Simultaneous Release of Silver Ions and 10–Undecenoic Acid from Silver Iron–Oxide Nanoparticles Impregnated Membranes
Abstract
:1. Introduction
- -
- Attachment to the surface cell membrane of the silver nanoparticle with dimensions below 10 nm, leading to disturbance of respiration and/or cell permeability [16];
- -
- Penetration of the cell membrane, having the effect of blocking the functions containing sulfur or phosphorus [17];
- -
- The release of silver ions by the nanoparticle, amplifying its local effect [18].
2. Materials and Methods
2.1. Reagents and Materials
2.1.1. Reagents
2.1.2. Materials
2.2. Methods
2.2.1. Obtaining and Characterizing Support Membranes
2.2.2. Obtaining and Characterizing Oxide Nanoparticles Containing Silver
2.2.3. Obtaining the Impregnated Membrane and the Procedure for Evaluating the Release Effect
2.3. Equipment
3. Results and Discussions
3.1. The Influence of the Organic Compound and the Polymeric Matrix on the Release of Silver Ions
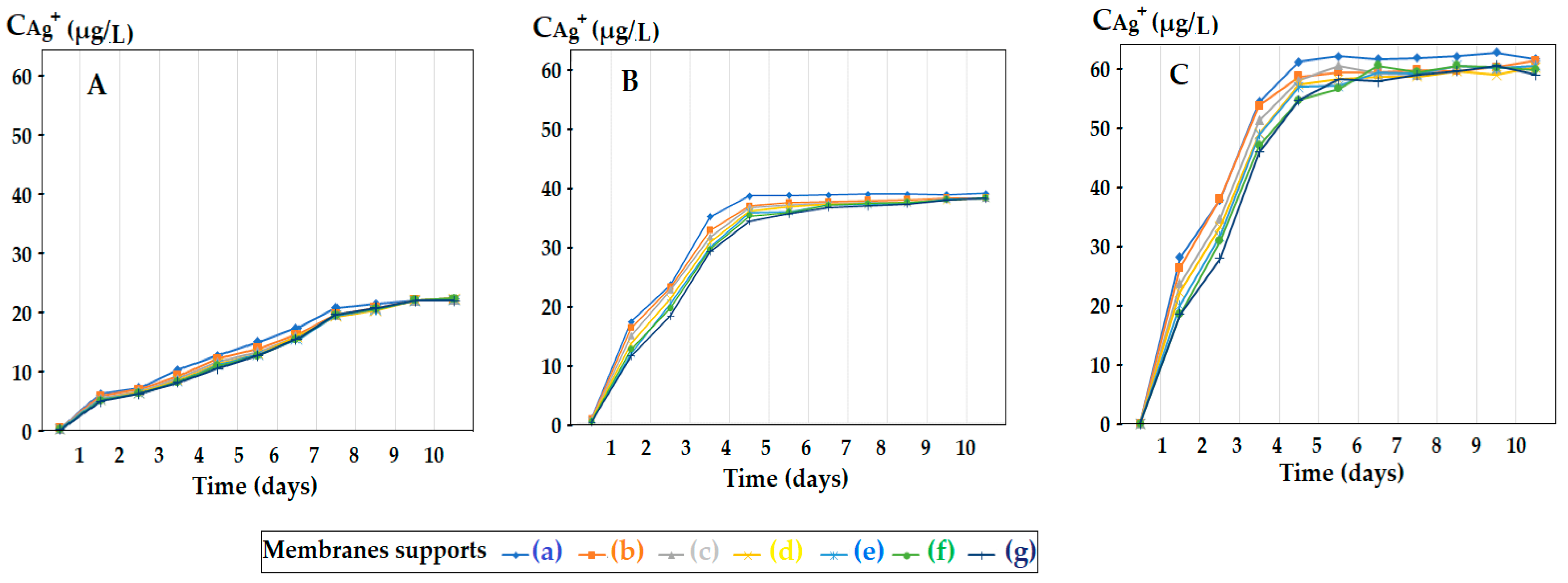
3.2. Influence of Membrane Support Morphology and Silver Content of Oxide Nanoparticles on the Release of Silver Ions in Aqueous Solution
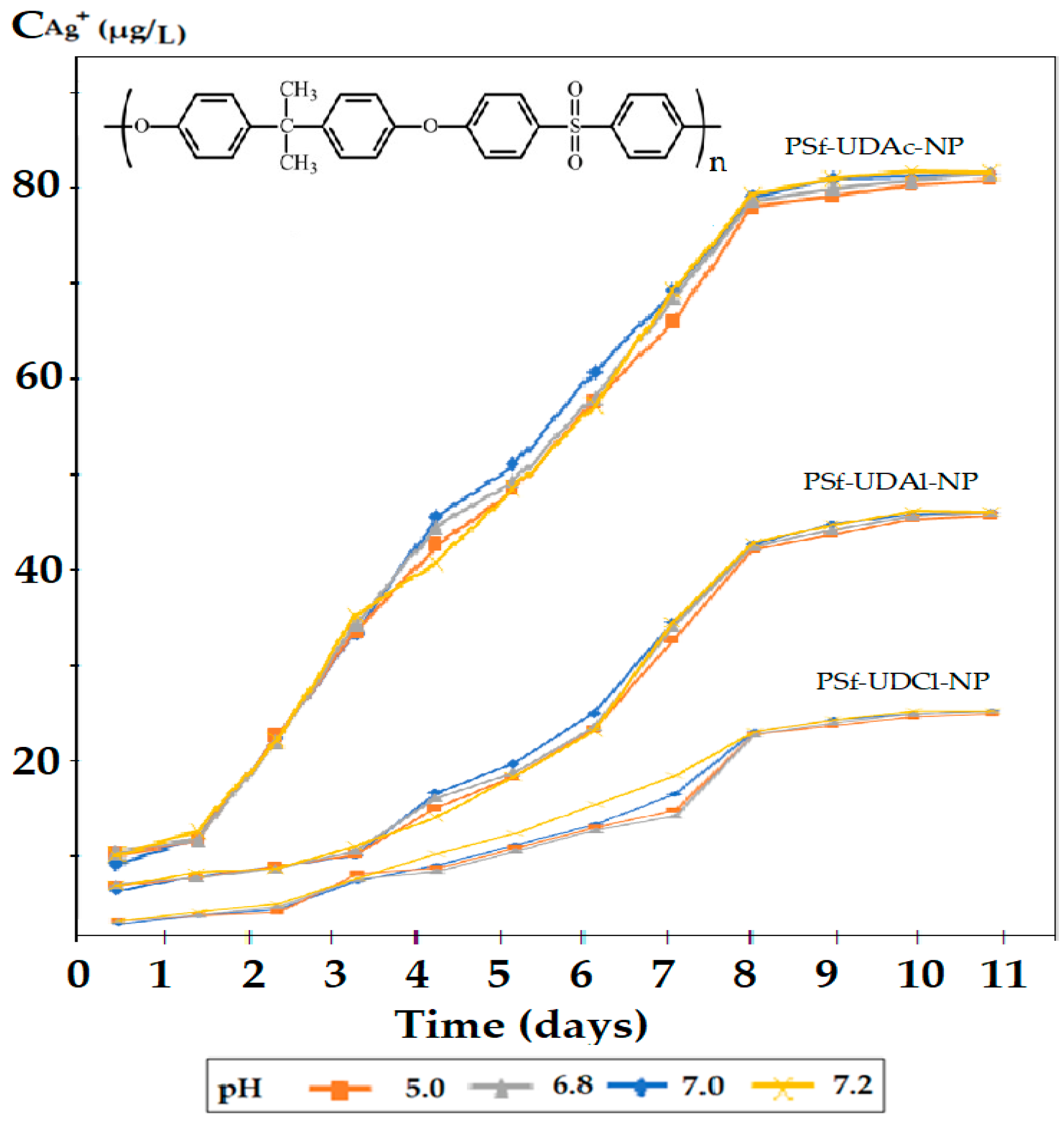
3.3. The Influence of the Receiving Phase pH of the Silver Ions and 10–Undecenoic Acid Simultaneous Release
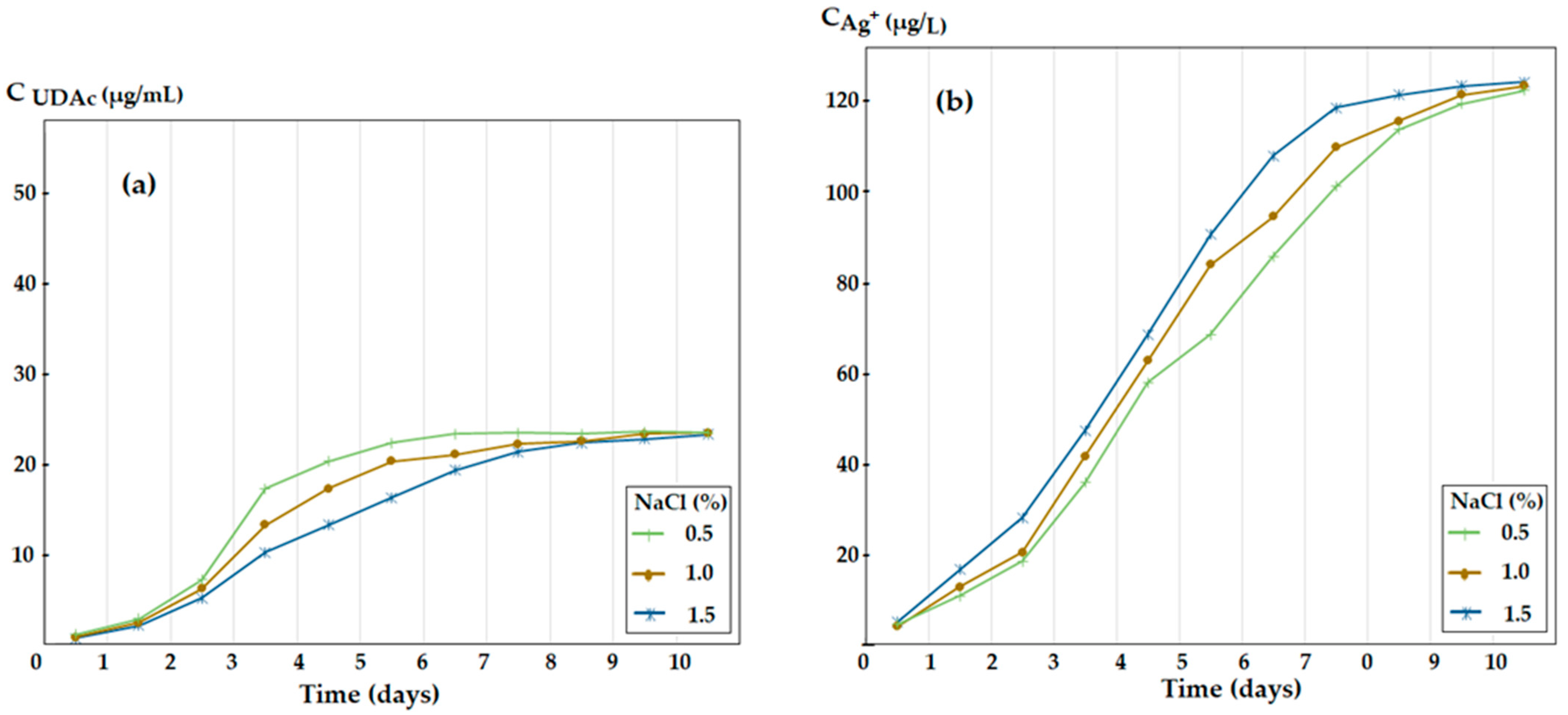
3.4. The Influence of the Ionic Strength of the Silver Ions and 10–Undecenoic Acid Simultaneous Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McHugh, A.J. The role of polymer membrane formation in sustained release drug delivery systems. J. Control. Release 2005, 109, 211–221. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Mogul, M.G.; Akin, H.; Hasirci, N.; Trantolo, D.J.; Gresser, J.D.; Wise, D.L. Controlled release of biologically active agents for purposes of agricultural crop management. Resour. Conserv. Recycl. 1996, 16, 289–320. [Google Scholar] [CrossRef]
- Jantzen, G.M.; Robinson, J.R. Sustained-and controlled-release drug delivery systems. Drugs Pharm. Sci. 2002, 121, 501–528. [Google Scholar]
- Toledano-Osorio, M.; Vallecillo, C.; Vallecillo-Rivas, M.; Manzano-Moreno, F.-J.; Osorio, R. Antibiotic-Loaded Polymeric Barrier Membranes for Guided Bone/Tissue Regeneration: A Mini-Review. Polymers 2022, 14, 840. [Google Scholar] [CrossRef]
- Candido, T.Z.; de Paiva, R.E.F.; Figueiredo, M.C.; de Oliveira Coser, L.; Frajácomo, S.C.L.; Abbehausen, C.; Cardinalli, I.A.; Lustri, W.R.; Carvalho, J.E.; Ruiz, A.L.T.G.; et al. Silver Nimesulide Complex in Bacterial Cellulose Membranes as an Innovative Therapeutic Method for Topical Treatment of Skin Squamous Cell Carcinoma. Pharmaceutics 2022, 14, 462. [Google Scholar] [CrossRef] [PubMed]
- Concha, L.; Resende Pires, A.L.; Moraes, A.M.; Mas-Hernández, E.; Berres, S.; Hernandez-Montelongo, J. Cost Function Analysis Applied to Different Kinetic Release Models of Arrabidaea chica Verlot Extract from Chitosan/Alginate Membranes. Polymers 2022, 14, 1109. [Google Scholar] [CrossRef]
- Shirkhanzadeh, M.; Azadegan, M.; Liu, G.Q. Bioactive delivery systems for the slow release of antibiotics: Incorporation of Ag+ ions into micro-porous hydroxyapatite coatings. Mater. Lett. 1995, 24, 7–12. [Google Scholar] [CrossRef]
- Totaro, G.; Cruciani, L.; Vannini, M.; Mazzola, G.; Di Gioia, D.; Celli, A.; Sisti, L. Synthesis of castor oil-derived polyesters with antimicrobial activity. Eur. Polym. J. 2014, 56, 174–184. [Google Scholar] [CrossRef]
- Kwiatkowska, A.; Drabik, M.; Lipko, A.; Grzeczkowicz, A.; Stachowiak, R.; Marszalik, A.; Granicka, L.H. Composite Membrane Dressings System with Metallic Nanoparticles as an Antibacterial Factor in Wound Healing. Membranes 2022, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Stetten, L.; Mackevica, A.; Tepe, N.; Hofmann, T.; von der Kammer, F. Towards Standardization for Determining Dissolution Kinetics of Nanomaterials in Natural Aquatic Environments: Continuous Flow Dissolution of Ag Nanoparticles. Nanomaterials 2022, 12, 519. [Google Scholar] [CrossRef]
- Sun, P.; Li, K.; Yi, S.; Li, H.; Chen, X. Effects of Silver Nanoparticles on Denitrification and Associated N2O Release in Estuarine and Marine Sediments. J. Ocean. Univ. China 2022, 21, 131–140. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Shih, Y.-S.; Chen, Z.-Y.; Cheng, F.-Y.; Lu, J.-Y.; Wu, Y.-H.; Wang, Y.-J. Toxic Effects and Mechanisms of Silver and Zinc Oxide Nanoparticles on Zebrafish Embryos in Aquatic Ecosystems. Nanomaterials 2022, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Prihandana, G.S.; Sriani, T.; Muthi’ah, A.D.; Machmudah, A.; Mahardika, M.; Miki, N. Study Effect of nAg Particle Size on the Properties and Antibacterial Characteristics of Polysulfone Membranes. Nanomaterials 2022, 12, 388. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Prakash, P.; Lee, W.-H.; Loo, C.-Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef]
- Milenin, S.A.; Drozdov, F.V.; Selezneva, E.V.; Ardabevskaia, S.N.; Buzin, M.I.; Muzafarov, A.M. Undecenoic acid-based polydimethylsiloxanes obtained by hydrosilylation and hydrothiolation reactions. J. Organomet. Chem. 2020, 907, 121074. [Google Scholar] [CrossRef]
- Hayes, D.G.; Mannam, V.K.; Ye, R.; Zhao, H.; Ortega, S.; Montiel, M.C. Modification of oligo-Ricinoleic Acid and Its Derivatives with 10-Undecenoic Acid via Lipase-Catalyzed Esterification. Polymers 2012, 4, 1037–1055. [Google Scholar] [CrossRef]
- Pawar, A.S.; Chattopadhyay, S. 10-Undecenoic Acid, an Inexpensive Source for the Synthesis of the Pheromones of Cotton Pests, Peach Tree Borer and Cherry Tree Borer. Molecules 1997, 2, 87–90. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Narra, N.; Kaki, S.S.; Prasad, R.B.N.; Misra, S.; Dhevendar, K.; Kontham, V.; Korlipara, P.V. Synthesis and evaluation of anti-oxidant and cytotoxic activities of novel 10-undecenoic acid methyl ester based lipoconjugates of phenolic acids. Beilstein J. Org. Chem. 2017, 13, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Maamoun, H.S.; Rabie, G.H.; Shaker, I.; Alaidaroos, B.A.; El-Sayed, A.S. Biochemical properties of tyrosinase from Aspergillus terreus and Penicillium copticola; Undecanoic Acid from Aspergillus flavus, an endophyte of Moringa oleifera, is a novel potent tyrosinase inhibitor. Molecules 2021, 26, 1309. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Agarwal, V. Tunable Upconversion Emission from Oil-based Carbon Nanodots. Mater. Lett. 2022, 313, 131640. [Google Scholar] [CrossRef]
- Shmool, T.A.; Constantinou, A.; Jirkas, A.; Zhao, C.; Georgiou, T.K.; Hallett, J. Next Generation Strategy for Tuning the Thermoresponsive Properties of Micellar and Hydrogel Drug Delivery Vehicles Using Ionic Liquids. Polym. Chem. 2022, 13, 2340–2350. [Google Scholar] [CrossRef]
- Nechifor, G.; Păncescu, F.M.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Ioan, M.-R.; Nechifor, A.C. Transport and Separation of the Silver Ion with n–decanol Liquid Membranes Based on 10–undecylenic Acid, 10–undecen–1–ol and Magnetic Nanoparticles. Membranes 2021, 11, 936. [Google Scholar] [CrossRef]
- Albu, P.C.; Ferencz, A.; Al-Ani, H.N.A.; Tanczos, S.-K.; Oprea, O.; Grosu, V.-A.; Nechifor, G.; Bungău, S.G.; Grosu, A.R.; Goran, A.; et al. Osmium Recovery as Membrane Nanomaterials through 10–Undecenoic Acid Reduction Method. Membranes 2022, 12, 51. [Google Scholar] [CrossRef]
- Ferencz, A.; Grosu, A.R.; Al-Ani, H.N.A.; Nechifor, A.C.; Tanczos, S.-K.; Albu, P.C.; Crăciun, M.E.; Ioan, M.-R.; Grosu, V.-A.; Nechifor, G. Operational Limits of the Bulk Hybrid Liquid Membranes Based on Dispersion Systems. Membranes 2022, 12, 190. [Google Scholar] [CrossRef]
- Scutariu, R.E.; Batrinescu, G.; Nechifor, G.; Popescu, M.; Tenea, A.G. Separation of the collagen protein by ultrafiltration: Effects of concentration on the membrane’s characteristics. Polym. Eng. Sci. 2020, 60, 2487–2495. [Google Scholar] [CrossRef]
- Batrinescu, G.; Scutariu, R.E.; Nechifor, G.; Ionescu, I.A.; Iancu, V.I. Comparative analysis of the processes of collagen concentration by ultrafiltration using different types of membranes. J. Appl. Polym. Sci. 2021, 138, 50055. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Cotorcea, S.; Bungău, C.; Albu, P.C.; Pașcu, D.; Oprea, O.; Grosu, A.R.; Pîrțac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Miricioiu, M.G.; Iacob, C.; Nechifor, G.; Niculescu, V.C. High selective mixed membranes based on mesoporous MCM-41 and MCM-41-NH2 particles in a polysulfone matrix. Front. Chem. 2019, 7, 332. [Google Scholar] [CrossRef] [Green Version]
- Nechifor, A.C.; Pîrțac, A.; Albu, P.C.; Grosu, A.R.; Dumitru, F.; Dimulescu Nica, I.A.; Oprea, O.; Pașcu, D.; Nechifor, G.; Bungău, S.G. Recuperative Amino Acids Separation through Cellulose Derivative Membranes with Microporous Polypropylene Fiber Matrix. Membranes 2021, 11, 429. [Google Scholar] [CrossRef]
- Dimulescu, I.A.; Nechifor, A.C.; Bǎrdacǎ, C.; Oprea, O.; Paşcu, D.; Totu, E.E.; Albu, P.C.; Nechifor, G.; Bungău, S.G. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials 2021, 11, 1204. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Bungău, C.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Păncescu, F.M.; Nechifor, G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes 2021, 11, 445. [Google Scholar] [CrossRef]
- Nechifor, G.; Totu, E.E.; Nechifor, A.C.; Constantin, L.; Constantin, A.M.; Cărăuşu, M.E.; Isildak, I. Added value recyclability of glass fiber waste as photo-oxidation catalyst for toxic cytostatic micropollutants. Sci. Rep. 2020, 10, 136. [Google Scholar] [CrossRef]
- Nechifor, G.; Eftimie Totu, E.; Nechifor, A.C.; Isildak, I.; Oprea, O.; Cristache, C.M. Non-Resorbable Nanocomposite Membranes for Guided Bone Regeneration Based on Polysulfone-Quartz Fiber Grafted with Nano-TiO2. Nanomaterials 2019, 9, 985. [Google Scholar] [CrossRef] [Green Version]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Pîrțac, A.; Albu, P.C.; Oprea, O.; Grosu, A.R.; Pașcu, D.; Păncescu, F.M.; Nechifor, G.; et al. Reactional Processes on Osmium–Polymeric Membranes for 5–Nitrobenzimidazole Reduction. Membranes 2021, 11, 633. [Google Scholar] [CrossRef]
- Hernández, D.; Cepriá, G.; Laborda, F.; Castillo, J.R. Detection and determination of released ions in the presence of nanoparticles: Selectivity or strategy? Electroanalysis 2019, 31, 405–410. [Google Scholar] [CrossRef]
- Hernández Arteaga, L.O.; Loredo Tovias, M.; Araujo Martínez, R.; Compeán Jasso, M.E.; de Alba Montero, I.; Ruiz, F. Determination of silver concentration in tomato seeds (Solanum lycopersicum L.) exposed to silver nanoparticles using AAS-F and a validated method. Acta Univ. 2018, 28, 58–65. [Google Scholar] [CrossRef]
- Nechifor, G.; Păncescu, F.M.; Grosu, A.R.; Albu, P.C.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Pîrțac, A.; Nechifor, A.C. Osmium Nanoparticles-Polypropylene Hollow Fiber Membranes Applied in Redox Processes. Nanomaterials 2021, 11, 2526. [Google Scholar] [CrossRef] [PubMed]
- No, A.M.C.T.B.; Analytical Methods Committee. What’s novel in the new Eurachem guide on uncertainty from sampling? Anal. Methods 2020, 12, 2295–2297. [Google Scholar]
- Al-Masri, M.S.; Amin, Y. Use of the Eurachem guide on method validation for determination of uranium in environmental samples. Accred. Qual. Assur. 2005, 10, 98–106. [Google Scholar] [CrossRef]
- Liao, J.; Hou, B.; Huang, H. Preparation, properties and drug controlled release of chitin-based hydrogels: An updated review. Carbohydr. Polym. 2022, 283, 119177. [Google Scholar] [CrossRef]
- Kang, S.; He, Y.; Yu, D.G.; Li, W.; Wang, K. Drug–zein@ lipid hybrid nanoparticles: Electrospraying preparation and drug extended release application. Colloids Surf. B Biointerfaces 2021, 201, 111629. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [Green Version]
- Arafat, M.; Sarfraz, M.; AbuRuz, S. Development and In Vitro Evaluation of Controlled Release Viagra® Containing Poloxamer-188 Using Gastroplus™ PBPK Modeling Software for In Vivo Predictions and Pharmacokinetic Assessments. Pharmaceuticals 2021, 14, 479. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety—Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culebras, M.; Pishnamazi, M.; Walker, G.M.; Collins, M.N. Facile Tailoring of Structures for Controlled Release of Paracetamol from Sustainable Lignin Derived Platforms. Molecules 2021, 26, 1593. [Google Scholar] [CrossRef] [PubMed]
- Neftci, E.O.; Averbeck, B.B. Reinforcement learning in artificial and biological systems. Nat. Mach. Intell. 2019, 1, 133–143. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Soltani, M.; Souri, M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J. Control. Release 2020, 327, 316–349. [Google Scholar] [CrossRef]
- Nechifor, A.; Panait, V.; Naftanaila, L.; Batalu, D.; Voicu, S.I. Symmetrically polysulfone membranes obtained by solvent evaporation using carbon nanotubes as additives. Synthesis, characterization and applications. Dig. J. Nanomater. Biostruc. 2013, 8, 875–884. [Google Scholar]
- Bărdacă Urducea, C.; Nechifor, A.C.; Dimulescu, I.A.; Oprea, O.; Nechifor, G.; Totu, E.E.; Isildak, I.; Albu, P.C.; Bungău, S.G. Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method. Nanomaterials 2020, 10, 2349. [Google Scholar] [CrossRef]
- Baek, Y.; Singh, N.; Arunkumar, A.; Borwankar, A.; Zydney, A.L. Mass balance model with donnan equilibrium accurately describes unusual pH and excipient profiles during diafiltration of monoclonal antibodies. Biotechnol. J. 2019, 14, 1800517. [Google Scholar] [CrossRef]
- Yustres, A.; Lopez-Vizcaino, R.; Cabrera, V.; Rodrigo, M.A.; Navarro, V. Donnan-ion hydration model to estimate the electroosmotic permeability of clays. Electrochim. Acta 2020, 355, 136758. [Google Scholar] [CrossRef]
- Gao, K.W.; Yu, X.; Darling, R.M.; Newman, J.; Balsara, N.P. Increased Donnan exclusion in charged polymer networks at high salt concentrations. Soft Matter 2022, 18, 282–292. [Google Scholar] [CrossRef]
- Voicu, S.I.; Aldea, F.; Radut, M.; Nechifor, G. Nanostructured polysulfone composite membranes. UPB Sci. Bull. Ser. B. 2008, 70, 39–46. [Google Scholar]
- Popescu, G.; Nechifor, G.; Albu, B.; Luca, N. Microporous polyethersulfone membranes. Rev. Roum. Chim. 1989, 34, 577–580. [Google Scholar]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef] [PubMed]
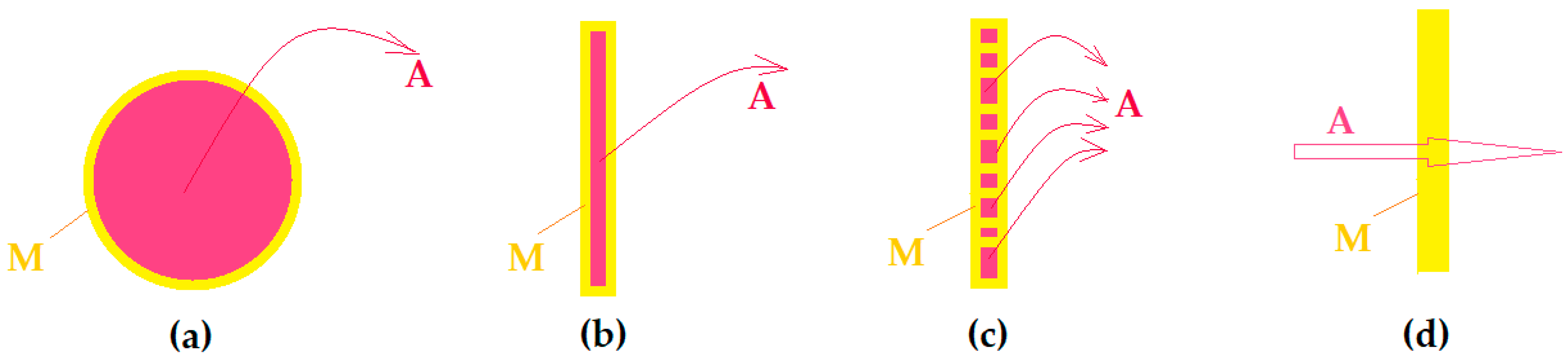


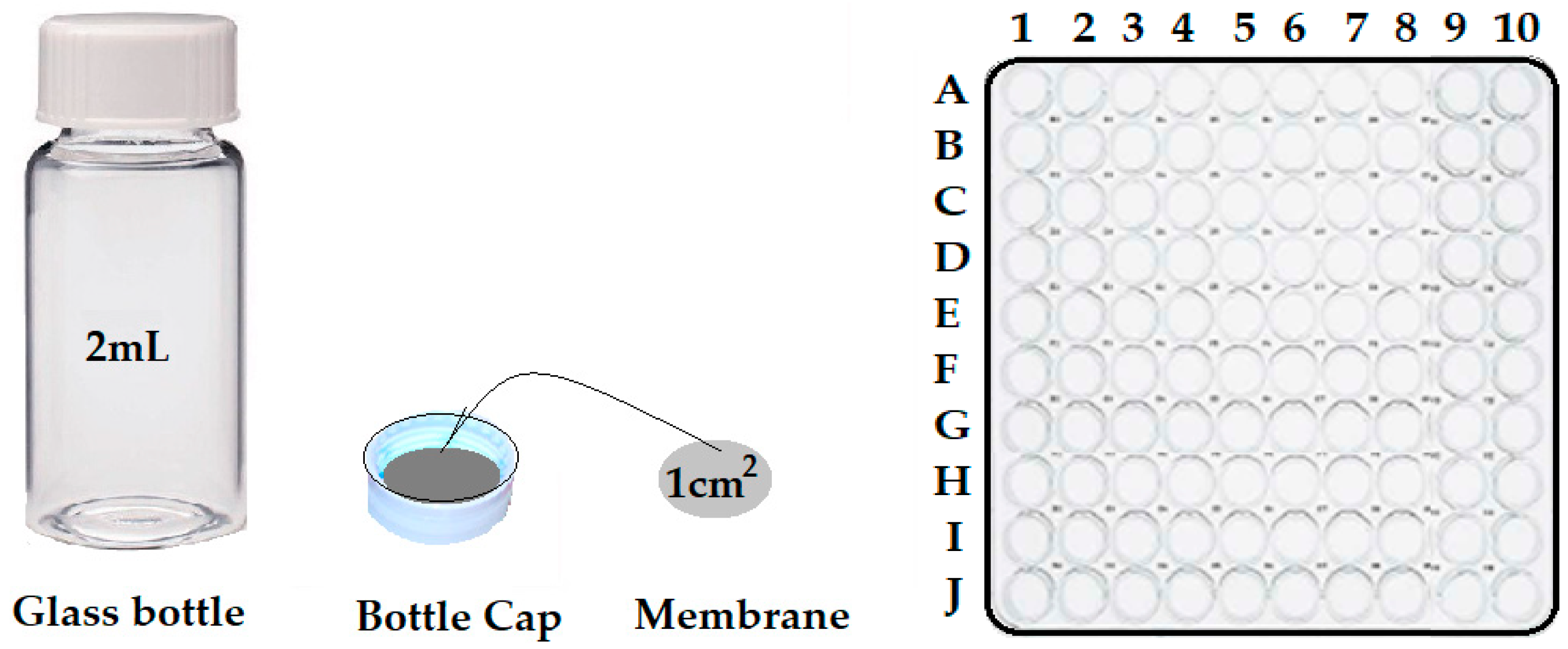




| Organic Compounds | Name and Symbol | Molar Mass (g/mol) | Density (g/mL) | Solubility in Water (g/L) | pKa |
|---|---|---|---|---|---|
 | 10–undecen–1–ol (UDAl) | 170.29 | 0.848 | 0.014 estimated 0.044 exp. | 16.84 |
 | 10–undecenoyl chloride (UDCl) | 202.72 | 0.944 | – | none |
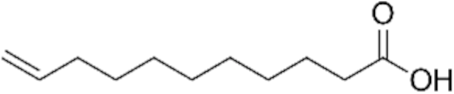 | 10–undecenoic acid (UDAc) | 184.28 | 0.912 | 0.019 estimated 0.0737 exp. | 5.02 |
| Polymer | Chemical Formula | Molar Weight (Da) | Membrane Symbols | Contact Angle *) (θ°) |
|---|---|---|---|---|
| Cellulose acetate (CA) | 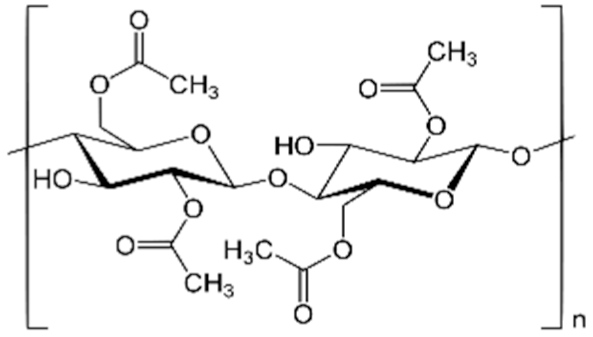 | 50,000 | CA-UDAc-NP CA-UDAl-NP CA-UDCl-NP | 43 ± 2 52 ± 2 60 ± 2 |
| Polysulfone (PSf) | 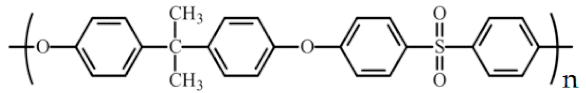 | 35,000 | PSf-UDAc-NP PSf-UDAl-NP PSf-UDCl-NP | 65 ± 2 71 ± 2 76 ± 2 |
| Membrane | Scanning Electron Microscopy (SEM) | Thickness (µm) | Porosity (%) | |
|---|---|---|---|---|
| Cross-Section | Bottom | |||
| Cellulose acetate (CA) | 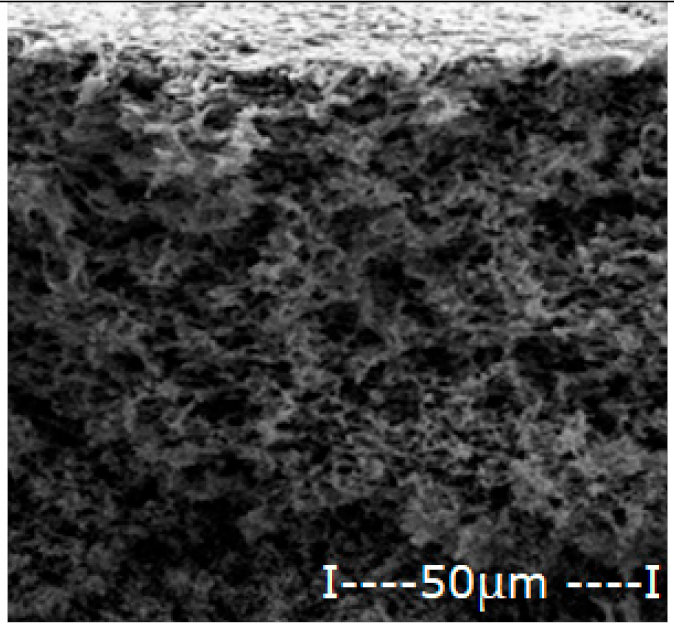 |  | 162 ± 5 | 82 ± 3 |
| Polysulfone (PSf) | 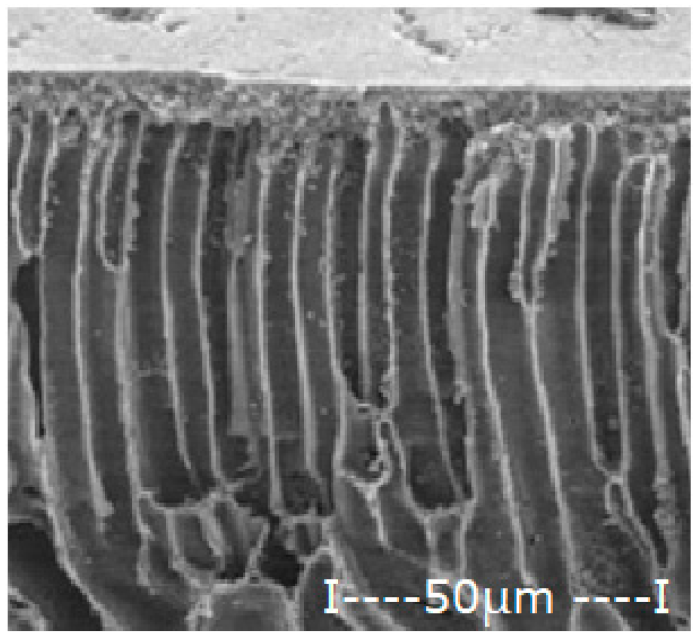 | 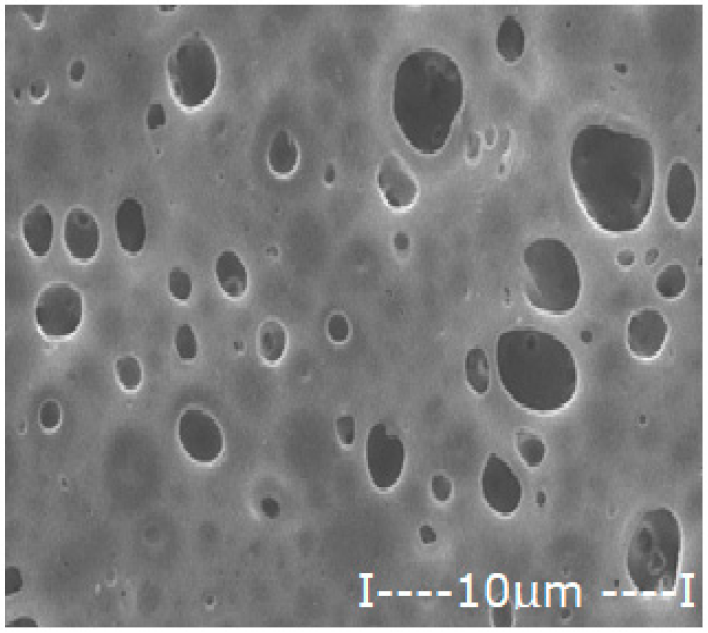 | 165 ± 3 | 76 ± 2 |
| Oxide Nanoparticle (Ag–NP) | Scanning Electron Microscopy (SEM) | Medium Diameter (nm) | Medium Silver Content (%) |
|---|---|---|---|
| NP0.55 | 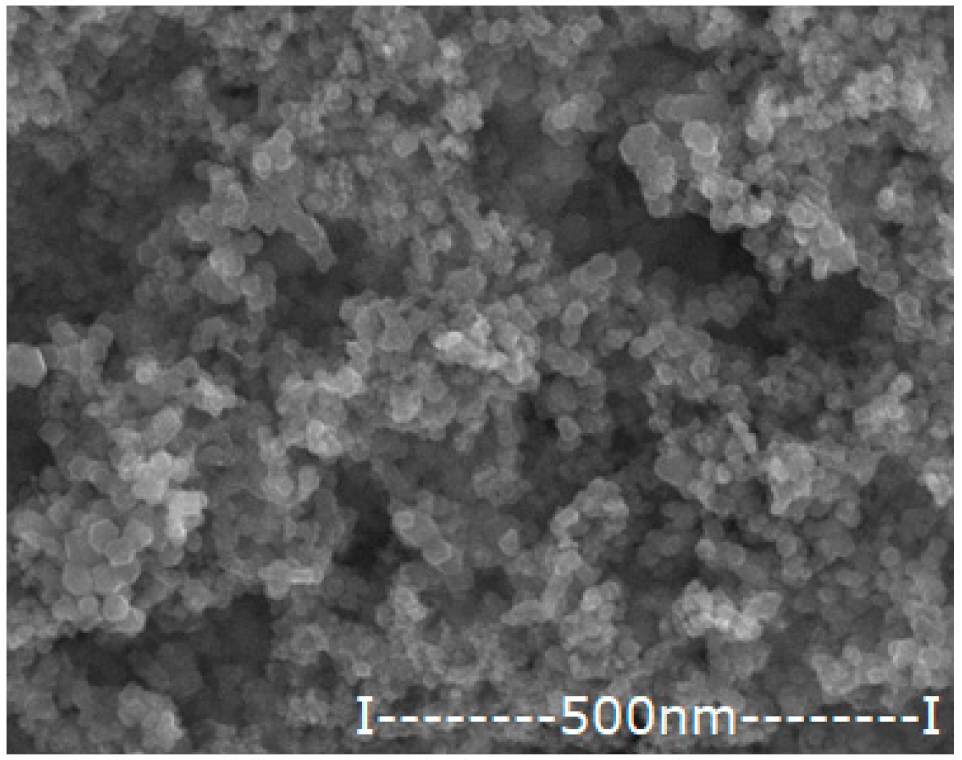 | 42.8 | 0.55 |
| NP1.12 | 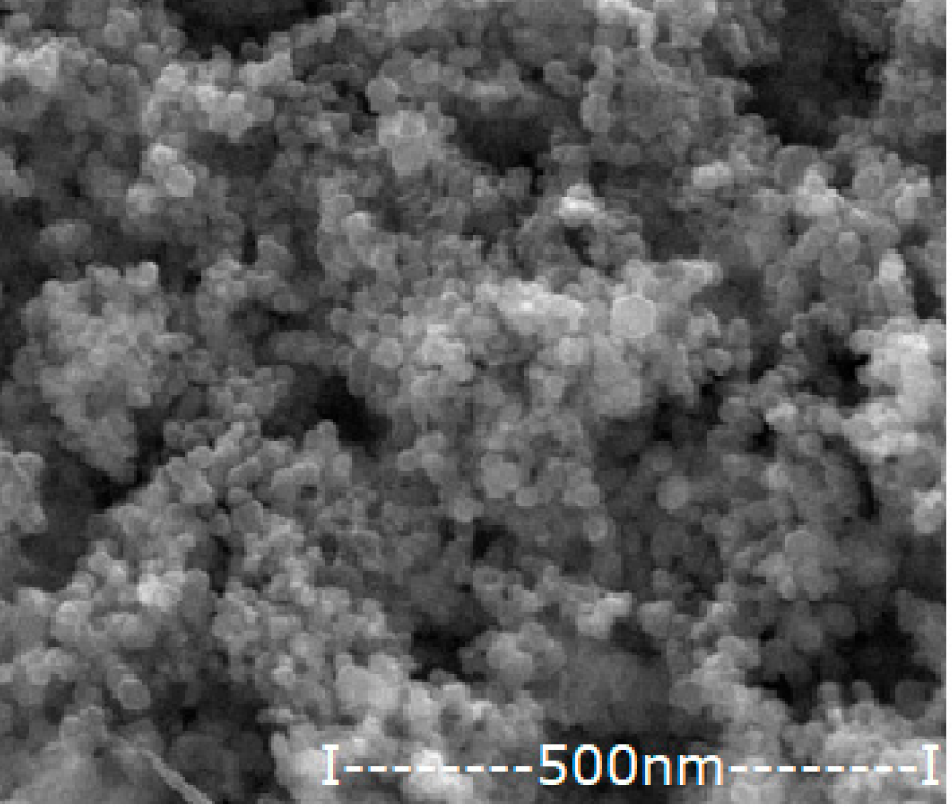 | 45.4 | 1.12 |
| NP1.63 | 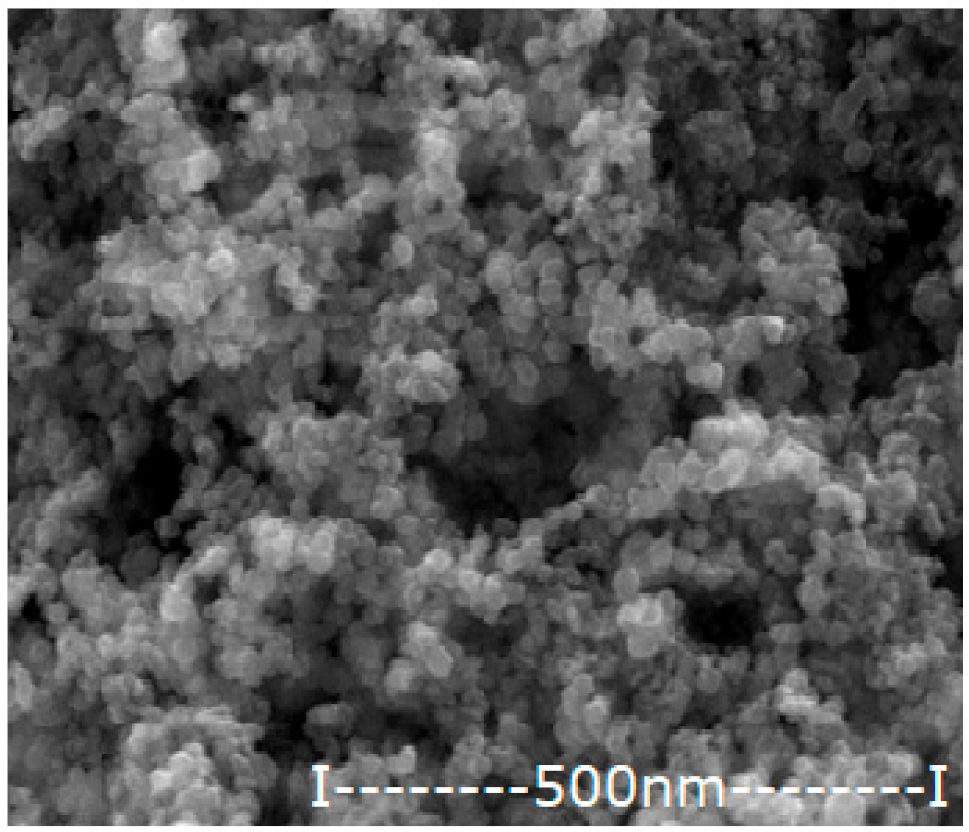 | 47.1 | 1.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechifor, G.; Grosu, A.R.; Ferencz, A.; Tanczos, S.-K.; Goran, A.; Grosu, V.-A.; Bungău, S.G.; Păncescu, F.M.; Albu, P.C.; Nechifor, A.C. Simultaneous Release of Silver Ions and 10–Undecenoic Acid from Silver Iron–Oxide Nanoparticles Impregnated Membranes. Membranes 2022, 12, 557. https://doi.org/10.3390/membranes12060557
Nechifor G, Grosu AR, Ferencz A, Tanczos S-K, Goran A, Grosu V-A, Bungău SG, Păncescu FM, Albu PC, Nechifor AC. Simultaneous Release of Silver Ions and 10–Undecenoic Acid from Silver Iron–Oxide Nanoparticles Impregnated Membranes. Membranes. 2022; 12(6):557. https://doi.org/10.3390/membranes12060557
Chicago/Turabian StyleNechifor, Gheorghe, Alexandra Raluca Grosu, Andreea Ferencz (Dinu), Szidonia-Katalin Tanczos, Alexandru Goran, Vlad-Alexandru Grosu, Simona Gabriela Bungău, Florentina Mihaela Păncescu, Paul Constantin Albu, and Aurelia Cristina Nechifor. 2022. "Simultaneous Release of Silver Ions and 10–Undecenoic Acid from Silver Iron–Oxide Nanoparticles Impregnated Membranes" Membranes 12, no. 6: 557. https://doi.org/10.3390/membranes12060557








