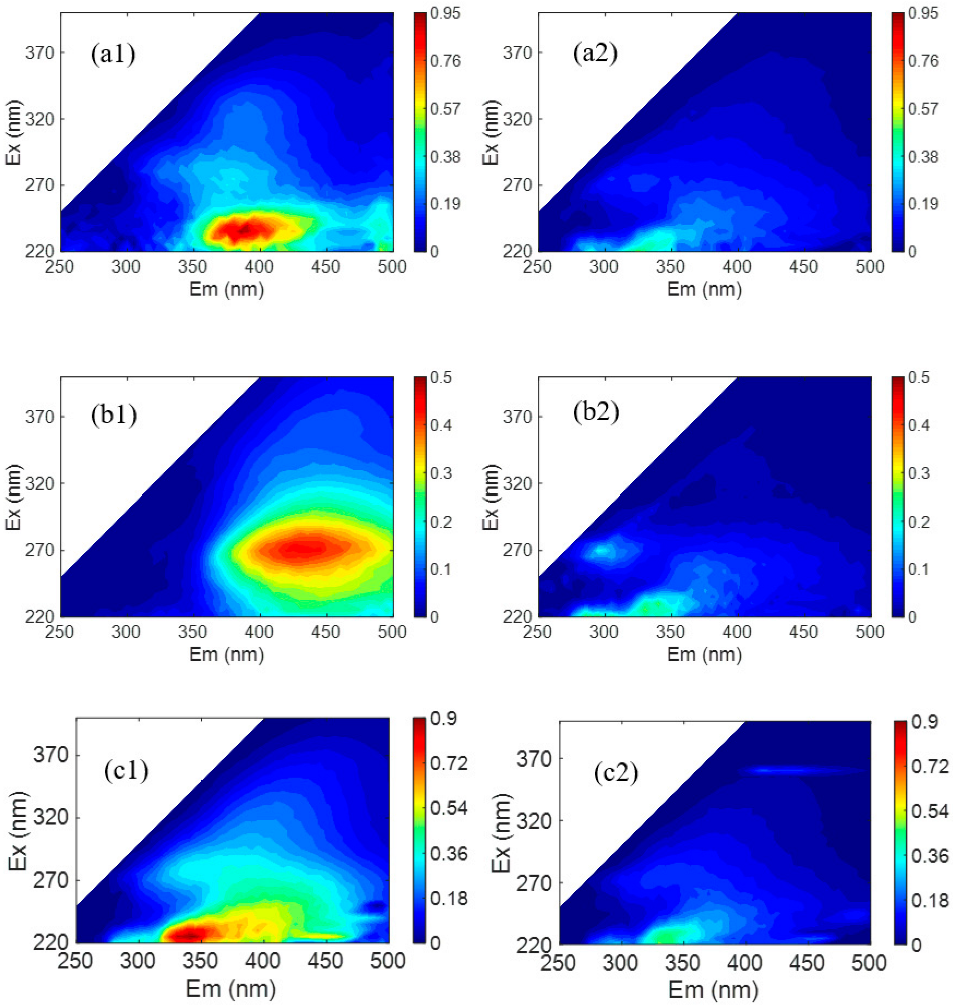
| Membrane | PES Concentration | PIP Concentration | TMC Concentration | MWCO (Da) | Water Permeance (L/m 2/h/bar) |
|---|---|---|---|---|---|
| LNF1 | 13 wt% | 0.8 wt% | 0.1 wt% | >2000 | 26.7 ± 1.3 |
| LNF2 | 15 wt% | 0.8 wt% | 0.1 wt% | 1050 | 23.0 ± 1.1 |
| LNF3 | 17 wt% | 0.8 wt% | 0.1 wt% | 530 | 16.7 ± 0.8 |
| LNF4 | 15 wt% | 0.4 wt% | 0.1 wt% | >2000 | 30.2 ± 1.8 |
| LNF5 | 15 wt% | 0.6 wt% | 0.1 wt% | >2000 | 25.4 ± 2.9 |
| LNF6 | 15 wt% | 1.2 wt% | 0.1 wt% | 400 | 17.1 ± 0.1 |
| Membrane Code | Supplier | Membrane Material | MWCO (Da) | Water Permeance (L/m2/h/bar) | References |
|---|---|---|---|---|---|
| CK | GE Osmonics | Cellulose acetate | ~2000 | 3.45 | [30] |
| GE | GE Osmonics | Polyamide | 1000 | 1.11 | [30] |
| GH | GE Osmonics | Polyamide | 2000 | 3.29 | [30] |
| GK | GE Osmonics | Polyamide | 2000 | 10.0 | [30] |
| NP010 | Microdyn Nadir | Polyether sulfone | ~1000 | >5.0 | [30] |
| TriSep SBNF | Microdyn Nadir | Cellulose acetate | 2000 | 12.0~17.7 | [30] |
| NFPES10 | Microdyn Nadir | Polyether sulfone | 1000 | 15.4 | [30] |
| Sepro NF6 | Ultura | Polyamide | 850 | 16.7 | [30] |
| NF2 * | – | Polyamide | 610 | 9.27 | [10] |
| NF3 * | – | Polyamide | 660 | 14.3 | [10] |
| NF4 * | TriSep | Polyamide | 970 | 16.9 | [10] |
| NF5 * | Synder | Polyamide | 1050 | 23.1 | [10] |
| NF6 * | – | Polyamide | 1240 | 20.5 | [10] |
| – | Lab-made | PES | 1250 | 20.0 | [31] |
| – | Lab-made | PES with sulfonated halloysite nanotubes | 706 | 18.7 | [32] |
| – | Lab-made | Diethylenetriamine-TMC | 800 | 4.5 | [33] |
| – | Lab-made | Sericin-TMC | 880 | 11.9 | [34] |
| – | Lab-made | Polyethyleneimine-Trimesic acid | 1000 | 19.1 | [35] |
| – | Lab-made | Triethanolamine-TMC | 1490 | 8.6 | [36] |
| – | Lab-made | (NH2-PEG600)- TMC | 678 | 13.2 | [37] |
| – | Lab-made | Chitin xanthate/H2O2 | 652 | 2.8~3.8 | [38] |
| – | Lab-made | Gallic acid-polyethyleneimine | 950 | 18.0 | [39] |
| – | Lab-made | Polydopamine | 1250 | 17.5 | [40] |
| – | Lab-made | Hybrid of poly(1,4-phenylene ether ether sulfone), polyacrylonitrile, poly(vinyl pyrrolidone), and SBA-15 mesoporous silica | 1000 | 1.3~13.3 | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Wang, K.; Liu, Y.; Zhang, T.; Wang, X. Fabrication of Loose Nanofiltration Membranes with High Rejection Selectivity between Natural Organic Matter and Salts for Drinking Water Treatment. Membranes 2022, 12, 887. https://doi.org/10.3390/membranes12090887
He Z, Wang K, Liu Y, Zhang T, Wang X. Fabrication of Loose Nanofiltration Membranes with High Rejection Selectivity between Natural Organic Matter and Salts for Drinking Water Treatment. Membranes. 2022; 12(9):887. https://doi.org/10.3390/membranes12090887
Chicago/Turabian StyleHe, Zhihai, Kunpeng Wang, Yanling Liu, Ting Zhang, and Xiaomao Wang. 2022. "Fabrication of Loose Nanofiltration Membranes with High Rejection Selectivity between Natural Organic Matter and Salts for Drinking Water Treatment" Membranes 12, no. 9: 887. https://doi.org/10.3390/membranes12090887






