Roles of Sodium Hydrogen Exchanger (NHE1) and Anion Exchanger (AE2) across Chondrocytes Plasma Membrane during Longitudinal Bone Growth
Abstract
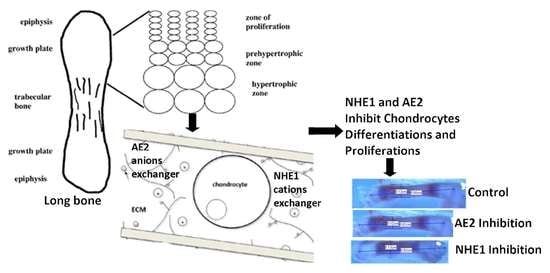
:1. Introduction
2. Materials and Methods
2.1. Biochemical Solutions Used
2.2. Animal Preparationand Grouping
2.3. Tibia and Metatarsal Growth Length and Velocity Measurements
2.4. Preparation of Growth Plate (GP) for Histology
2.5. Quantitative Histology
2.6. Tissue Immunohistochemistry
2.7. Data Analysis
3. Results
3.1. Effects of the Membrane Inhibitors on the Whole Bone Length and Growth Rate and Velocity
3.2. Growth Inhibitory Effects on GP Length, HCZ Length, and Chondrocyte Density
3.3. Immunoperoxidase and Immunofluorescence Labeling of NHE1 and AE2 in Tibial GP
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.L.; Oh, S.; Sung, Y.; Dasari, R.R.; Marc, W.; Tabin, C.J. Multiple phases of chondrocytes enlargement underlie differences in Skeletal Proportions. Nature 2013, 495, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Staines, K.A.; Pollard, A.S.; McGonnell, I.M.; Farquharson, C.; Pitsillides, A.A. Cartilage to bone transitions in health and disease. J. Endocrinol. 2013, 219, R1–R12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, F.; Chung, U.; Ohba, S.; McMahon, J.; Kronenberg, H.M.; McMahon, A.P. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development 2004, 131, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Emons, J.; Chagin, A.S.; Hultenby, K.; Zhivotovsky, B.; Wit, J.M.; Karperien, M.S.; Vendahl, L. Epiphyseal fusion in the human growth plate does not involve classical apoptosis. Pediatr. Res. 2009, 66, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.S.; Shapiro, I.M. The Fate of the Terminally Differentiated Chondrocyte: Evidence for Microenvironmental Regulation of Chondrocyte Apoptosis. Crit. Rev. Oral Biol. Med. 2002, 13, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Joeng, K.S.; Long, F. Wnt7b can replace Ihh to induce hypertrophic cartilage vascularization but not osteoblast differentiation during endochondral bone development. Bone Res. 2014, 2, 14004. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Naznin, A.; Hossen, M.J.; Rajat, S.B.; Sikder, M.A. TGF-β/BMP Signaling and Other Molecular Events: Regulation of Osteoblastogenesis and Bone Formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [Green Version]
- Rauch, F. Bone growth in length and width: The Yin and Yang of bone stability. J. Musculoskelet. Neuronal Interact. 2005, 5, 194–201. [Google Scholar]
- Loqman, M.Y.; Bush, P.G.; Farquharson, C.; Hall, A.C. Suppression of mammalian bone growth by membrane transport inhibitors. J. Cell Biochem. 2013, 114, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, A.A.; Noordin, M.M.; Azmi, T.I.; Kaka, U.; Loqman, M.Y. The use of rats and mice as animal models in ex vivo bone growth and development studies. Bone Jt. Res. 2016, 5, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Iglesias, A.; Fuente, R.; Gil-Peña, H.; Alonso-Duran, L.; García-Bengoa, M.; Santos, F.; López, J.M. A simple method based on confocalmicroscopy and thick sectionsrecognizes seven subphases ingrowth plate chondrocytes. Sci. Rep. 2020, 10, 6935. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J.; Tatarczuch, L.; Mirams, M. The skeleton: A multi-functional complex organ. The growth plate chondrocyte and endochondral ossification. J. Endocrinol. 2011, 211, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Baron, J. Fundamental limits on longitudinal bone growth: Growth plate senescence and epiphyseal fusion. Trends Endocrinol. Metabs. 2004, 15, 370–374. [Google Scholar] [CrossRef]
- Amini, S.; Veilleux, D.; Villemure, I. Three-dimensional in situ zonal morphology of viable growth plate chondrocytes: A confocal microscopy study. J. Orthop. Res. 2011, 29, 710–717. [Google Scholar] [CrossRef]
- Chagin, A.S.; Karimian, E.; Sundström, K.; Eriksson, E.; Sävendahl, L. Catch-up growth after dexamethasone withdrawal occurs in cultured postnatal rat metatarsal bones. J. Endocrinol. 2010, 204, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, I.M.; Adams, C.S.; Freeman, T.; Srinivas, V. Fate of the hypertrophic chondrocyte: Microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res. Part CEmbryo Today Rev. 2005, 75, 330–339. [Google Scholar] [CrossRef]
- Caramés, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.J.; Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. ArthritisRheum 2010, 62, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Bush, P.G.; Pritchard, M.; Loqman, M.Y.; Damron, T.A.; Hall, A.C. A key role for membrane transporter NKCC1 in mediating chondrocyte volume increase in the mammalian growth plate. J. Bone Miner. Res. 2010, 25, 1594–1603. [Google Scholar] [CrossRef] [Green Version]
- Malo, M.E.; Fliegel, L. Physiological role and regulation of the Na+/H+ exchanger. Can. J. Physiol. Pharmacol. 2006, 84, 1081–1095. [Google Scholar] [CrossRef] [Green Version]
- Coppini, R.; Ferrantini, C.; Mazzoni, L.; Sartiani, L.; Olivotto, I.; Poggesi, C.; Mugelli, A. Regulation of intracellular Na 1 in health and disease: Pathophysiological mechanisms and implications for treatment. Glob. Cardiol. Sci. Pract. 2013, 3, 222–242. [Google Scholar] [CrossRef] [PubMed]
- Alka, K.; Casey, J.R. Bicarbonate transport in health and disease. IUBMB Life 2014, 66, 596–615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wiederkehr, M.R.; Fan, L.; Collazo, R.L.; Crowder, L.A.; Moe, O.W. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase A and NHE-3 phosphoserines 552 and 605. J. Biol. 1999, 274, 3978–3987. [Google Scholar] [CrossRef] [Green Version]
- Medina, J.F.; Recalde, S.; Prieto, J.; Lecanda, J.; Saez, E.; Funk, C.D.; Vecino, P.; van Roon, M.A.; Ottenhoff, R.; Bosma, P.J.; et al. Anion exchanger 2 is essential for spermiogenesis in mice. Proc. Natl. Acad. Sci. USA 2002, 100, 15847–15852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.J.; Hwang, J.M.; Wu, T.T.; Hsieh, Y.H.; Wu, C.C.; Hsieh, Y.S.; Liu, J.Y. Anion exchanger inhibitor DIDS induces human poorly-differentiated malignant hepatocellular carcinoma HA22T cell apoptosis. Mol. Cell Biochem. 2008, 308, 117–125. [Google Scholar] [CrossRef]
- Hwang, J.M.M.; Kao, S.H.H.; Hsieh, Y.H.H.; Li, K.L.L.; Wang, P.H.H.; Hsu, L.S.S.; Liu, J.Y.Y. Reduction of anion exchanger 2 expression induces apoptosis of human hepatocellular carcinoma cells. Mol. Cell Biochem. 2009, 327, 135–144. [Google Scholar] [CrossRef]
- Fliegel, L. Regulation of the Na+/H+ exchanger in the healthy and diseased myocardium. Expert Opin. Ther. Targets 2009, 13, 55–68. [Google Scholar] [CrossRef]
- Yu, L.; Hales, C.A. Silencing of sodium-hydrogen exchanger 1 attenuates the proliferation, hypertrophy, and migration of pulmonary artery smooth muscle cells via E2F1. Am. J. Respir. Cell Mol. Biol. 2011, 45, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Hosogi, S.; Miyazaki, H.; Nakajima, K.I.; Ashihara, E.; Niisato, N.; Kusuzaki, K.; Marunaka, Y. An inhibitor of Na+/H+ exchanger (NHE), ethyl-isopropyl amiloride (EIPA), diminishes proliferation of MKN28 human gastric cancer cells by decreasing the cytosolic Cl-concentration via DIDS-sensitive pathways. Cell PhysiolBiochem. 2012, 30, 1241–1253. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Burghardt, B.; Best, L.; Steward, M.C. Role of anion exchangers in Cl− and HCO3− secretion by the human airway epithelial cell line Calu-3. Am. J. Physiol. Cell Physiol. 2014, 307, C208–C219. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, E.C.; Debnath, S.; Gundry, S.; Gundry, S.; Uyar, U.; Fata, J.E. Intracellular pH regulation by Na+/H+ exchanger-1 (NHE1) is required for growth factor-induced mammary branching morphogenesis. Dev. Biol. 2012, 365, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, A.R.; Lopez, M.; Ardura-Fabregat, A.; Medina, J.F. Role of AE2 for pHi regulation in biliary epithelial cells. Front. Physiol. 2014, 4, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valles, P.G.; Bocanegra, V.; Gil-Lorenzo, A.; Costantino, V.V. Physiological Functions and Regulation of the Na+/H+ Exchanger [NHE1] in Renal Tubule Epithelial Cells. Kidney Blood Press. Res. 2015, 40, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.A.; Ibrahim, S.M.; Ali, A.K.; Handool, K.O.; Khan, S.M.; Noordin, M.M.; Ibrahim, T.A.; Kaka, U.; Loqman, M.Y. Postnatal ex vivo rat model for longitudinal bone growth investigations. Anim. Models Exp. Med. 2019, 2, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mårtensson, K.; Chrysis, D.; Sävendahl, S. Interleukin-1β and TNF-α Act in Synergy to Inhibit Longitudinal Growth in Fetal Rat Metatarsal Bones. J. Bone Miner. Res. 2004, 19, 1805–1812. [Google Scholar] [CrossRef]
- Loqman, M.Y.; Bush, P.G.; Farquharson, C.; Hall, A.C. A cell shrinkage artefact in growth plate chondrocytes with common fixative solutions: Importance of fixative osmolarity for maintaining morphology. Eur. Cells Mater. 2010, 19, 214–224. [Google Scholar] [CrossRef]
- Dettmeyer, R.B. Staining techniques and microscopy. In Forensic Histopathology; Springer: Cham, Switzerland, 2018; pp. 17–45. [Google Scholar] [CrossRef]
- Pastoureau, P.C.; Hunziker, E.B.; Pelletier, J.P. Cartilage, bone and synovial histomorphometry in animal models of osteoarthritis. Osteoarthr. Cartil. 2010, 18, S106–S112. [Google Scholar] [CrossRef] [Green Version]
- Wilsman, N.J.; Bernardini, E.S.; Leiferman, E.; Noonan, K.; Farnum, C.E. Age and pattern of the onset of differential growth among growth plates in rats. J. Orthop. Res. 2008, 26, 1457–1465. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.M.G.; Beier, F. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth Defects Res. C Embryo 2014, 102, 74–82. [Google Scholar] [CrossRef]
- Renshaw, S. Immunochemical staining techniques. In Immunohistochemistry: Methods Express; Scion Publishing Ltd.: Bloxham, UK, 2007; pp. 45–96. [Google Scholar]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Karmazyn, M.; Sawyer, M.; Fliegel, L. The Na+/H+ Exchanger: A Target for Cardiac Therapeutic Intervention. CardiovasHematol. Disord. Drug Targets 2005, 5, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Kidd, J.F.; Thorn, P. Intracellular Ca2+ and Cl− channel activation in secretory cells. Annu. Rev. Physiol. 2000, 62, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H. New light on the “old” chloride channel blocker DIDS. ACS Chem. Biol. 2008, 3, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Rangel, E.; López-Méndez, M.; García, L.; Guerrero-Hernández, A. DIDS (4,4′-Diisothiocyanatostilbene-2,2′-disulfonate) directly inhibits caspase activity in HeLa cell lysates. Cell Death Discov. 2014, 1, 15037. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.; Feetham, C.H.; Barrett-jolley, R. Cell Volume Regulation in Chondrocytes. Cell Physiol.Biochem. 2011, 28, 1111–1122. [Google Scholar] [CrossRef]






| Treatments | Metatarsal Growth Rate (%) | Tibial Growth Rate (%) |
|---|---|---|
| DMSO control | 18.12 ±1.93 a | 15.63 ± 5.54 a |
| EIPA (444 µM) | 5.50 ± 0.96 b | 4.56 ± 0.755 b |
| KHCO3 control | 16.34 ± 1.49 b | 16.13 ± 3.85 b |
| DIDS (250 µM) | 7.43 ± 1.41 a | 7.52 ± 1.15 a |
| Treatments | Total GP Length (µm) | HCZ Length (µm) | HCZ (% of Total) |
|---|---|---|---|
| DMSO control (n = 5) | 632 ± 33 b | 143 ± 5 ab | 24.33 ± 1.75 a |
| EIPA (444 µM; n = 5) | 516 ± 30 ab | 129 ± 4 b | 23.08 ± 1.32 a |
| KHCO3 control (n = 5) | 603 ± 33 ab | 137 ± 6 ab | 23.95 ± 0.94 a |
| DIDS (250 µM; n = 5) | 510 ± 29 a | 121 ± 5 a | 23.21 ± 1.50 a |
| Treatments | Total GP Chondrocyte Densities (Cells/mm2) | HCZ Chondrocyte Densities (Cells/mm2) |
|---|---|---|
| DMSO control (n = 5) | 2409 ± 21 b | 1122 ± 16 b |
| EIPA (444 µM; n = 5) | 1609 ± 40 a | 742 ± 18 a |
| KHCO3 control (n = 5) | 2500 ± 22 a | 1151 ± 13 a |
| DIDS (250 µM; n = 5) | 1656 ± 33 c | 789 ± 10 b |
| Treatments | Number of Slides Viewed (n) | Median IP Scores | p Values |
|---|---|---|---|
| DMSO; (n = 4) | 12 | 4.0 | 0.001 |
| EIPA (444 µM); (n = 4) | 12 | 1.0 | |
| KHCO3; (n = 4) | 12 | 3.5 | 0.001 |
| DIDS (250 µM); (n = 4) | 12 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abubakar, A.A.; Ali, A.K.; Ibrahim, S.M.; Handool, K.O.; Khan, M.S.; Mustapha, N.M.; Ibrahim, T.A.T.; Kaka, U.; Yusof, L.M. Roles of Sodium Hydrogen Exchanger (NHE1) and Anion Exchanger (AE2) across Chondrocytes Plasma Membrane during Longitudinal Bone Growth. Membranes 2022, 12, 707. https://doi.org/10.3390/membranes12070707
Abubakar AA, Ali AK, Ibrahim SM, Handool KO, Khan MS, Mustapha NM, Ibrahim TAT, Kaka U, Yusof LM. Roles of Sodium Hydrogen Exchanger (NHE1) and Anion Exchanger (AE2) across Chondrocytes Plasma Membrane during Longitudinal Bone Growth. Membranes. 2022; 12(7):707. https://doi.org/10.3390/membranes12070707
Chicago/Turabian StyleAbubakar, Adamu Abdul, Ahmed Khalaf Ali, Sahar Mohammed Ibrahim, Kareem Obayes Handool, Mohammad Shuaib Khan, Noordin Mohamed Mustapha, Tengku Azmi Tengku Ibrahim, Ubedullah Kaka, and Loqman Mohamad Yusof. 2022. "Roles of Sodium Hydrogen Exchanger (NHE1) and Anion Exchanger (AE2) across Chondrocytes Plasma Membrane during Longitudinal Bone Growth" Membranes 12, no. 7: 707. https://doi.org/10.3390/membranes12070707