Treatment of Boiler Condensate by Ultrafiltration for Reuse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effluent
2.2. Membranes
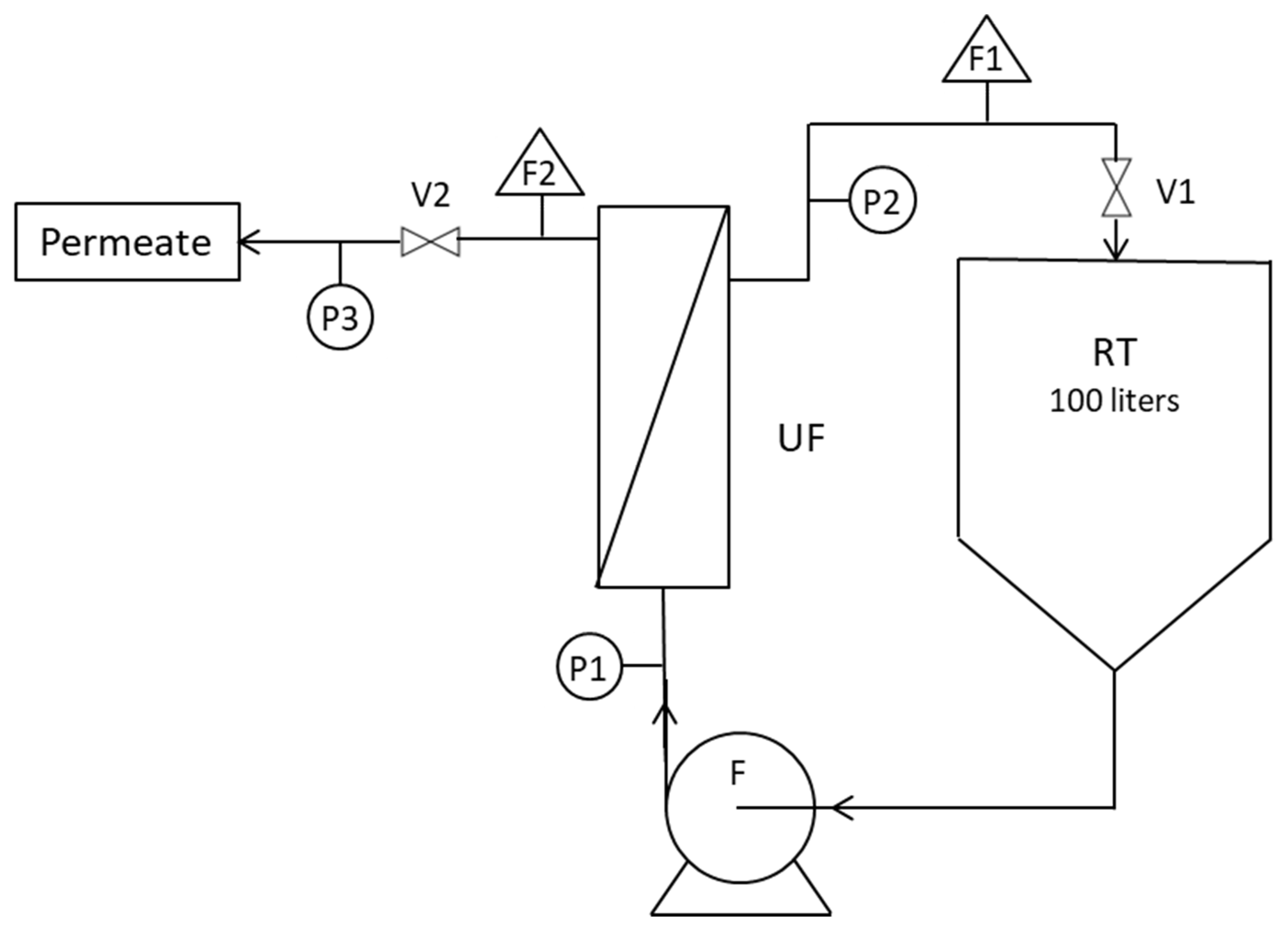
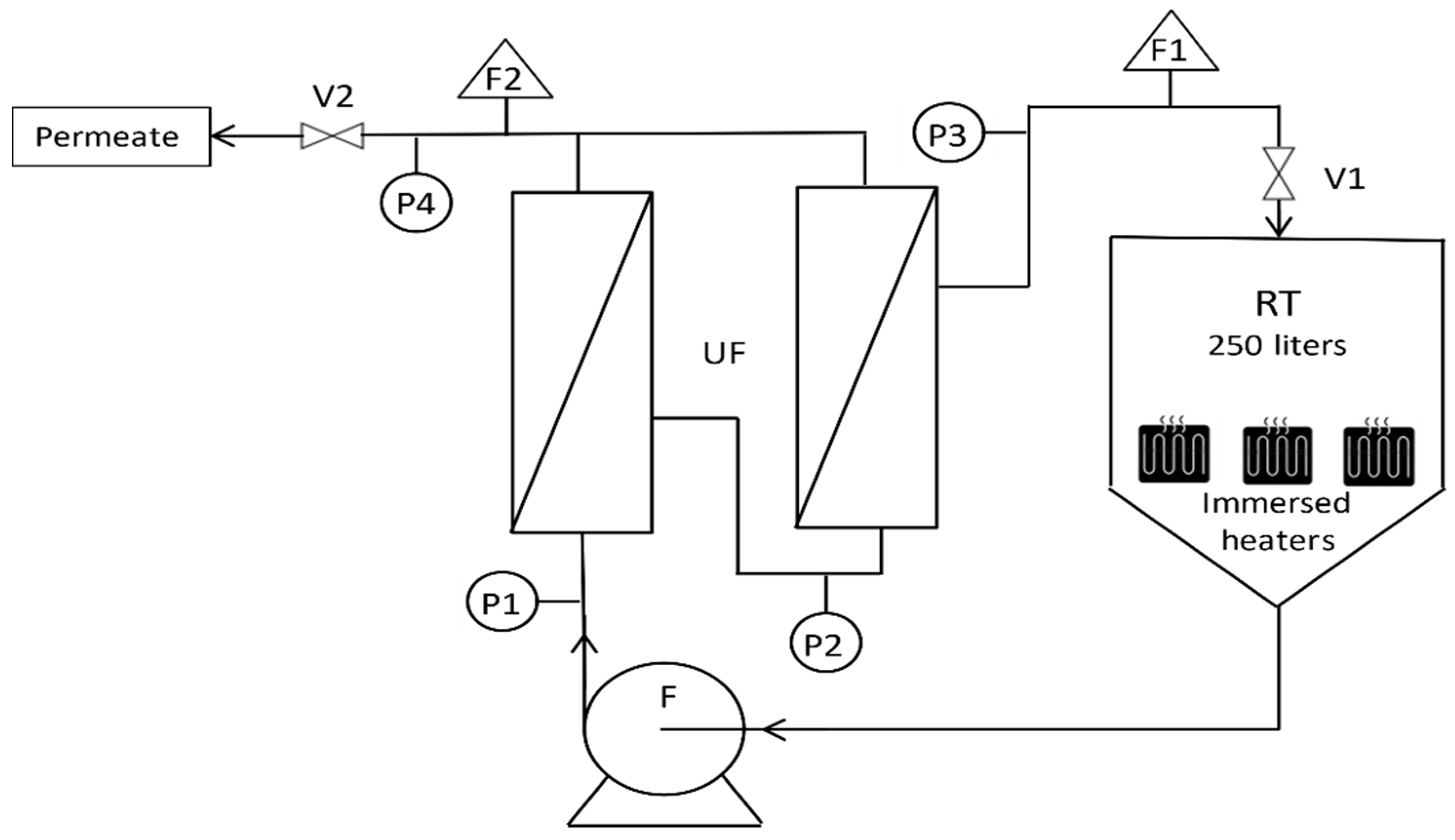
2.3. Apparatus
3. Results
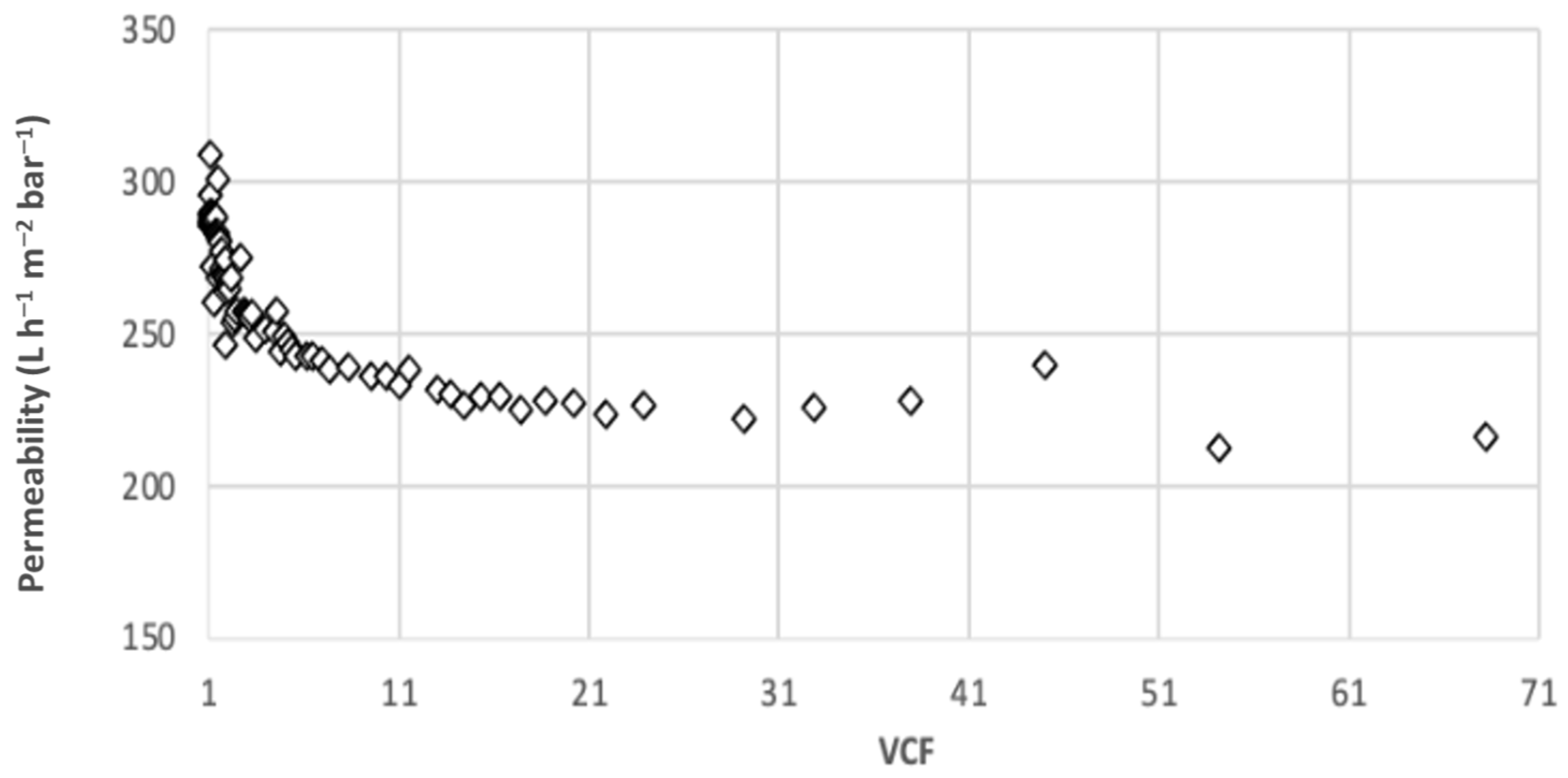
3.1. Filtration at Laboratory Scale
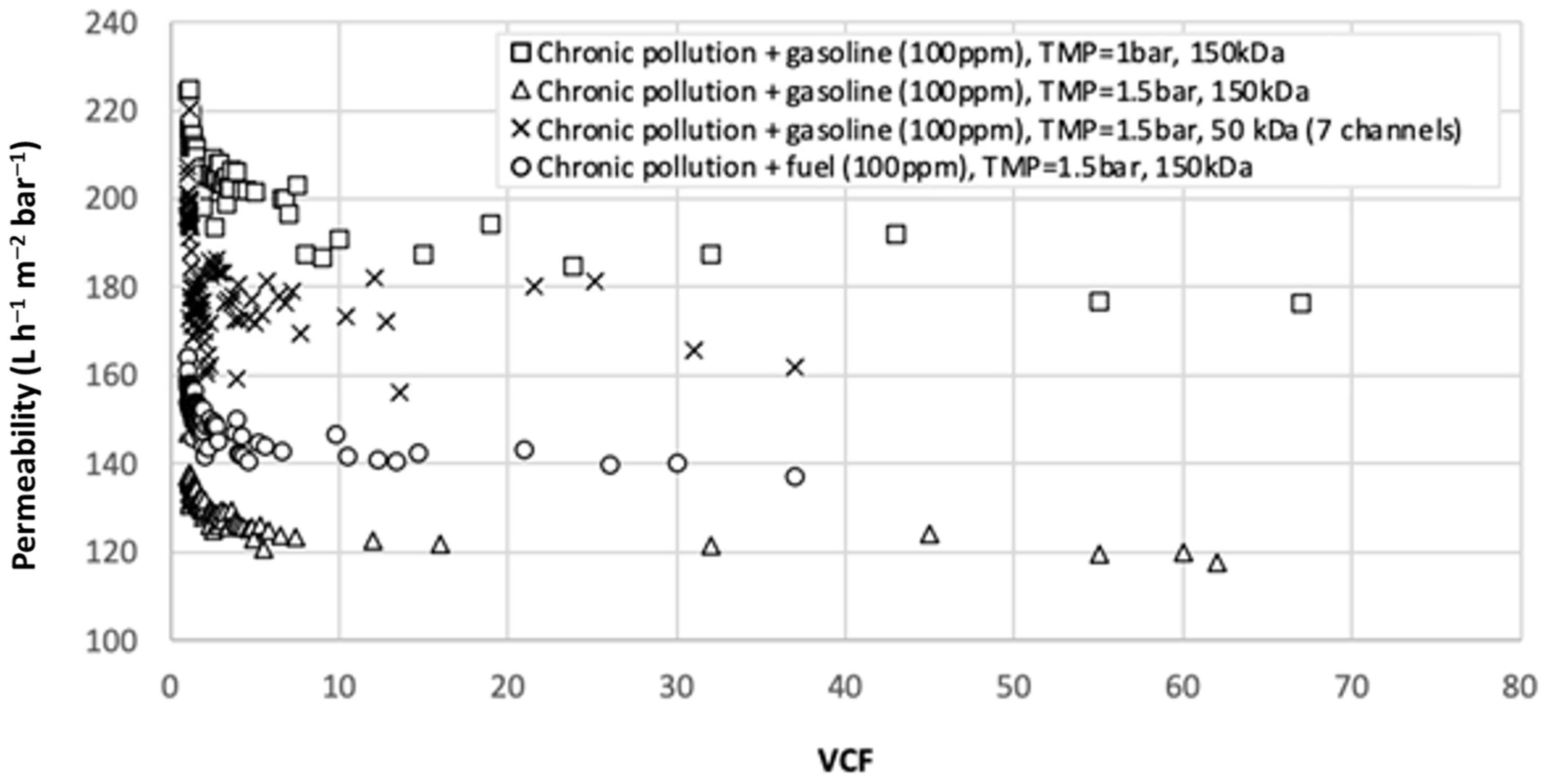
3.2. Filtration with Semi-Industrial Pilot Plant at High Temperature
3.3. Filtration with Semi-Industrial Pilot Plant on Line
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fakhru’L-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. A review of oily wastewater treatment using ultrafiltration membrane: A parametric study to enhance the membrane performance. J. Water Process Eng. 2020, 36, 101289. [Google Scholar] [CrossRef]
- Salahi, A.; Abbasi, M.; Mohammadi, T. Permeate flux decline during UF of oily wastewater: Experimental and modeling. Desalination 2010, 251, 153–160. [Google Scholar] [CrossRef]
- Sayegh, A.; Merkert, S.; Zimmermann, J.; Horn, H.; Saravia, F. Treatment of Hydrothermal-Liquefaction Wastewater with Crossflow UF for Oil and Particle Removal. Membranes 2022, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Abadi, S.R.H.; Sebzari, M.R.; Hemati, M.; Rekabdar, F.; Mohammadi, T. Ceramic membrane performance in microfiltration of oily wastewater. Desalination 2011, 265, 222–228. [Google Scholar] [CrossRef]
- Handbook of Refractory Practice; Harbison-Walker: Moom Township, PA, USA, 2005; Available online: https://www.mha-net.org/docs/Harbison%20Walker%202005%20Handbook.pdf (accessed on 20 November 2022).
- Zuo, H.-R.; Shi, P.; Duan, M. A review on thermally stable membranes for water treatment: Material, fabrication, and application. Sep. Purif. Technol. 2020, 236, 116223. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Ghidossi, R.; Veyret, D.; Scotto, J.L.; Jalabert, T.; Moulin, P. Ferry oily wastewater treatment. Sep. Purif. Technol. 2009, 64, 296–303. [Google Scholar] [CrossRef]
- Listiarini, K.; Chun, W.; Sun, D.D.; Leckie, J.O. Fouling mechanism and resistance analyses of systems containing sodium alginate, calcium, alum and their combination in dead-end fouling of nanofiltration membranes. J. Membr. Sci. 2009, 344, 244–251. [Google Scholar] [CrossRef]
- Basso, R.C.; Viotto, L.A.; Gonçalves, L.A.G. Cleaning process in ceramic membrane used for the ultrafiltration of crude soybean oil. Desalination 2006, 200, 85–86. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Application of ultrafiltration ceramic membrane for separation of oily wastewater generated by maritime transportation. Sep. Purif. Technol. 2021, 261, 118259. [Google Scholar] [CrossRef]
- Dos Santos, J.D.; Veit, M.T.; Palácio, S.M.; da Cunha Gonçalves, G.; Fagundes-Klen, M.R. Evaluation of the Combined Process of Coagulation/Flocculation and Microfiltration of Cassava Starch Wastewater: Removal Efficiency and Membrane Fouling. Water Air Soil Pollut. 2017, 228, 238. [Google Scholar] [CrossRef]
- Widiasa, I.N.; Wenten, I.G. Study on Membrane Fouling and Solutes Rejection During Ultrafiltration of Starcy Wastewater. In Proceedings of the Chemical Engineering Rekayasa Kim, Semarang, Indonesia, 21–22 July 2004. [Google Scholar]
- Wang, Z.; Ding, J.; Xie, P.; Chen, Y.; Wan, Y.; Wang, S. Formation of halogenated by-products during chemical cleaning of humic acid-fouled UF membrane by sodium hypochlorite solution. Chem. Eng. J. 2018, 332, 76–84. [Google Scholar] [CrossRef]
- Widiasa, I.N.; Wenten, I.G. Fouling Behaviour During Cross Flow Ultrafiltration of Cassava Starch Hydrolysate Using Polyacrilonitrile Membrane. J. Appl. Membr. Sci. Technol. 2005, 1, 29–45. [Google Scholar]
- Kim, S.; Park, C. Fouling behavior and cleaning strategies of ceramic ultrafiltration membranes for the treatment and reuse of laundry wastewater. J. Water Process Eng. 2022, 48, 102840. [Google Scholar] [CrossRef]
- Kestin, J.; Sokolov, M.; Wakeham, W.A. Viscosity of Liquid Water in the Range—8 C to 150 C. J. Phys. Chem. Ref. Data 1978, 7, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Bilstad, T.; Espedal, E. Membrane separation of produced water. Water Sci. Technol. 1996, 34, 239–246. [Google Scholar] [CrossRef]
- Weschenfelder, S.E.; Louvisse, A.M.; Borges, C.P.; Meabe, E.; Izquierdo, J.; Campos, J.C. Evaluation of ceramic membranes for oilfield produced water treatment aiming reinjection in offshore units. J. Pet. Sci. Eng. 2015, 131, 51–57. [Google Scholar] [CrossRef]
- Hua, F.L.; Tsang, Y.F.; Wang, Y.J.; Chan, S.Y.; Chua, H.; Sin, S.N. Performance study of ceramic microfiltration membrane for oily wastewater treatment. Chem. Eng. J. 2007, 128, 169–175. [Google Scholar] [CrossRef]


| Effluent | Chronicle Pollution | Accidental Pollution | Petrochemical Plant |
|---|---|---|---|
| Hydrocarbon (mg L−1) | 0.2 | >50 | 0.2 |
| Turbidity (NTU) | 0.66 | 1.13 | 2.12 |
| Conductivity (µS cm−1) | 96.4 | 12.5 | 4.41 |
| pH | 5.95 | 6.98 | 6.3 |
| Molecular Weight Cut Off | 150 kDa | 50 kDa | 50 kDa |
|---|---|---|---|
| Number of channels | 19 | 7 | 52 |
| Surface (m2) | 0.24 | 0.15 | 0.42 |
| Channel diameter (mm) | 3.5 | 6 | 2.2 |
| Length (m) | 1178 | 1178 | 1178 |
| Volume Concentration Factor | Sample | pH | Conductivity (µS cm−1) | Turbidity (NTU) | [HC] (mg L−1) |
|---|---|---|---|---|---|
| 1 | Concentrate | 5.95 | 96.4 | 0.66 | 0.2 |
| Permeate | 6.65 | 24.3 | 0.21 | 0.4 | |
| 2 | Concentrate | 6.5 | 42.1 | 1.73 | 0.8 |
| Permeate | 5.95 | 88.5 | 0.14 | 0.3 | |
| 70 | Concentrate | 6.3 | 96.4 | 26.7 | 15 |
| Permeate | 5.82 | 52.3 | 0.34 | 0.2 |
| Experiment | Transmembrane Pressure (bar) | pH | Conductivity (µS cm−1) | Turbidity Retention (%) | Permeate [HC] (mg L−1) | ||
|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||||
| Chronic pollution + gasoline (100 ppm), 150 kDa | 1 | 6.3 | 54.1 | 52 | 97.5 | <0.1 | <0.1 |
| Chronic pollution + gasoline (100 ppm), 150 kDa | 1.5 | 6.2 | 41.8 | 87.5 | 97.9 | <0.1 | <0.1 |
| Chronic pollution + fuel (100 ppm), 150 kDa | 1.5 | 6.2 | 28.5 | 68.3 | 99.4 | <0.1 | <0.1 |
| Chronic pollution + gasoline (100 ppm), 50 kDa (7 channels) | 1.5 | 6.8 | 35.9 | 93.8 | 99.8 | <0.1 | <0.1 |
| Experiment | Transmembrane Pressure (bar) | pH | Conductivity (µS cm−1) | Turbidity Retention (%) | Permeate [HC] (mg L−1) | ||
|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||||
| Accidental pollution 50 kDa (7 channels) | 1 | 6.7 | 55.2 | 31.3 | 97.4 | <0.1 | <0.1 |
| Accidental pollution 50 kDa (52 channels) | 1 | 6.9 | 12.5 | 79.6 | 90.6 | <0.1 | <0.1 |
| Initial Volume (L) | Transmembrane Pressure (bar) | pH | Conductivity (µS cm−1) | Turbidity Retention (%) | Permeate [HC] (mg L−1) | ||
|---|---|---|---|---|---|---|---|
| Initial | Final | Initial VCF | Final VCF | ||||
| 400 | 2 | 6.7 | 4.4 | 45.6 | 96.1 | <0.1 | <0.1 |
| 1500 | 2 | 6.8 | 6.6 | 81 | 92.8 | <0.1 | <0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano, G.; Moulin, P. Treatment of Boiler Condensate by Ultrafiltration for Reuse. Membranes 2022, 12, 1285. https://doi.org/10.3390/membranes12121285
Cano G, Moulin P. Treatment of Boiler Condensate by Ultrafiltration for Reuse. Membranes. 2022; 12(12):1285. https://doi.org/10.3390/membranes12121285
Chicago/Turabian StyleCano, Grégory, and Philippe Moulin. 2022. "Treatment of Boiler Condensate by Ultrafiltration for Reuse" Membranes 12, no. 12: 1285. https://doi.org/10.3390/membranes12121285






