MOF/Polymer Mixed-Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. MOF Synthesis
2.3. Membrane Synthesis
2.4. Characterization
2.4.1. X-ray Diffraction (XRD)
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Differential Scanning Calorimetry (DSC)
2.5. Gas Separation Performance
3. Results and Discussion
3.1. THF-Based Membranes: Effect of Polymer and MOF Concentration, Casting Solution Volume, and Solvent Evaporation Rate
3.1.1. Visual Assessment
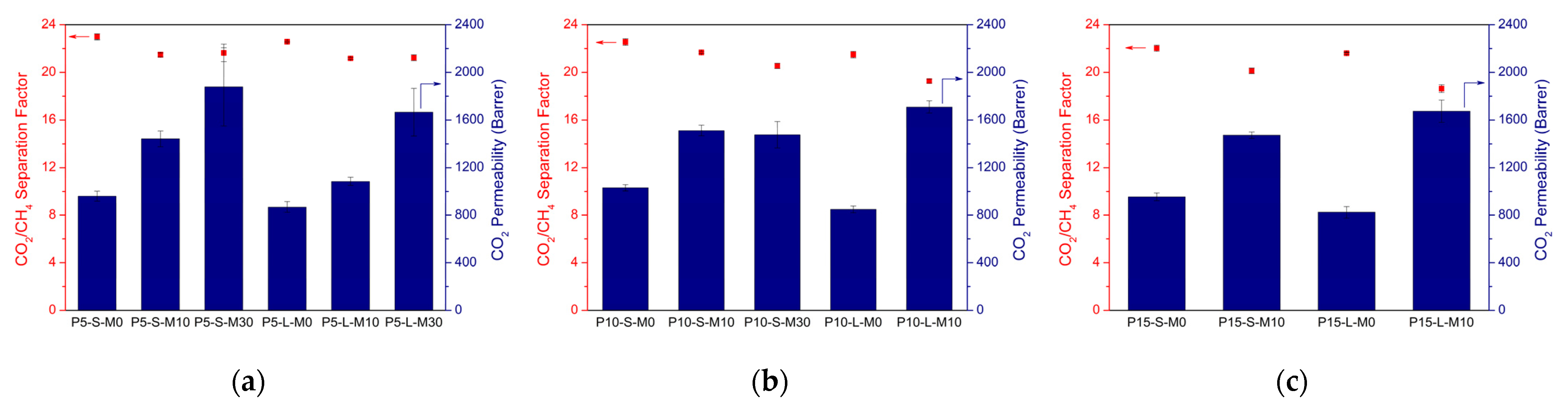
3.1.2. Gas Separation Performances
3.2. Effect of Solvent Type
3.2.1. Visual Assessment
3.2.2. Gas Separation Performances
3.2.3. Characterization of MMMs
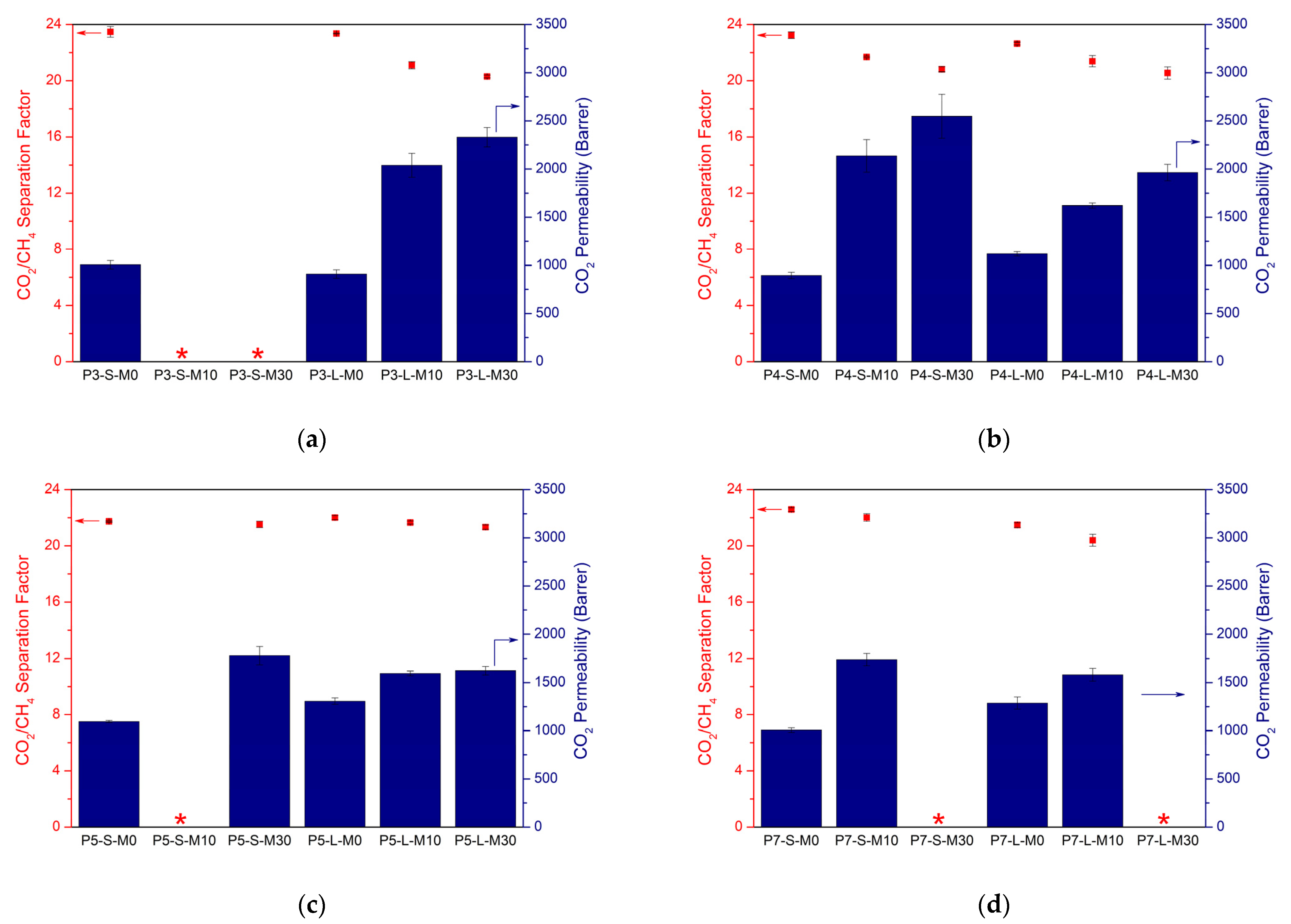
3.3. Optimized Synthesis of Chloroform-Based Membranes
3.3.1. Visual Assessment
3.3.2. Gas Separation Performance
3.3.3. Characterization
3.4. Overall Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Japip, S.; Jiang, S.-D.; Chung, T.-S.; Zhang, S.; Zhao, D. Advanced Porous Materials in Mixed Matrix Membranes. Adv. Mater. 2018, 30, e1802401. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liang, B.; Lin, R.-B.; Zhang, C.; Chen, B. Gas Separation via Hybrid Metal-Organic Framework/Polymer Membranes. Trends Chem. 2020, 2, 254–269. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Wong, D.; Yang, J. Recent Advances in Polymer-Inorganic Mixed Matrix Membranes for CO2 Separation. Polymers 2021, 13, 2539. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chernikova, V.; Liu, Y.; Zhang, K.; Belmabkhout, Y.; Shekhah, O.; Zhang, C.; Yi, S.; Eddaoudi, M.; Koros, W.J. Mixed matrix formulations with MOF molecular sieving for key energy-intensive separations. Nat. Mater. 2018, 17, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kertik, A.; Wee, L.H.; Pfannmöller, M.; Bals, S.; Martens, J.A.; Vankelecom, I.F.J. Highly selective gas separation membrane using in situ amorphised metal–organic frameworks. Energy Environ. Sci. 2017, 10, 2342–2351. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Vanherck, K.; Aerts, A.; Martens, J.; Vankelecom, I. Hollow filler based mixed matrix membranes. Chem. Commun. 2010, 46, 2492–2494. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Zhang, C.; Qiu, W.; Yi, S.; Chernikova, V.; Chen, Z.; Belmabkhout, Y.; Shekhah, O.; Eddaoudi, M.; et al. Enhanced CO2/CH4 Separation Performance of a Mixed Matrix Membrane Based on Tailored MOF-Polymer Formulations. Adv. Sci. 2018, 5, 1800982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Liu, G.; Labreche, Y.; Chernikova, V.; Shekhah, O.; Zhang, C.; Belmabkhout, Y.; Eddaoudi, M.; Koros, W.J. Zeolite-like MOF nanocrystals incorporated 6FDA-polyimide mixed-matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2018, 565, 186–193. [Google Scholar] [CrossRef]
- Winarta, J.; Meshram, A.; Zhu, F.; Li, R.; Jafar, H.; Parmar, K.; Liu, J.; Mu, B. Metal–organic framework-based mixed-matrix membranes for gas separation: An overview. J. Appl. Polym. Sci. 2020, 58, 2518–2546. [Google Scholar] [CrossRef]
- Qian, Q.; Wu, A.X.; Chi, W.S.; Asinger, P.A.; Lin, S.; Hypsher, A.; Smith, Z.P. Mixed-Matrix Membranes Formed from Imide-Functionalized UiO-66-NH2 for Improved Interfacial Compatibility. ACS Appl. Mater. Interfaces 2019, 11, 31257–31269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Muñoz, R.; Ahmad, M.Z.; Fíla, V. Tuning of Nano-Based Materials for Embedding into Low-Permeability Polyimides for a Featured Gas Separation. Front. Chem. 2020, 7, 897. [Google Scholar] [CrossRef] [Green Version]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Yuan, S.; Zhou, J.; Wang, B. Challenges and recent advances in MOF–polymer composite membranes for gas separation. Inorg. Chem. Front. 2016, 3, 896–909. [Google Scholar] [CrossRef]
- Seoane, B.; Coronas, J.; Gascon, I.; Benavides, M.E.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal-organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef] [Green Version]
- Aroon, M.A.; Ismail, A.; Matsuura, T.; Montazer-Rahmati, M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Kamble, A.R.; Patel, C.M.; Murthy, Z. A review on the recent advances in mixed matrix membranes for gas separation processes. Renew. Sustain. Energy Rev. 2021, 145, 111062. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Martin-Gil, V.; Dujardin, W.; Sysel, P.; Koeckelberghs, G.; Vankelecom, I.; Fila, V. Effect of benzoic acid content on aging of 6FDA copolyimides based thin film composite (TFC) membranes in CO2/CH4 environment. Sep. Purif. Technol. 2019, 210, 616–626. [Google Scholar] [CrossRef]
- Jain, A.; Ahmad, M.; Linkès, A.; Martin-Gil, V.; Castro-Muñoz, R.; Izak, P.; Sofer, Z.; Hintz, W.; Fila, V. 6FDA-DAM:DABA Co-Polyimide Mixed Matrix Membranes with GO and ZIF-8 Mixtures for Effective CO2/CH4 Separation. Nanomaterials 2021, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Xu, L.; Chen, C.-C.; Paul, D.R.; Koros, W.J. Gas separation performance of 6FDA-based polyimides with different chemical structures. Polymer 2013, 54, 6226–6235. [Google Scholar] [CrossRef]
- Eguchi, H.; Kim, D.J.; Koros, W.J. Chemically cross-linkable polyimide membranes for improved transport plasticization resistance for natural gas separation. Polymer 2015, 58, 121–129. [Google Scholar] [CrossRef]
- Thur, R.; Van Velthoven, N.; Lemmens, V.; Bastin, M.; Smolders, S.; De Vos, D.E.; Vankelecom, I.F.J. Modulator-Mediated Functionalization of MOF-808 as a Platform Tool to Create High-Performance Mixed-Matrix Membranes. ACS Appl. Mater. Interfaces 2019, 11, 44792–44801. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.H.; Chitale, S.K.; Kim, S.-J.; Cha, G.-Y.; Hong, D.-Y.; Ryu, S.G.; Chang, J.-S.; Hwang, Y.K. Adsorptive removal of nerve-agent simulant with zirconium-based metal-organic frameworks modified by hydrophobic monocarboxylic acids. Microporous Mesoporous Mater. 2019, 285, 61–69. [Google Scholar] [CrossRef]
- Zheng, H.-Q.; Liu, C.-Y.; Zeng, X.-Y.; Chen, J.; Lü, J.; Lin, R.-G.; Cao, R.; Lin, Z.-J.; Su, J.-W. MOF-808: A Metal–Organic Framework with Intrinsic Peroxidase-Like Catalytic Activity at Neutral pH for Colorimetric Biosensing. Inorg. Chem. 2018, 57, 9096–9104. [Google Scholar] [CrossRef] [PubMed]
- Van Velthoven, N.; Waitschat, S.; Chavan, S.M.; Liu, P.; Smolders, S.; Vercammen, J.; Bueken, B.; Bals, S.; Lillerud, K.P.; Stock, N.; et al. Single-site metal–organic framework catalysts for the oxidative coupling of arenes via C–H/C–H activation. Chem. Sci. 2019, 10, 3616–3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Mu, Y.; Zhang, W.; Zhao, S.; Wang, Y. PVC-based hybrid membranes containing metal-organic frameworks for Li+/Mg2+ separation. J. Membr. Sci. 2020, 596, 117724. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Insight Studies on Metal-Organic Framework Nanofibrous Membrane Adsorption and Activation for Heavy Metal Ions Removal from Aqueous Solution. ACS Appl. Mater. Interfaces 2018, 10, 18619–18629. [Google Scholar] [CrossRef] [PubMed]
- Healey, K.; Liang, W.; Southon, P.D.; Church, T.L.; D’Alessandro, D.M. Photoresponsive spiropyran-functionalised MOF-808: Postsynthetic incorporation and light dependent gas adsorption properties. J. Mater. Chem. A 2016, 4, 10816–10819. [Google Scholar] [CrossRef]
- Park, J.M.; Yoo, D.K.; Jhung, S.H. Selective CO2 adsorption over functionalized Zr-based metal organic framework under atmospheric or lower pressure: Contribution of functional groups to adsorption. Chem. Eng. J. 2020, 402, 126254. [Google Scholar] [CrossRef]
- Thür, R.; Van Havere, D.; Van Velthoven, N.; Smolders, S.; Lamaire, A.; Wieme, J.; Van Speybroeck, V.; De Vos, D.E.; Vankelecom, I.F.J. Correlating MOF-808 parameters with mixed-matrix membrane (MMM) CO2 permeation for a more rational MMM development. J. Mater. Chem. A 2021, 9, 12782–12796. [Google Scholar] [CrossRef]
- Thür, R.; Van Velthoven, N.; Slootmaekers, S.; Didden, J.; Verbeke, R.; Smolders, S.; Dickmann, M.; Egger, W.; De Vos, D.; Vankelecom, I.F. Bipyridine-based UiO-67 as novel filler in mixed-matrix membranes for CO2-selective gas separation. J. Membr. Sci. 2019, 576, 78–87. [Google Scholar] [CrossRef]
- Didden, J.; Thür, R.; Volodin, A.; Vankelecom, I.F.J. Blending PPO-based molecules with Pebax MH 1657 in membranes for gas separation. J. Appl. Polym. Sci. 2018, 135, 46433. [Google Scholar] [CrossRef]
- Khan, A.; Basu, S.; Odena, A.; Vankelecom, I.F. Novel high throughput equipment for membrane-based gas separations. J. Membr. Sci. 2010, 354, 32–39. [Google Scholar] [CrossRef]
- Zheng, H.-Q.; Zeng, Y.-N.; Chen, J.; Lin, R.-G.; Zhuang, W.-E.; Cao, R.; Lin, Z.-J. Zr-Based Metal–Organic Frameworks with Intrinsic Peroxidase-Like Activity for Ultradeep Oxidative Desulfurization: Mechanism of H2O2 Decomposition. Inorg. Chem. 2019, 58, 6983–6992. [Google Scholar] [CrossRef] [PubMed]
- Reinsch, H.; Waitschat, S.; Chavan, S.M.; Lillerud, K.P.; Stock, N. A Facile “Green” Route for Scalable Batch Production and Continuous Synthesis of Zirconium MOFs. Eur. J. Inorg. Chem. 2016, 2016, 4490–4498. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Li, Z.; Wang, X.; Xu, Y.; Chen, S.; Wang, Z. Optimized synthesis of Zr(iv) metal organic frameworks (MOFs-808) for efficient hydrogen storage. New J. Chem. 2019, 43, 4092–4099. [Google Scholar] [CrossRef]
- Gevers, L.; Aldea, S.; Vankelecom, I.; Jacobs, P. Optimisation of a lab-scale method for preparation of composite membranes with a filled dense top-layer. J. Membr. Sci. 2006, 281, 741–746. [Google Scholar] [CrossRef]
- Isanejad, M.; Azizi, N.; Mohammadi, T. Pebax membrane for CO2/CH4 separation: Effects of various solvents on morphology and performance. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ahmad, J.; Hägg, M.-B. Preparation and characterization of polyvinyl acetate/zeolite 4A mixed matrix membrane for gas separation. J. Membr. Sci. 2013, 427, 73–84. [Google Scholar] [CrossRef]
- Rodenas, T.; Van Dalen, M.; García-Pérez, E.; Crespo, P.S.; Zornoza, B.; Kapteijn, F.; Gascon, J. Visualizing MOF Mixed Matrix Membranes at the Nanoscale: Towards Structure-Performance Relationships in CO2/CH4 Separation Over NH2-MIL-53(Al)@PI. Adv. Funct. Mater. 2013, 24, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 85th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley: Weinheim, Germany, 2002. [Google Scholar]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry, 3rd ed.; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Xuan, K.; Pu, Y.; Li, F.; Luo, J.; Zhao, N.; Xiao, F. Metal-organic frameworks MOF-808-X as highly efficient catalysts for direct synthesis of dimethyl carbonate from CO2 and methanol. Chin. J. Catal. 2019, 40, 553–566. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Park, S.; Bang, J.; Choi, J.; Lee, S.H.; Lee, J.-H.; Lee, J.S. 3-Dimensionally disordered mesoporous silica (DMS)-containing mixed matrix membranes for CO2 and non-CO2 greenhouse gas separations. Sep. Purif. Technol. 2014, 136, 286–295. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Martin-Gil, V.; Supinkova, T.; Lambert, P.; Castro-Muñoz, R.; Hrabanek, P.; Kocirik, M.; Fila, V. Novel MMM using CO2 selective SSZ-16 and high-performance 6FDA-polyimide for CO2/CH4 separation. Sep. Purif. Technol. 2021, 254, 117582. [Google Scholar] [CrossRef]
- Lively, R.P.; Dose, M.E.; Xu, L.; Vaughn, J.T.; Johnson, J.; Thompson, J.A.; Zhang, K.; Lydon, M.E.; Lee, J.-S.; Liu, L.; et al. A high-flux polyimide hollow fiber membrane to minimize footprint and energy penalty for CO2 recovery from flue gas. J. Membr. Sci. 2012, 423–424, 302–313. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. High pressure gas separation performance of mixed-matrix polymer membranes containing mesoporous Fe(BTC). J. Membr. Sci. 2014, 459, 33–44. [Google Scholar] [CrossRef]
- Ding, S.; Li, X.; Ding, S.; Zhang, W.; Guo, R.; Zhang, J. Ionic liquid-decorated nanocages for cooperative CO2 transport in mixed matrix membranes. Sep. Purif. Technol. 2020, 239, 116539. [Google Scholar] [CrossRef]
- Ge, L.; Zhou, W.; Rudolph, V.; Zhu, Z. Mixed matrix membranes incorporated with size-reduced Cu-BTC for improved gas separation. J. Mater. Chem. A 2013, 1, 6350–6358. [Google Scholar] [CrossRef]



| S-Version | L-Version | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 wt.% MOF Loading | 30 wt.% MOF Loading | 10 wt.% MOF Loading | 30 wt.% MOF Loading | ||||||||||
| Evap. Rate | Fast | Medium | Slow | Fast | Medium | Slow | Fast | Medium | Slow | Fast | Medium | Slow | |
| Pol. Con. | |||||||||||||
| 3 wt.% | − | − | + | − | + | + | − | + | + | − | − | − | |
| 5 wt.% | + | ++ | ++ | − | ++ | ++ | + | ++ | ++ | − | ++ | ++ | |
| 10 wt.% | + | ++ | ++ | − | − | ++ | + | ++ | ++ | NA | NA | NA | |
| 15 wt.% | − | ++ | ++ | NA | NA | NA | + | ++ | ++ | NA | NA | NA | |
| Solvent | Boiling Point (°C) | Density a (g/cm3) | Viscosity b (mPa·s) | Vapor Pressure c (kPa) | Surface Tension d (mN/m) | Relative Polarity | Dispersion of Filler e |
|---|---|---|---|---|---|---|---|
| THF | 65 | 0.8892 | 0.456 | 21.6 | 26.40 | 0.207 | Fair |
| DCM | 40 | 1.3266 | 0.413 | 58.2 | 27.20 | 0.309 | Good |
| Chloroform | 61 | 1.4832 | 0.537 | 26.2 | 26.67 | 0.259 | Good |
| MEK | 80 | 0.8054 | 0.405 | 12.6 | 23.97 | 0.327 | Poor |
| S-Version | L-Version | |||||
|---|---|---|---|---|---|---|
| Solvent | 0 wt.% MOF | 10 wt.% MOF | 30 wt.% MOF | 0 wt.% MOF | 10 wt.% MOF | 30 wt.% MOF |
| THF | ++ | ++ | ++ | ++ | ++ | ++ |
| DCM | ++ | ++ | − | ++ | ++ | − |
| Chloroform | ++ | + | ++ | ++ | ++ | ++ |
| MEK | + | + | + | + | + | + |
| Polymer Concentration | S-Version | L-Version | ||||
|---|---|---|---|---|---|---|
| 0 wt.% MOF | 10 wt.% MOF | 30 wt.% MOF | 0 wt.% MOF | 10 wt.% MOF | 30 wt.% MOF | |
| 3 wt.% | ++ | + | + | ++ | + | ++ |
| 4 wt.% | ++ | + | + | ++ | + | ++ |
| 5 wt.% | ++ | + | ++ | ++ | ++ | ++ |
| 7 wt.% | ++ | + | ++ | + | ++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulak, H.; Thür, R.; Vankelecom, I.F.J. MOF/Polymer Mixed-Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance. Membranes 2022, 12, 425. https://doi.org/10.3390/membranes12040425
Kulak H, Thür R, Vankelecom IFJ. MOF/Polymer Mixed-Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance. Membranes. 2022; 12(4):425. https://doi.org/10.3390/membranes12040425
Chicago/Turabian StyleKulak, Harun, Raymond Thür, and Ivo F. J. Vankelecom. 2022. "MOF/Polymer Mixed-Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance" Membranes 12, no. 4: 425. https://doi.org/10.3390/membranes12040425
APA StyleKulak, H., Thür, R., & Vankelecom, I. F. J. (2022). MOF/Polymer Mixed-Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance. Membranes, 12(4), 425. https://doi.org/10.3390/membranes12040425







