Membrane Bioreactor for Simultaneous Synthesis and Fractionation of Oligosaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological and Chemical Materials
2.2. Enzyme Assays
2.2.1. Enzyme Activity
2.2.2. Total Protein Concentration
2.3. Sugar Analysis
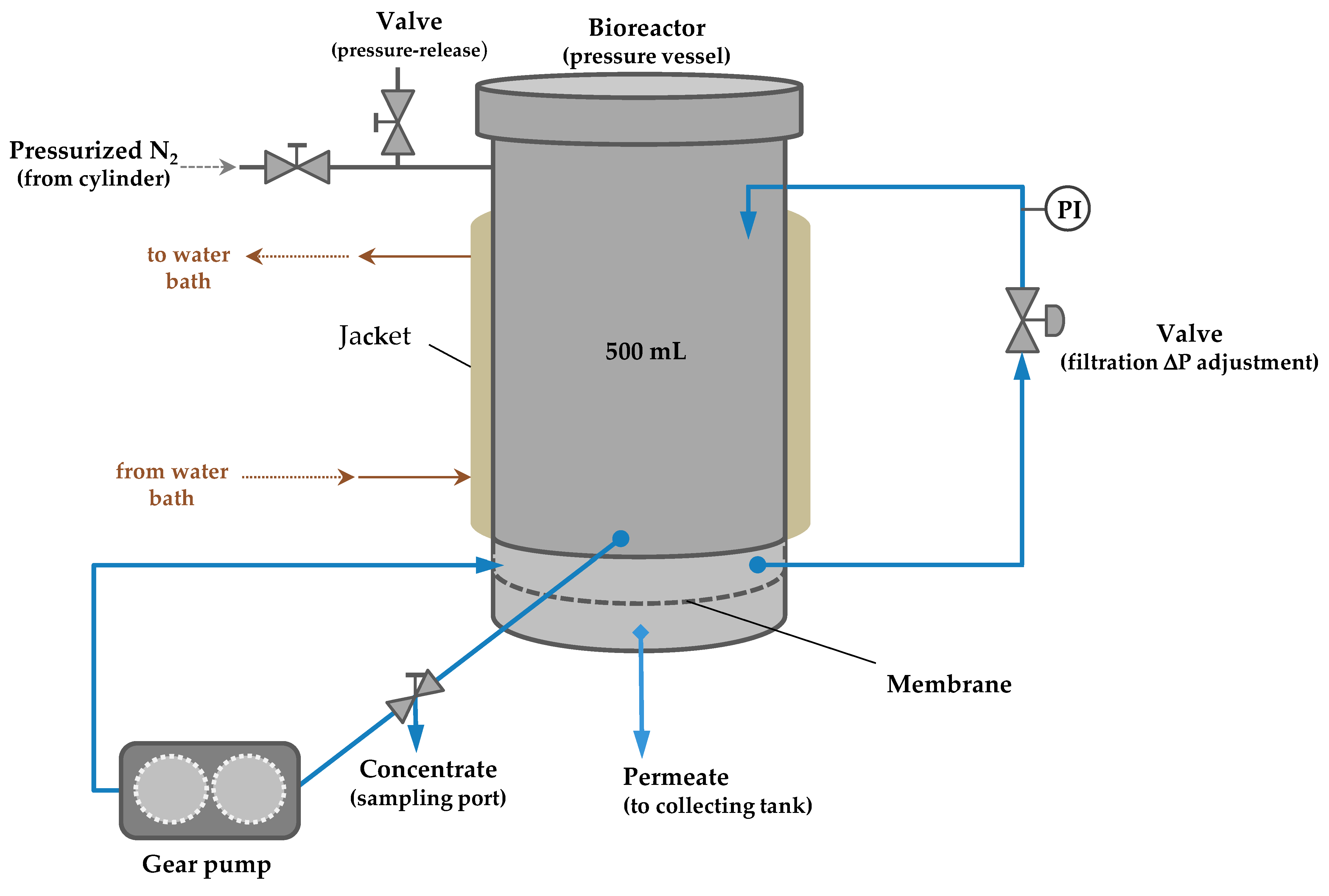
2.4. Membrane Bioreactor
2.4.1. Experimental Set-Up
2.4.2. Membranes
2.4.3. Process Operation Conditions
2.5. Membrane Regeneration
3. Results
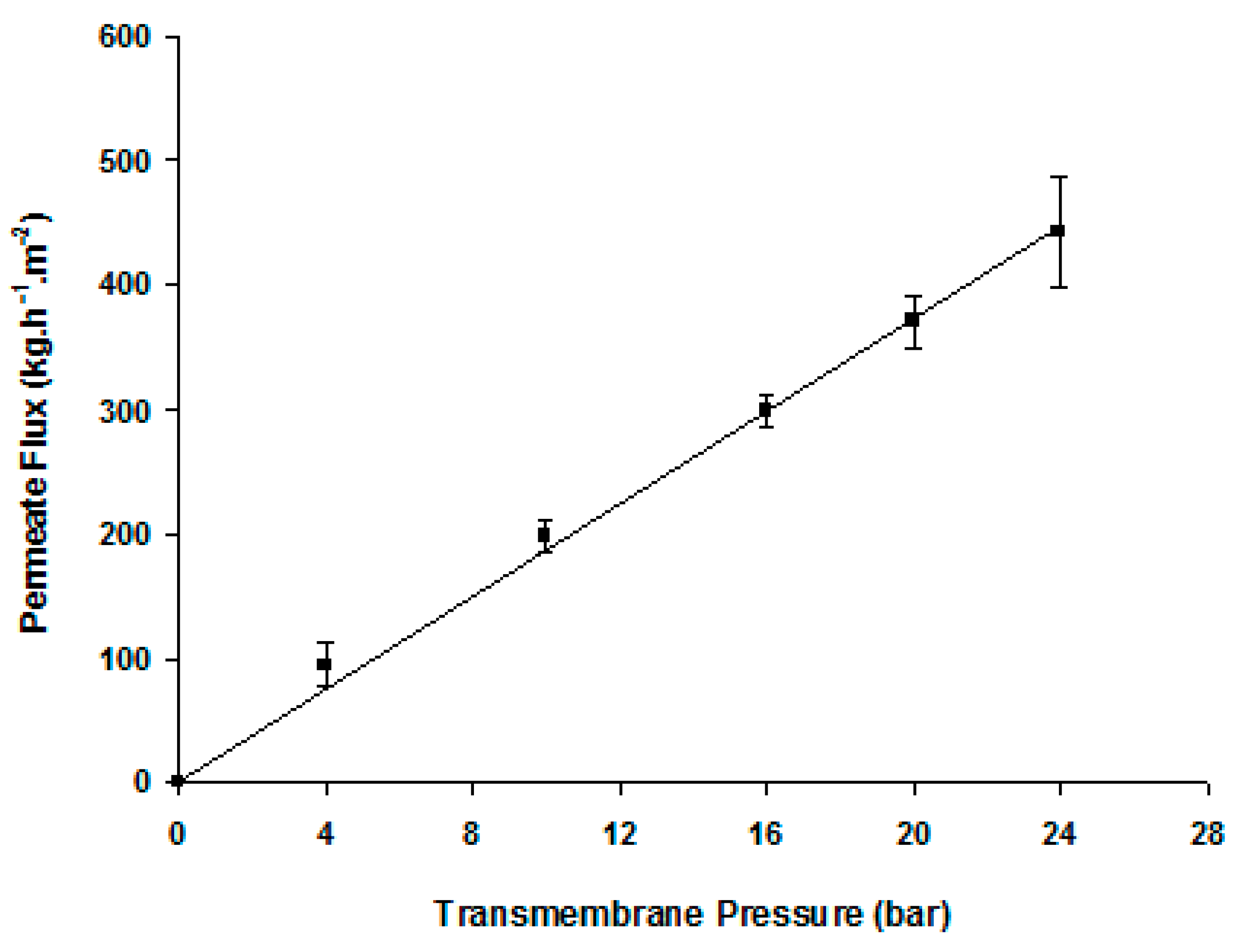
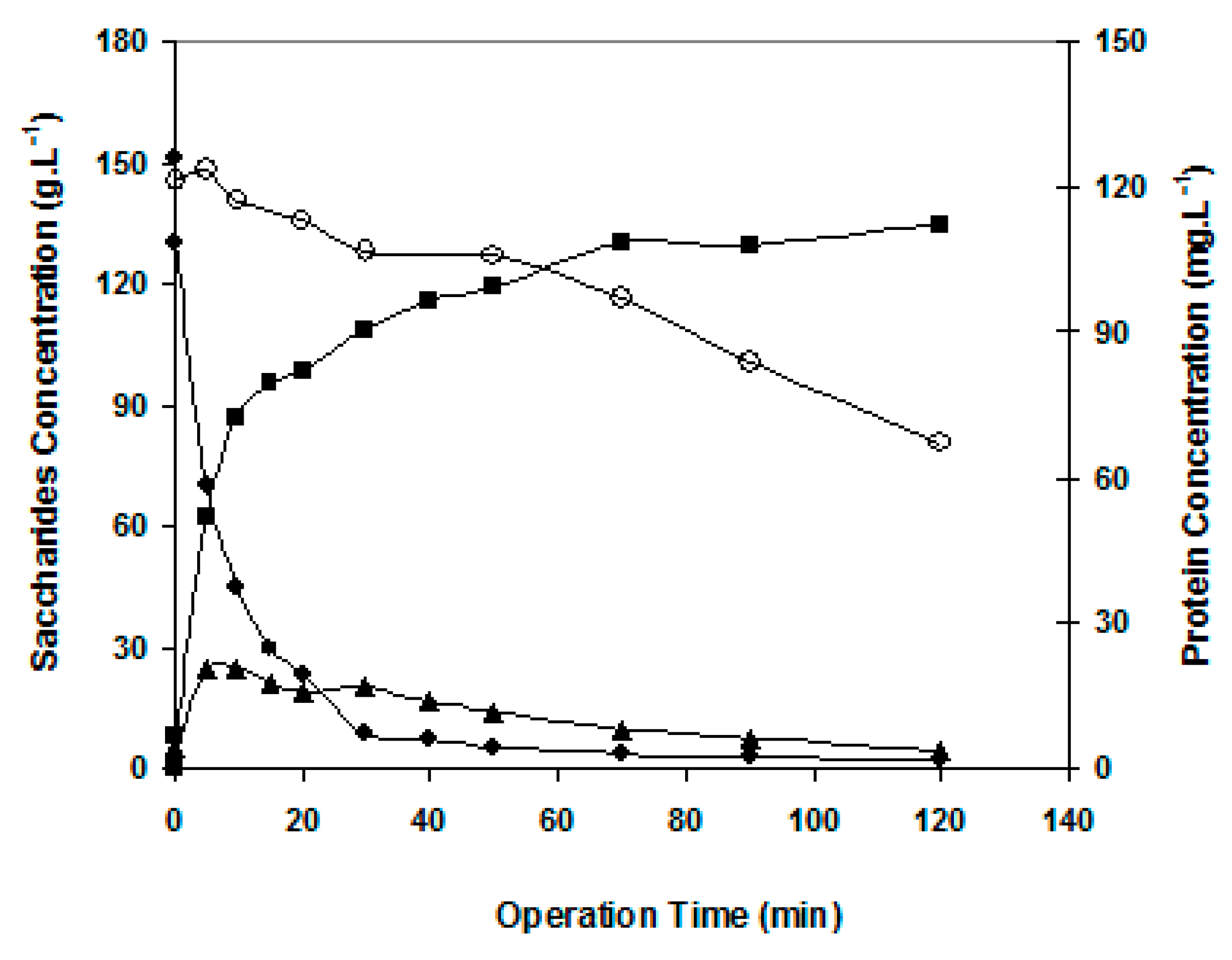
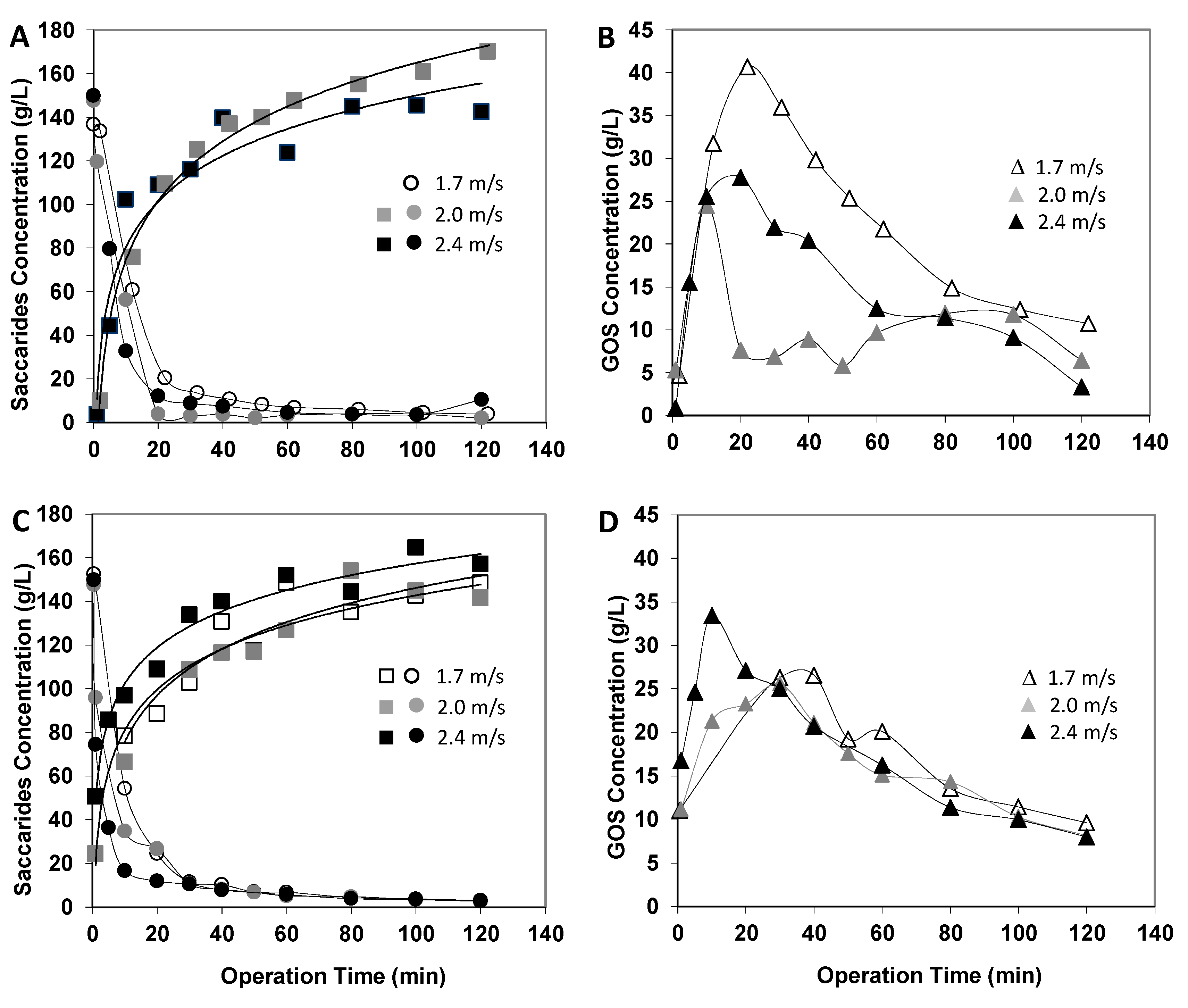
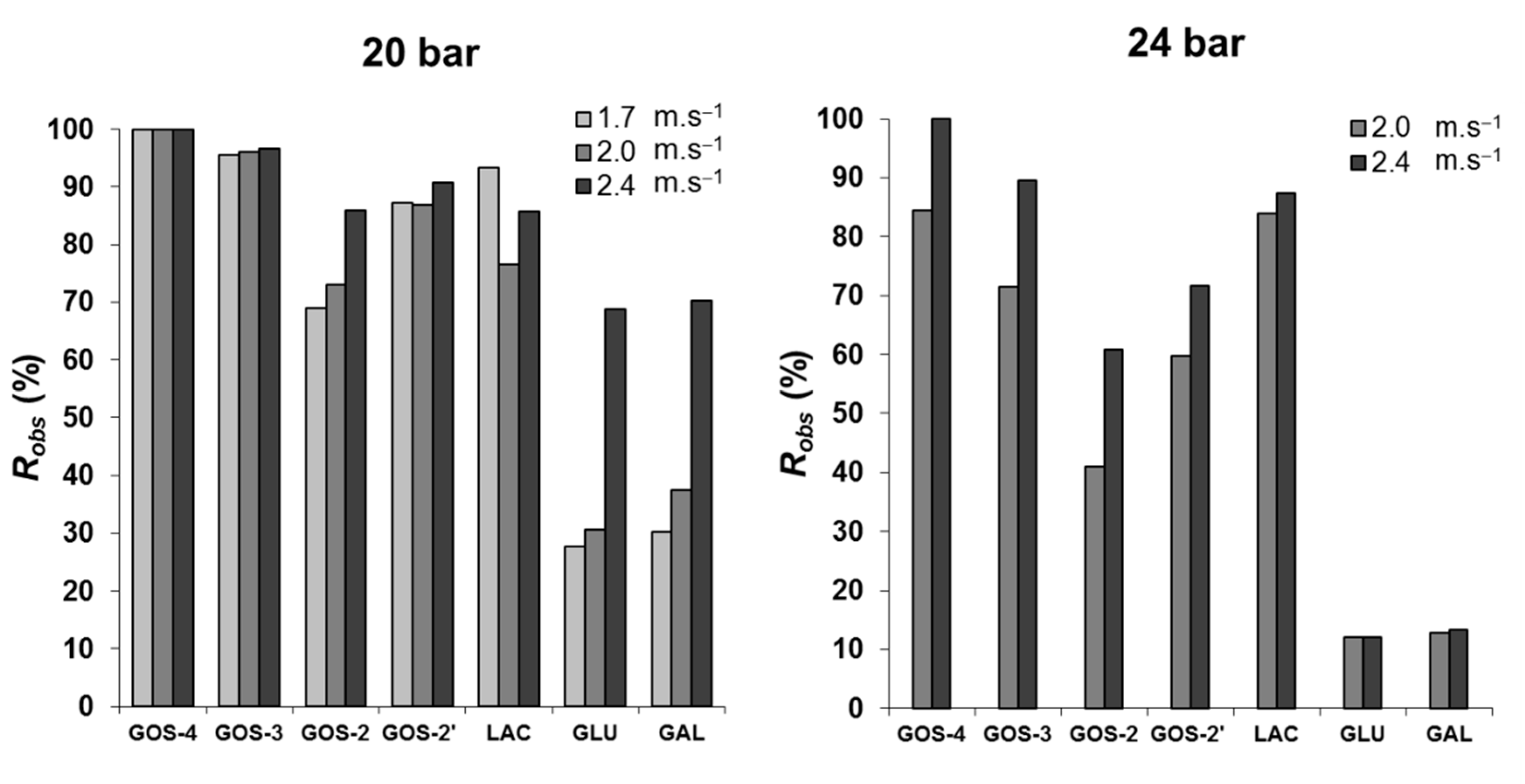
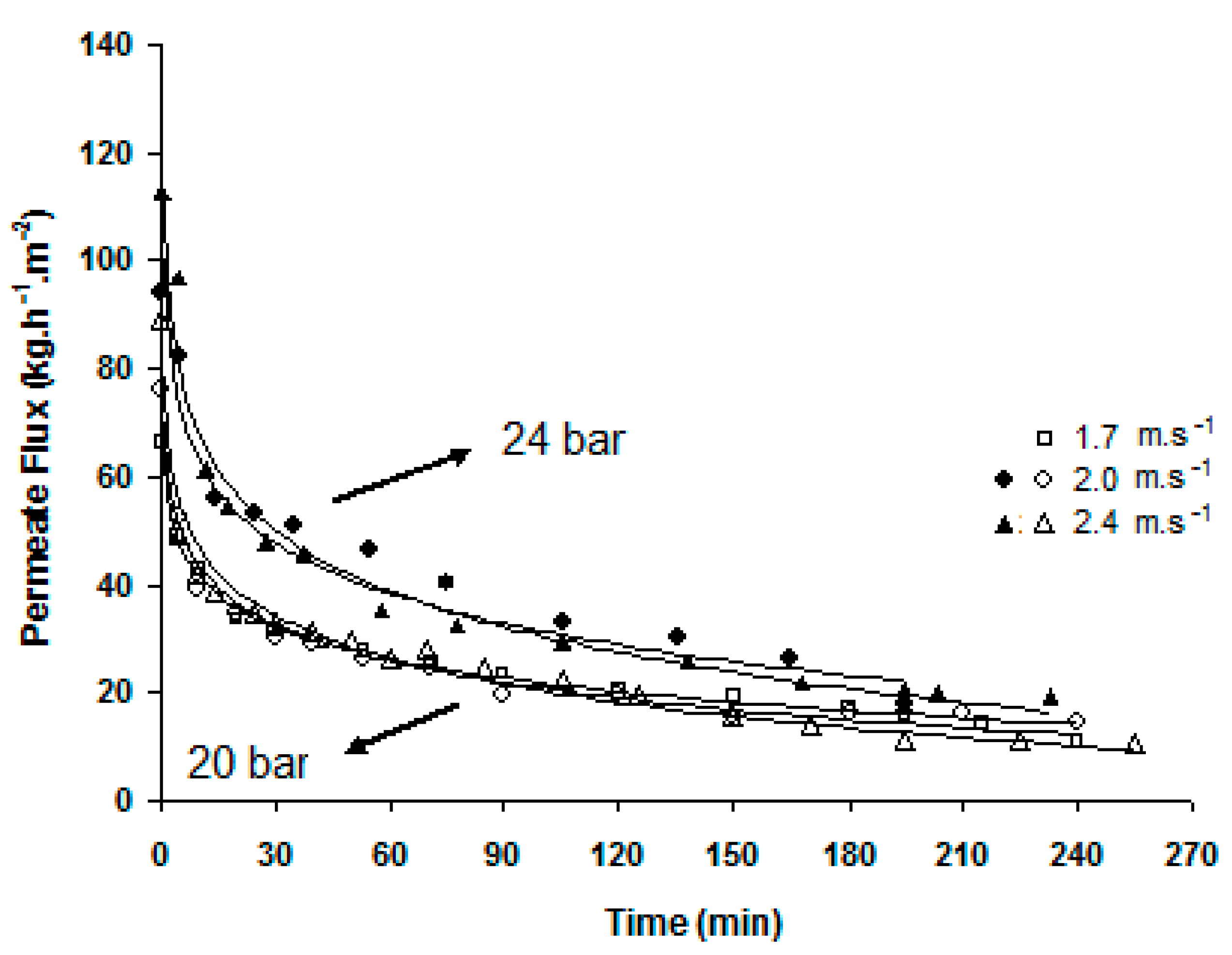
3.1. Membrane Bioreactor Characterization and Optimization
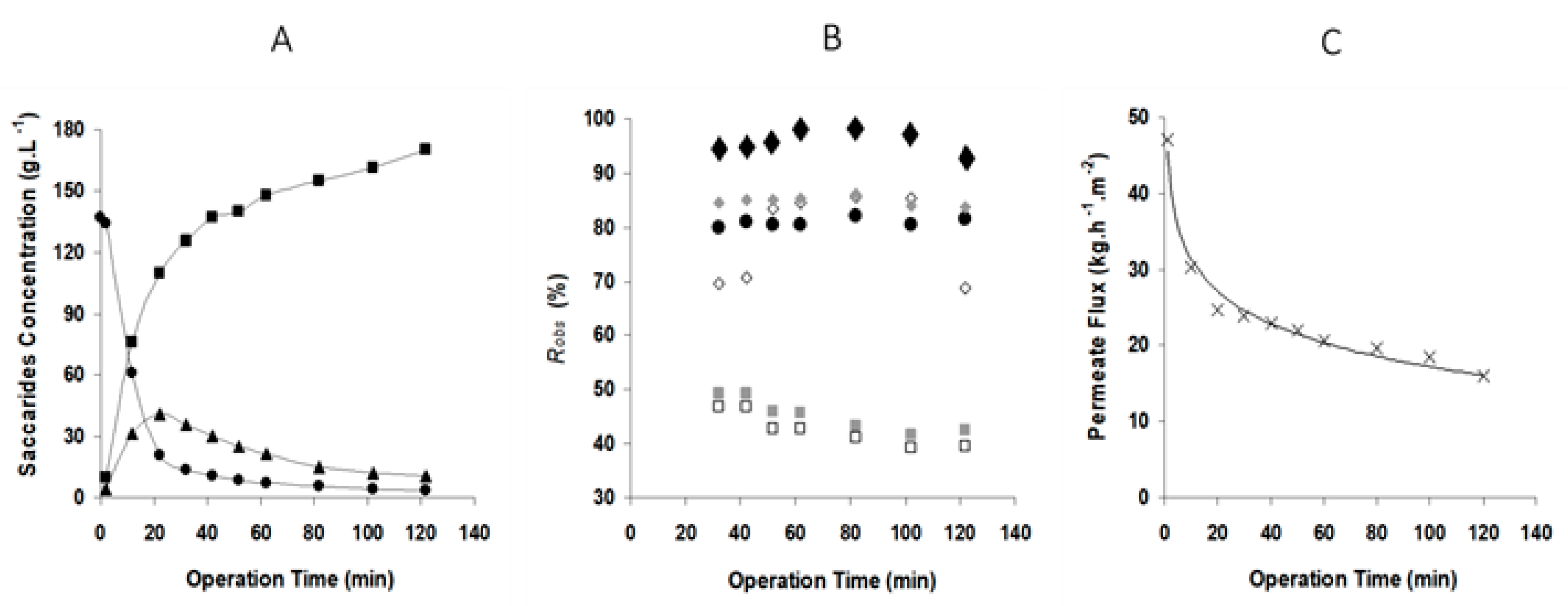
3.2. Membrane Regeneration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Fischer, C.; Kleinschmidt, T. Synthesis of galactooligosaccharides in milk and whey: A review. Compr. Rev. Food. Sci. Food Saf. 2018, 17, 678–697. [Google Scholar] [CrossRef]
- Botelho-Cunha, V.A.; Mateus, M.; Petrus, J.C.C.; Pinho, M.N. Tailoring the enzymatic synthesis and nanofiltration fractionation of galacto-oligosaccharides. Biochem. Eng. J. 2010, 50, 29–36. [Google Scholar] [CrossRef]
- Boon, M.A.; van′t Riet, K.; Janssen, A.E.M. Enzymatic synthesis of oligosaccharides: Product removal during a kinetically controlled reaction. Biotechnol. Bioeng. 2000, 70, 411–420. [Google Scholar] [CrossRef]
- Vera, C.; Córdova, A.; Aburto, C.; Guerrero, C.; Suárez, S.; Illanes, A. Synthesis and purification of galacto-oligosaccharides: State of the art. World J. Microbiol. Biotechnol. 2016, 32, 197. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, L.; Córdova, A.; Astudillo-Castro, C.; Illanes, A. Effect of the lactose hydrolysis on galacto-oligosaccharides mixtures subjected to nanofiltration: A detailed fractionation analysis. Sep. Purif. Technol. 2019, 222, 342–351. [Google Scholar] [CrossRef]
- Catarino, I.; Minhalma, M.; Beal, L.L.; Mateus, M.; Pinho, M.N. Assessment of saccharide fractionation by ultrafiltration and nanofiltration. J. Membr. Sci. 2008, 312, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.; Pázmándi, M.; Galambos, I.; Kovács, Z. Continuous production of galacto-oligosaccharides by an enzyme membrane reactor utilizing free enzymes. Membranes 2020, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Astudillo, C.; Santibañez, L.; Cassano, A.; Ruby-Figueroa, R.; Illanes, A. Purification of galacto-oligosaccharides (GOS) by three-stage serial nanofiltration units under critical transmembrane pressure conditions. Chem. Eng. Res. Des. 2017, 117, 488–499. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Illanes, A. Membrane technology for the purification of enzymatically produced oligosaccharides. In Separation of Functional Molecules in Food by Membrane Technology; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 113–153. [Google Scholar] [CrossRef]
- Su, Z.; Luo, J.; Li, X.; Pinelo, M. Enzyme membrane reactors for production of oligosaccharides: A review on the interdependence between enzyme reaction and membrane separation. Sep. Purif. Technol. 2020, 243, 116840. [Google Scholar] [CrossRef]
- Singh, R.D.; Muir, J.; Arora, A. Concentration of xylooligosaccharides with a low degree of polymerization using membranes and their effect on bacterial fermentation. Biofuels Bioprod. Biorefining 2021, 15, 61–73. [Google Scholar] [CrossRef]
- Fan, R.; Burghardt, J.P.; Dresler, J.; Czermak, P. Process design for the production of prebiotic oligosaccharides in an enzyme membrane bioreactor: Interaction between enzymatic reaction and membrane filtration. Chem. Ing. Tech. 2021, 93, 306–310. [Google Scholar] [CrossRef]
- Maugard, T.; Gaunt, D.; Legoy, M.D.; Besson, T. Microwave-assisted synthesis of galacto-oligosaccharides from lactose with immobilized β-galactosidase from Kluyveromyces lactis. Biotechnol. Lett. 2003, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, R.R. Galactosyl-oligosaccharide formation during lactose hydrolysis: A review. Food Chem. 1998, 63, 147–154. [Google Scholar] [CrossRef]
- Bouchoux, A.; Balmann, H.R.; Lutin, F. Nanofiltration of glucose and sodium lactate solutions: Variations of retention between single- and mixed-solute solutions. J. Membr. Sci. 2005, 258, 123–132. [Google Scholar] [CrossRef] [Green Version]
- De, S.; Bhattacharya, P.K. Modeling of ultrafiltration process for a two-component aqueous solution of low and high (gel-forming) molecular weight solutes. J. Membr. Sci. 1997, 136, 57–69. [Google Scholar] [CrossRef]
- Morison, K.R.; Mackay, F.M. Viscosity of lactose and whey protein solutions. Int. J. Food Prop. 2001, 4, 441–454. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; (reprint); Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Winter, J.; Barbeau, B.; Bérubé, P. Nanofiltration and tight ultrafiltration membranes for natural organic matter removal—Contribution of fouling and concentration polarization to filtration resistance. Membranes 2017, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Drews, A. Membrane fouling in membrane bioreactors—Characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363, 1–28. [Google Scholar] [CrossRef]


| Reference Solutes | NaCl | Na2HPO4 | Glucose | Lactose | Raffinose |
|---|---|---|---|---|---|
| Rejection (%) | 3 | 25 | 8 | 3 | 25 |
| Pressure | Velocity | Concentration (g·L−1) | |||||
|---|---|---|---|---|---|---|---|
| (bar) | (m.s−1) | LAC | GOS-4 | GOS-3 | GOS-2t ¥ | GLU | GAL |
| 1.7 | 7.6 | 0.3 | 1.8 | 4.5 | 89.2 | 90.9 | |
| 20 | 2.0 | 10.3 | 0.4 | 1.9 | 4.7 | 79.6 | 85.7 |
| 2.4 | 5.6 | 0.5 | 3.9 | 10.5 | 94.4 | 96.7 | |
| 24 | 2.0 | 12.0 | 0.6 | 3.0 | 6.3 | 90.9 | 91.9 |
| 2.4 | 9.5 | 0.7 | 3.3 | 6.6 | 93.3 | 94.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botelho, V.A.; Mateus, M.; Petrus, J.C.C.; de Pinho, M.N. Membrane Bioreactor for Simultaneous Synthesis and Fractionation of Oligosaccharides. Membranes 2022, 12, 171. https://doi.org/10.3390/membranes12020171
Botelho VA, Mateus M, Petrus JCC, de Pinho MN. Membrane Bioreactor for Simultaneous Synthesis and Fractionation of Oligosaccharides. Membranes. 2022; 12(2):171. https://doi.org/10.3390/membranes12020171
Chicago/Turabian StyleBotelho, Vanessa A., Marília Mateus, José C. C. Petrus, and Maria Norberta de Pinho. 2022. "Membrane Bioreactor for Simultaneous Synthesis and Fractionation of Oligosaccharides" Membranes 12, no. 2: 171. https://doi.org/10.3390/membranes12020171






