Characteristics of a PVDF–Tin Dioxide Membrane Assisted by Electric Field Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
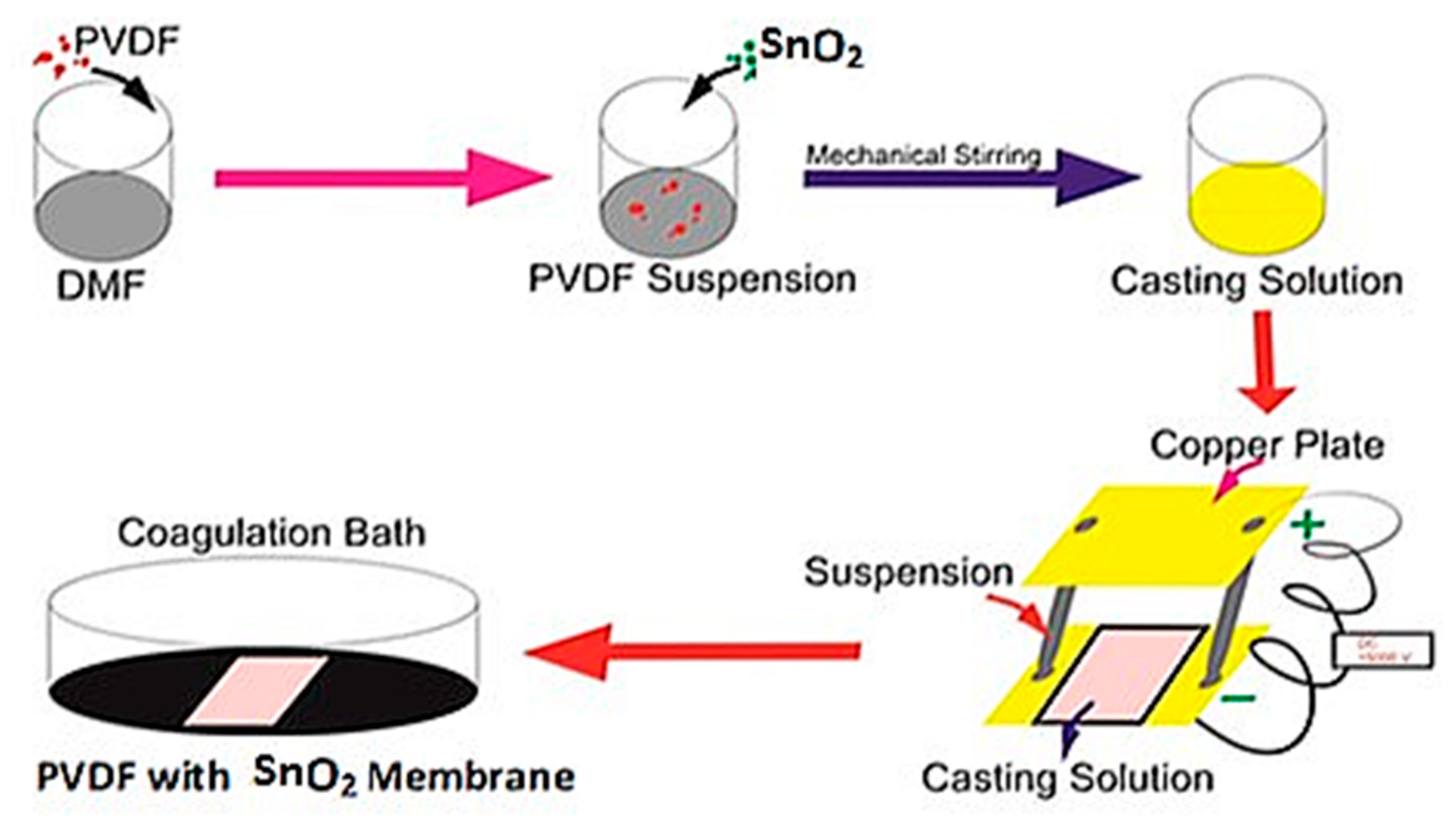
2.2. Membrane Preparation
2.3. Membrane Characterization
3. Results and Discussion
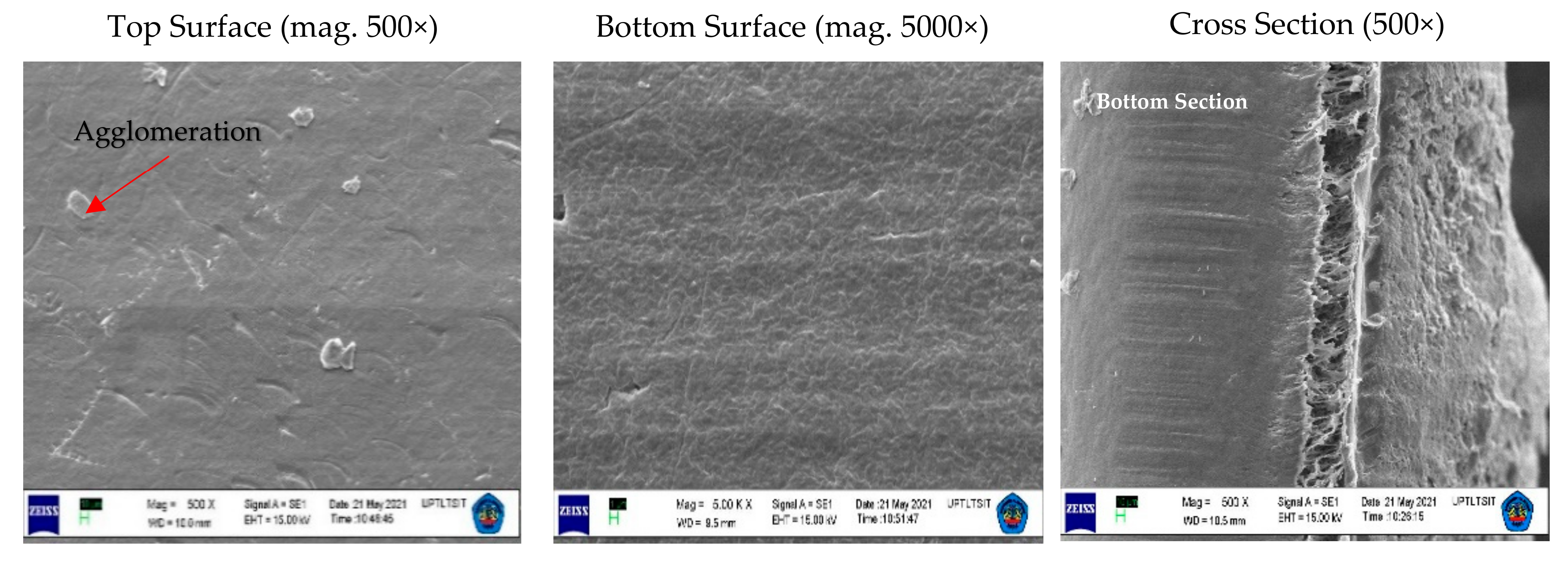
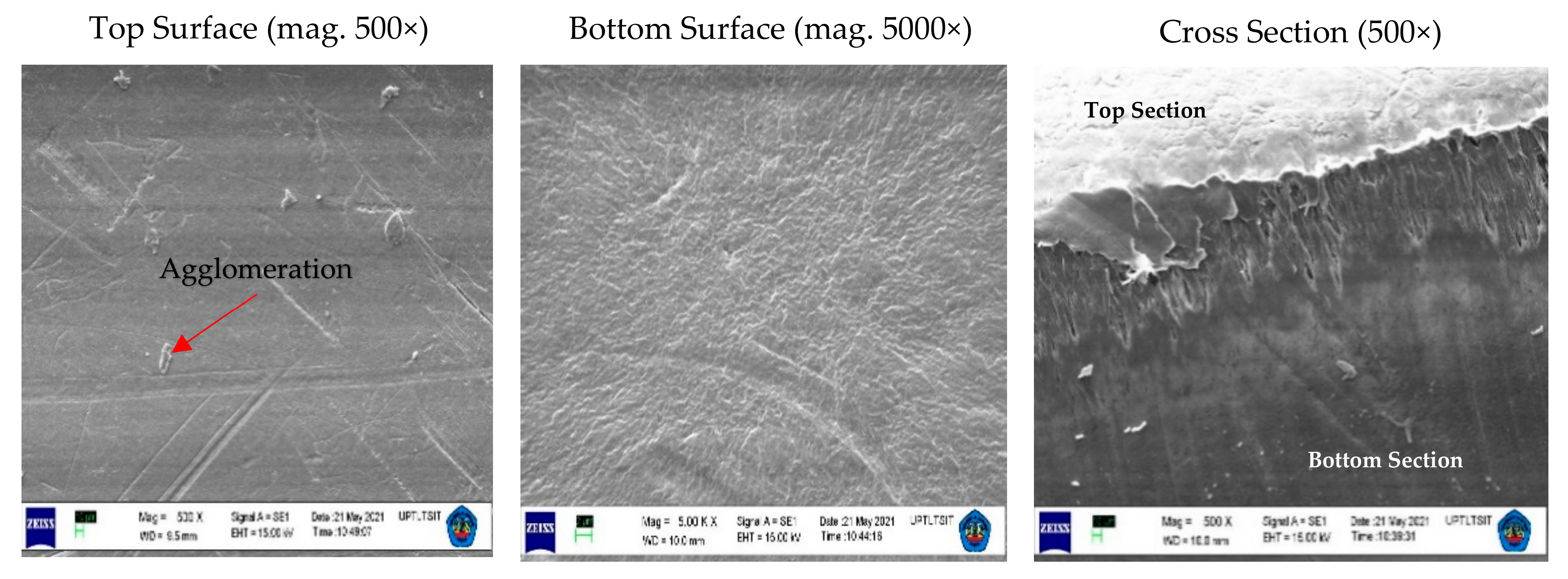
3.1. Membrane Structure
3.2. Mechanical Properties
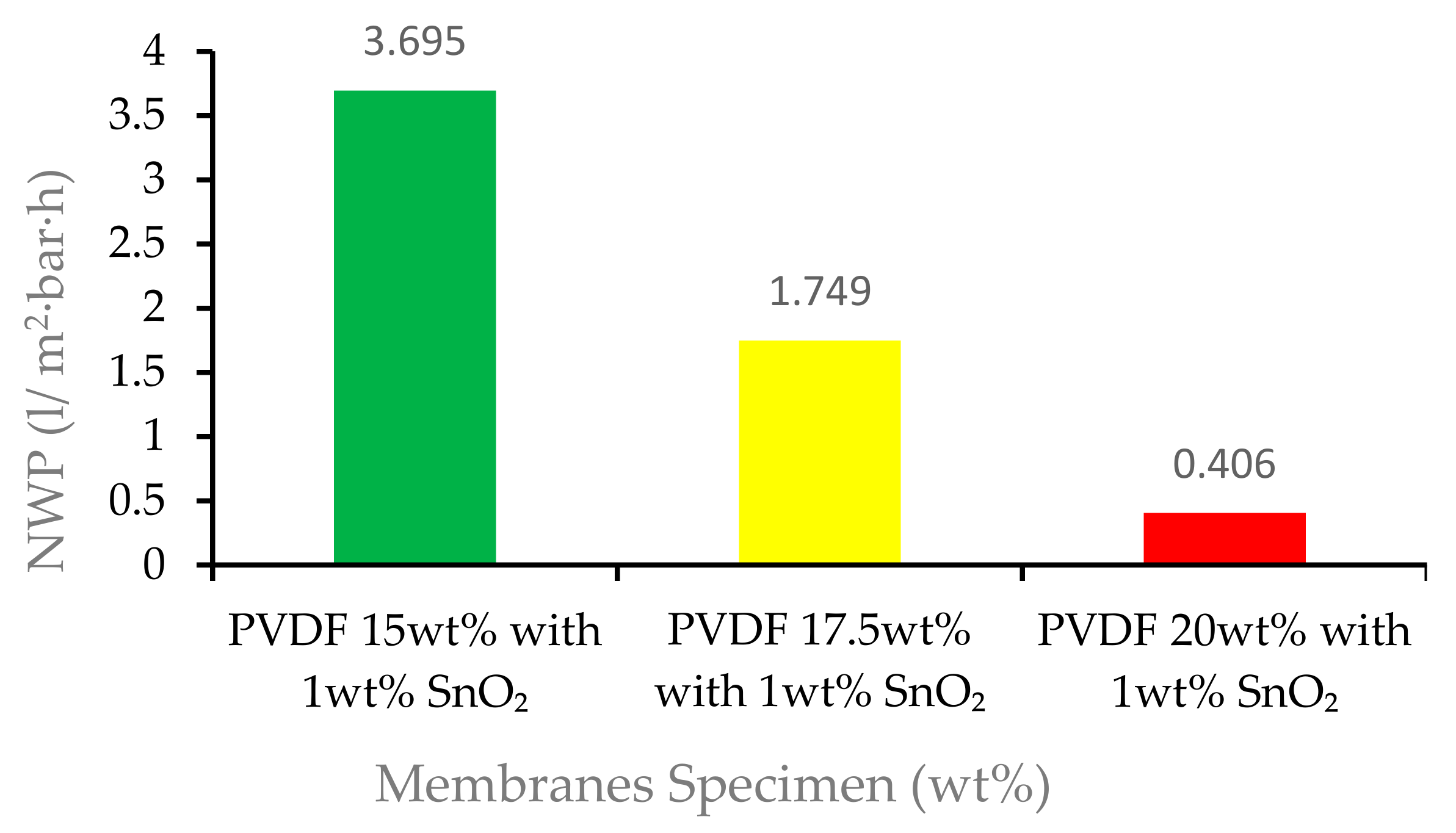
3.3. Membrane Permeability Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R. Introduction to membrane technology. In Hybrid Membrane Systems for Water; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; pp. 1–56. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Vu, M.-T.; Monsalve-Bravo, G.; Lin, R.; Li, M.; Bhatia, S.; Smart, S. Mitigating the Agglomeration of Nanofiller in a Mixed Matrix Membrane by Incorporating an Interface Agent. Membranes 2021, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Costantino, F.; Armirotti, A.; Carzino, R.; Gavioli, L.; Athanassiou, A.; Fragouli, D. In situ formation of SnO2 nanoparticles on cellulose acetate fibrous membranes for the photocatalytic degradation of organic dyes. J. Photochem. Photobiol. A Chem. 2020, 398, 112599. [Google Scholar] [CrossRef]
- Staude, E. Industrial membrane separation technology. J. Membr. Sci. 1997, 130, 285–286. [Google Scholar] [CrossRef]
- Zare, S.; Kargari, A. Membrane Properties in Membrane Distillation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–156. [Google Scholar] [CrossRef]
- Langhe, D.; Ponting, M. Gas Transport, Mechanical, Interphase, and Interdiffusion Properties in Coextruded-Multilayered Films; Elsevier: Amsterdam, The Netherlands, 2016; pp. 46–116. [Google Scholar] [CrossRef]
- Nagy, E. Membrane Materials, Characterization, and Transport Properties. In Basic Equations of Mass Transport Through a Membrane Layer; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–10. [Google Scholar] [CrossRef]
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. Introduction to Membranes: Filtration for water and wastewater treatment. Filtr. Sep. 2018, 25, 24–27. [Google Scholar]
- Zhang, L.; Shu, Z.; Yang, N.; Wang, B.; Dou, H.; Zhang, N. Improvement in antifouling and separation performance of PVDF hybrid membrane by incorporation of room-temperature ionic liquids grafted halloysite nanotubes for oil-water separation. J. Appl. Polym. Sci. 2018, 135, 46278. [Google Scholar] [CrossRef]
- Suryandari, E.T. Sintesis Membran Komposit PVDF-Zeolit untuk Penghilangan Metilen Biru. al-Kimiya 2020, 6, 58–66. [Google Scholar] [CrossRef]
- Mertens, M.; Van Dyck, T.; Van Goethem, C.; Gebreyohannes, A.; Vankelecom, I.F. Development of a polyvinylidene difluoride membrane for nanofiltration. J. Membr. Sci. 2018, 557, 24–29. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Naddeo, V.; Banat, F.; Hasan, S.W. Preparation of novel polyvinylidene fluoride (PVDF)-Tin(IV) oxide (SnO2) ion exchange mixed matrix membranes for the removal of heavy metals from aqueous solutions. Sep. Purif. Technol. 2020, 250, 117250. [Google Scholar] [CrossRef]
- Neves, D.C.; da Silva, A.L.; Romano, R.C.D.O.; Gouvêa, D. Fe2O3-doped SnO2 membranes with enhanced mechanical resistance for ultrafiltration application. J. Eur. Ceram. Soc. 2020, 40, 5959–5966. [Google Scholar] [CrossRef]
- Ranjani, M.; Al-Sehemi, A.G.; Pannipara, M.; Aziz, A.; Phang, S.-M.; Ng, F.-L.; Kumar, G.G. SnO2 nanocubes/bentonite modified SPEEK nanocomposite composite membrane for high performance and durable direct methanol fuel cells. Solid State Ion. 2020, 353, 115318. [Google Scholar] [CrossRef]
- Dou, P.; Song, J.; Zhao, S.; Xu, S.; Li, X.; He, T. Novel low cost hybrid extraction-distillation-reverse osmosis process for complete removal of N,N-dimethylformamide from industrial wastewater. Process. Saf. Environ. Prot. 2019, 130, 317–325. [Google Scholar] [CrossRef]
- Mataram, A.; Septano, G.D. Pengujian Tarik Dan Struktrur Mikro Membran Polyvinylidene Fluoride; Sriwijya University: Palembang, Indonesia, 2017; pp. 1–6. [Google Scholar]
- Mataram, A.; Rizal, S.; Pujiono, E. Physical and mechanical properties of membrane polyvinilidene flouride with the addition of silver nitrate. MATEC Web Conf. 2018, 156, 08014. [Google Scholar] [CrossRef] [Green Version]
- Mazumder, A.; Chowdhury, Z.; Sen, D.; Bhattacharjee, C. Electric field assisted membrane separation for oily wastewater with a novel and cost-effective electrocoagulation and electroflotation enhanced membrane module (ECEFMM). Chem. Eng. Process. Process. Intensif. 2020, 151, 107918. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, J.; Wang, Y.; Zhang, J. Electric field processing to control the structure of titanium oxide/sulfonated poly (ether ether ketone) hybrid proton exchange membranes. J. Membr. Sci. 2013, 437, 65–71. [Google Scholar] [CrossRef]
- Wang, X.; Feng, M.; Liu, Y.; Deng, H.; Lu, J. Fabrication of graphene oxide blended polyethersulfone membranes via phase inversion assisted by electric field for improved separation and antifouling performance. J. Membr. Sci. 2019, 577, 41–50. [Google Scholar] [CrossRef]
- Febriasari, A.; Purnawan, I.; Chalid, M.; Ismojo, I.; Kartohardjono, S. A Direct Comparison between Poly(vinylidene) Flouride and Polysulfone Flat Sheet Membrane; Characterization and Mechanical Strength. IOP Conf. Ser. Earth Environ. Sci. 2020, 442, 012002. [Google Scholar] [CrossRef]
- Rosato, D.; Rosato, D.V. Design Parameter. In Plastics Engineered Product Design; Elsevier: Amsterdam, The Netherlands, 2003; pp. 161–197. [Google Scholar] [CrossRef]
- Lee, K.-J.; Park, H.-D. The most densified vertically-aligned carbon nanotube membranes and their normalized water permeability and high pressure durability. J. Membr. Sci. 2016, 501, 144–151. [Google Scholar] [CrossRef]
- Cyganowski, J.; Lutz, H. Post-processing. In Ultrafiltration for Bioprocessing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 131–149. [Google Scholar] [CrossRef]
- Yang, F.; Ding, G.; Wang, J.; Liang, Z.; Gao, B.; Dou, M.; Xu, C.; Li, S. Self-cleaning, antimicrobial, and antifouling membrane via integrating mesoporous graphitic carbon nitride into polyvinylidene fluoride. J. Membr. Sci. 2020, 606, 118146. [Google Scholar] [CrossRef]
- Ali, I.; Bamaga, O.A.; Gzara, L.; Bassyouni, M.; Abdel-Aziz, M.H.; Soliman, M.F.; Drioli, E.; Albeirutty, M. Assessment of Blend PVDF Membranes, and the Effect of Polymer Concentration and Blend Composition. Membranes 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.I. Gas Sweetening; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zhou, L.; Yang, S.-Y.; Chen, W.; Wang, X.-B.; Yuan, Z.-L.; Yang, L.-J.; Wu, H. Chemical agglomeration properties of fine particles immersed in solutions and the reduction in fine particle emission by adding emulsion polymers. Fuel Process. Technol. 2018, 175, 44–53. [Google Scholar] [CrossRef]
- Sayago, I.; Hontañón, E.; Aleixandre, M. Preparation of Tin Oxide Nanostructures by Chemical Vapor Deposition; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–280. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics (D 638-02a)—SCAN VERSION. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2003; Volume 8, pp. 46–58. [CrossRef]
- Wang, K.; Abdala, A.A.; Hilal, N.; Khraisheh, M.K. Membranes; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Zhao, B.; Hu, J.; Chen, W.; Chen, J.; Jing, Z. A nonlinear uniaxial stress-strain constitutive model for viscoelastic membrane materials. Polym. Test. 2020, 90, 106633. [Google Scholar] [CrossRef]
- Ligia, G.; Deodato, R. Physicochemical Behavior and Supramolecular Organization of Polymers; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Arahman, N.; Arifin, B.; Razi, F. Profil Permeabilitas Berdasarkan Struktur Morfologi Membran Polietersulfon pada Pemekatan Larutan Tokoferol. Agritech 2016, 36, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Singh, R. Introduction to Membrane Technology. In Membrane Technology and Engineering for Water Purification; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–80. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, Y.; Wang, R.; Chen, D. Poly(vinylidene fluoride) grafted polystyrene (PVDF-g-PS) membrane based on in situ polymerization for solvent resistant nanofiltration. RSC Adv. 2017, 7, 33201–33207. [Google Scholar] [CrossRef] [Green Version]
- Ariono, D.; Aryanti, P.T.P.; Subagjo, S.; Wenten, I.G. The Effect of Polymer Concentration on Flux Stability of Polysulfone Membrane; American Institute of Physics: College Park, MD, USA, 2017; Volume 1788. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.A.; Felgueiras, H.P.; De Amorim, M.T.P. Carbon based membranes with modified properties: Thermal, morphological, mechanical and antimicrobial. Cellulose 2019, 27, 1497–1516. [Google Scholar] [CrossRef]


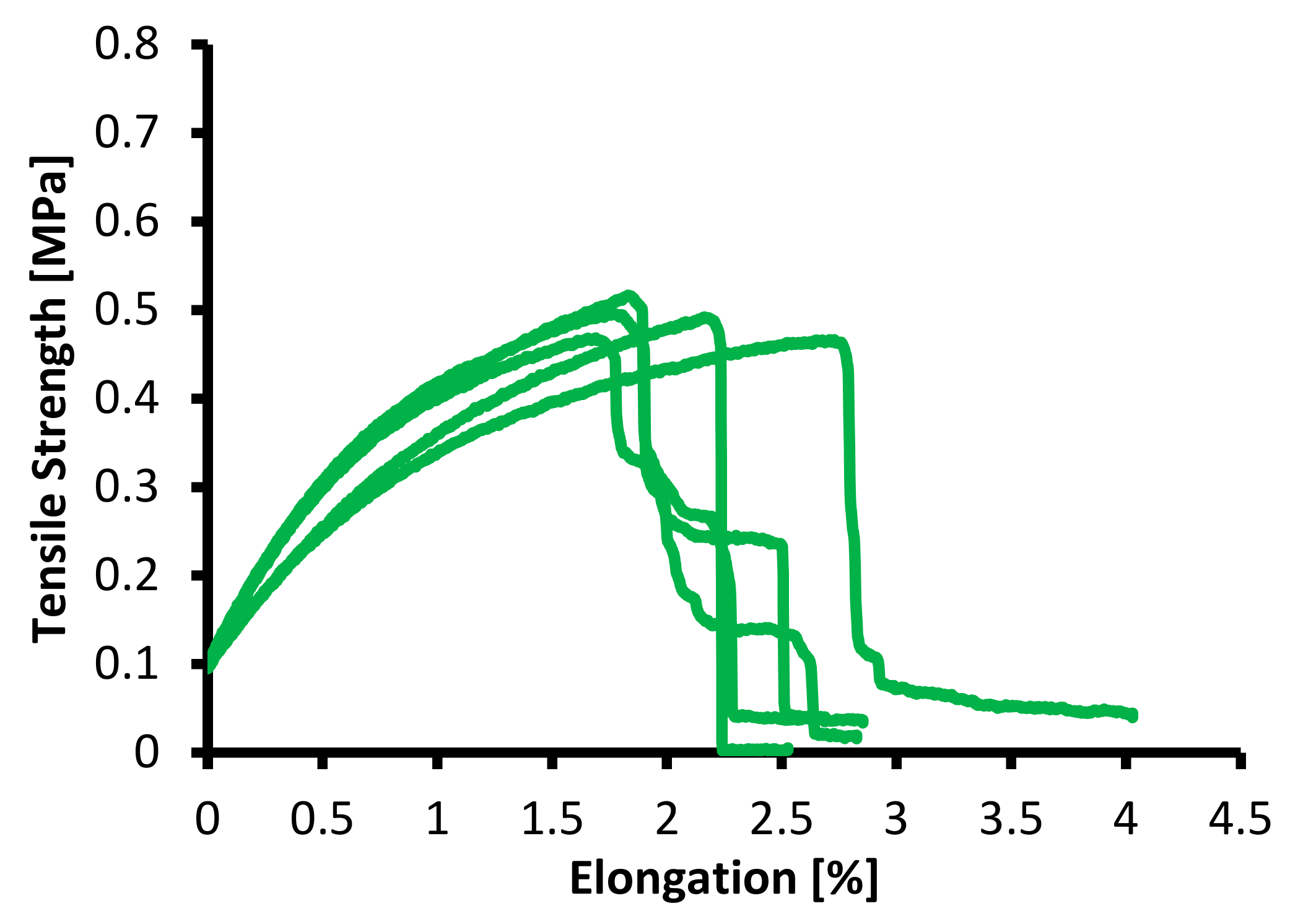


| Membrane Specimen | Samples | Yield Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| 15 wt% PVDF with 1% tin dioxide | A1 | 0.468 | 1.68 |
| A2 | 0.496 | 1.76 | |
| A3 | 0.492 | 2.16 | |
| A4 | 0.466 | 2.70 | |
| A5 | 0.516 | 1.83 | |
| Average | 0.488 | 2.03 | |
| Median | 0.494 | 1.99 | |
| Range | 0.050 | 1.02 | |
| Standard Deviation | 0.0209 | 0.4187 | |
| 17.5 wt% PVDF with 1% tin dioxide | B1 | 0.657 | 1.97 |
| B2 | 0.693 | 1.74 | |
| B3 | 0.724 | 2.12 | |
| B4 | 0.578 | 2.43 | |
| B5 | 0.594 | 2.28 | |
| Average | 0.649 | 2.11 | |
| Median | 0.657 | 2.12 | |
| Range | 0.146 | 0.69 | |
| Standard Deviation | 0.062 | 0.418 | |
| 20 wt% PVDF with 1% tin dioxide | C1 | 0.714 | 3.57 |
| C2 | 0.745 | 3.57 | |
| C3 | 0.779 | 3.61 | |
| C4 | 0.757 | 3.82 | |
| C5 | 0.735 | 4.17 | |
| Average | 0.746 | 3.75 | |
| Median | 0.745 | 3.61 | |
| Range | 0.065 | 0.60 | |
| Standard Deviation | 0.024 | 0.25 | |
| Membrane Specimen | Samples (n) | NWP (L/m2·h·bar) |
|---|---|---|
| 15 wt% PVDF with 1% tin dioxide | 1 | 3.837 |
| 2 | 4.030 | |
| 3 | 3.942 | |
| 4 | 3.713 | |
| 5 | 2.953 | |
| Average | 3.695 | |
| Median | 3.837 | |
| Range | 1.077 | |
| Standard Deviation | 0.431 | |
| 17.5 wt% PVDF with 1% tin dioxide | 1 | 1.850 |
| 2 | 1.719 | |
| 3 | 1.652 | |
| 4 | 1.690 | |
| 5 | 1.835 | |
| Average | 1.7492 | |
| Median | 1.7190 | |
| Range | 0.1980 | |
| Standard Deviation | 0.0886 | |
| 20 wt% PVDF with 1% tin dioxide | 1 | 0.402 |
| 2 | 0.410 | |
| 3 | 0.405 | |
| 4 | 0.407 | |
| 5 | 0.405 | |
| Average | 0.406 | |
| Median | 0.405 | |
| Range | 0.0079 | |
| Standard Deviation | 0.0029 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasution, M.S.; Mataram, A.; Yani, I.; Septano, G.D. Characteristics of a PVDF–Tin Dioxide Membrane Assisted by Electric Field Treatment. Membranes 2022, 12, 772. https://doi.org/10.3390/membranes12080772
Nasution MS, Mataram A, Yani I, Septano GD. Characteristics of a PVDF–Tin Dioxide Membrane Assisted by Electric Field Treatment. Membranes. 2022; 12(8):772. https://doi.org/10.3390/membranes12080772
Chicago/Turabian StyleNasution, Muhammad Syahrul, Agung Mataram, Irsyadi Yani, and Gurruh Dwi Septano. 2022. "Characteristics of a PVDF–Tin Dioxide Membrane Assisted by Electric Field Treatment" Membranes 12, no. 8: 772. https://doi.org/10.3390/membranes12080772






