Polyhexanide-Releasing Membranes for Antimicrobial Wound Dressings: A Critical Review
Abstract
:1. General Introduction and Aims
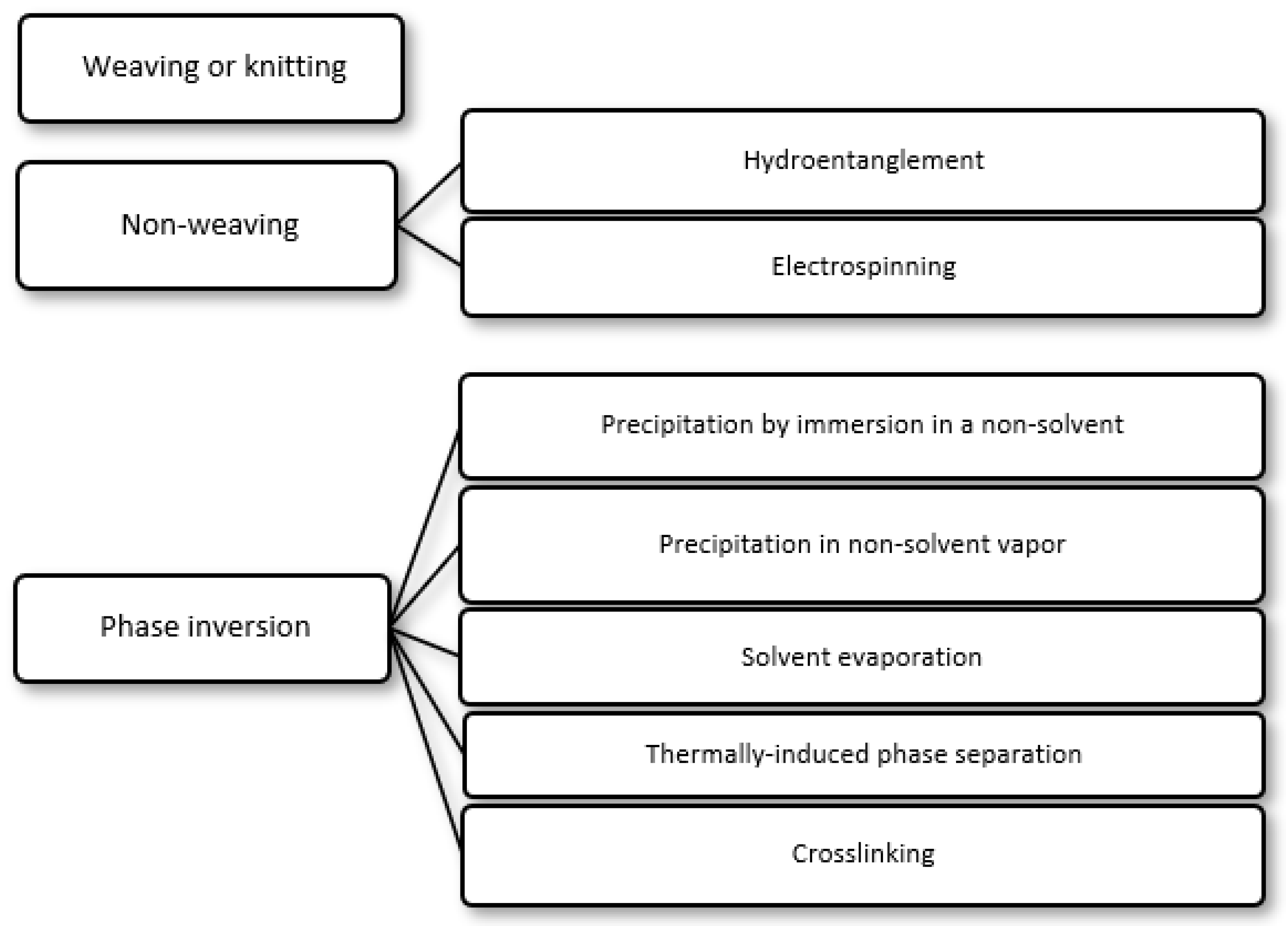
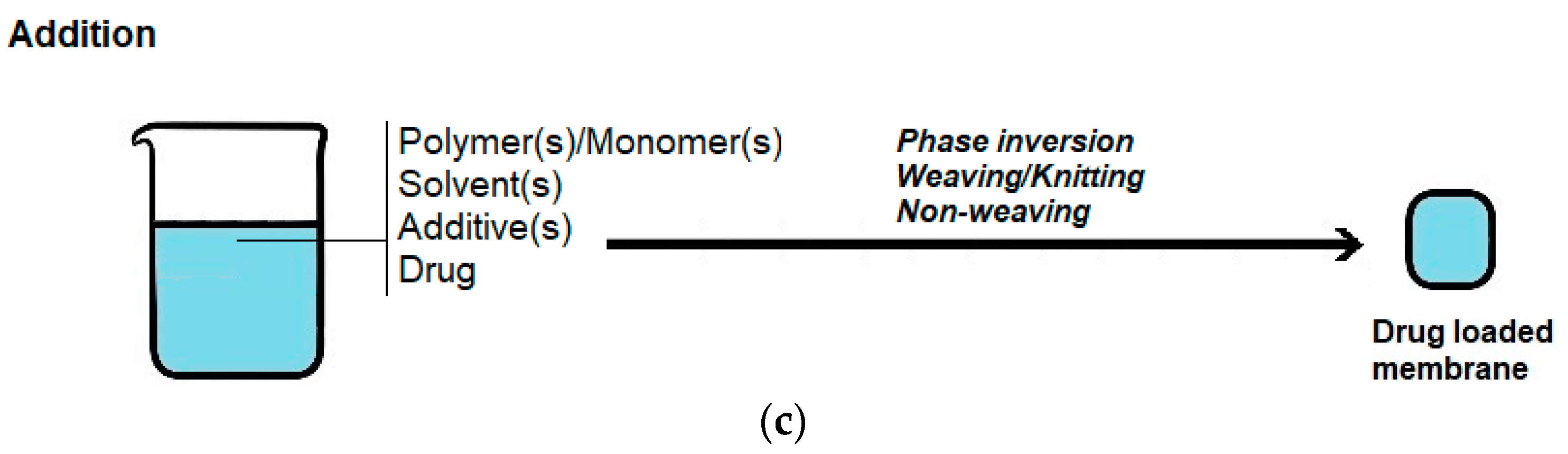
2. Preparation of Selective Polymer Membranes for Wound Dressings
- (i)
- Precipitation by immersion in a non-solvent, in which precipitation occurs due to the exchange of the solvent in the polymer solution by a non-solvent miscible with the solvent present in a coagulation bath;
- (ii)
- Precipitation in non-solvent vapor, in which phase inversion occurs inside a closed chamber in which the non-solvent is in the vapor phase and causes precipitation of the polymer in the solution;
- (iii)
- Solvent evaporation (also known as solution casting), in which the solvent of a polymer solution cast on a substrate is allowed to evaporate or is removed by heating the polymer solution in an oven;
- (iv)
- Thermally induced phase separation, in which a solvent that dissolves the polymer at a given temperature loses this ability when the temperature is decreased, with the solvent being subsequently removed by extraction, evaporation or lyophilization (freeze-drying);
- (v)
- Crosslinking, in which a polymer in solution is insolubilized by the formation of chemical bonds or by physical interactions between its molecular chains.
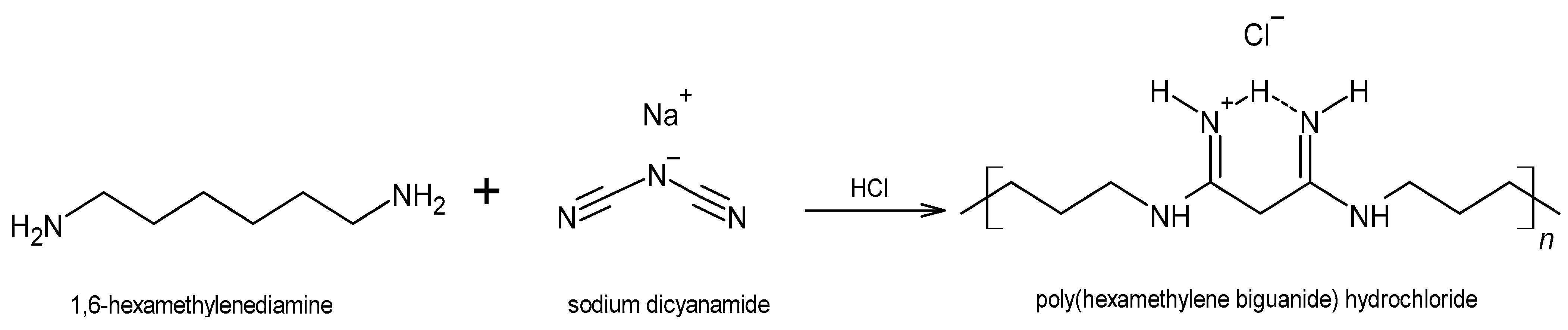
3. The Antiseptic Polyhexanide (PHMB)
4. Commercially Available PHMB-Releasing Wound Dressings (PRWDs)
5. PHMB-Releasing Membranes (PRMs) for Antimicrobial Dressings (AMDs)
5.1. Composition and Preparation Methods
5.1.1. Preparation of the Polymer Membrane
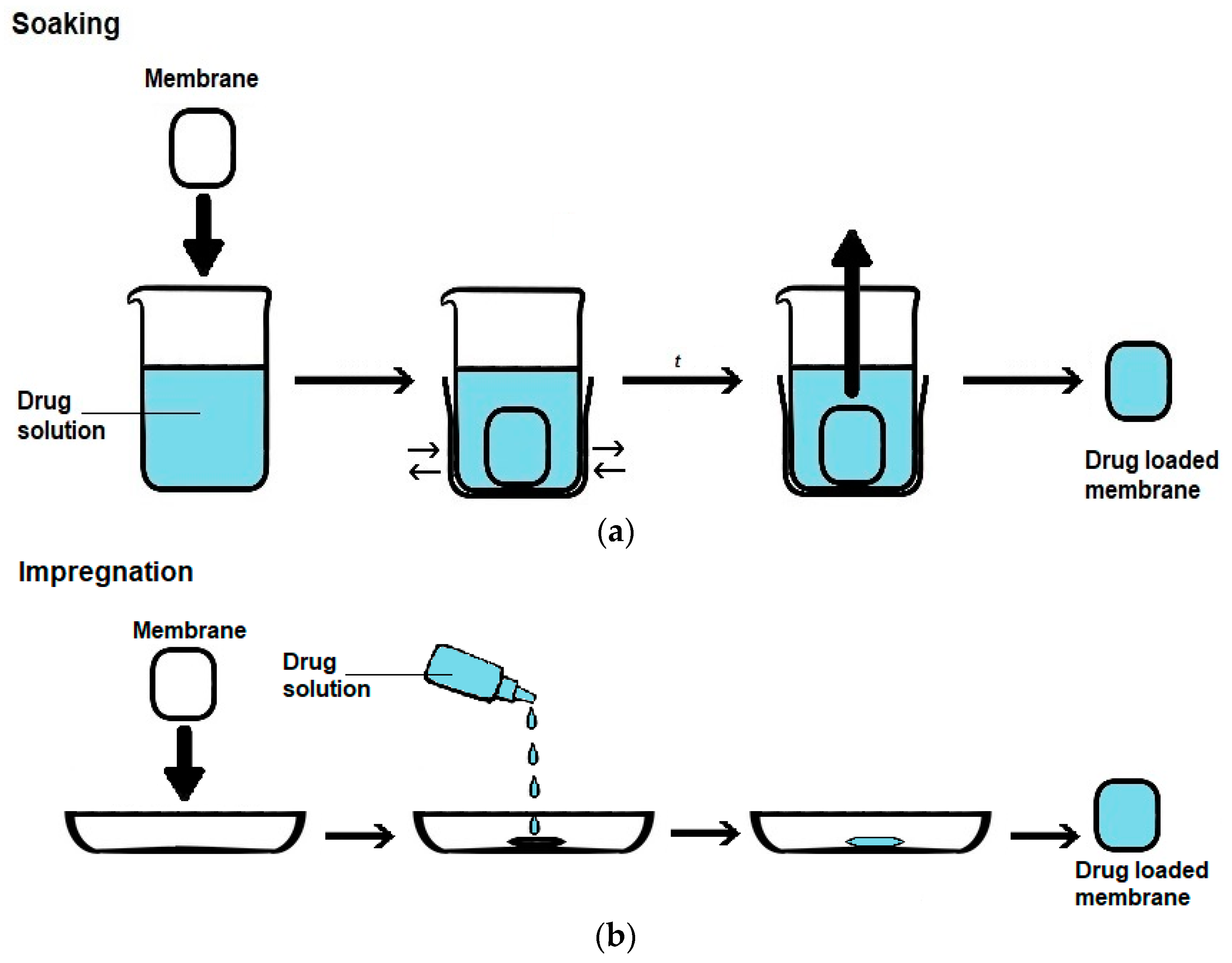
5.1.2. PHMB Loading
5.1.3. Sterilization
5.2. Characterization
5.2.1. Physical Properties
5.2.2. Drug Release
- (i)
- Distilled water;
- (ii)
- A salt solution composed of NaCl 8.6 g/L, KCl 0.3 g/L and CaCl2·2H2O 0.33 g/L;
- (iii)
- A 50 mM tris(hydroxymethyl)aminomethane (TRIS) solution at pH 7.4;
- (iv)
- PBS. The exact composition varies slightly according to the laboratory; a common composition is 140 mM NaCl, 10 mM phosphate buffer, and 3 mM KCl, with a pH of 7.4 at 25 °C [159];
- (v)
- “Simulated body fluid”, whose composition was not provided.
5.2.3. Biological Evaluation: Antimicrobial Activity
5.2.4. Biological Evaluation: Biocompatibility
5.2.5. Biological Evaluation: Wound Healing
5.2.6. Clinical Trials and Patents
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Graves, N.; Phillips, C.J.; Harding, K. A narrative review of the epidemiology and economics of chronic wounds. Br. J. Dermatol. 2021, 187, 141–148. [Google Scholar] [CrossRef]
- Gray, D.; Barrett, S.; Battacharyya, M.; Butcher, M.; Enoch, S.; Fumerola, S.; Stephen-Haynes, J.; Edwards-Jones, V.; Leaper, D.; Strohal, R.; et al. PHMB and its potential contribution to wound management. Wounds 2010, 6, 40–46. [Google Scholar]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on wound antisepsis: Update 2018. Skin. Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Op’t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F. Design considerations for hydrogel wound dressings: Strategic and molecular advances. Tissue Eng. Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.B. The history of wound care. J. Am. Col. Certif. Wound Spec. 2011, 3, 65–66. [Google Scholar] [CrossRef] [Green Version]
- Broughton, G.; Janis, J.E.; Attinger, C.E. A brief history of wound care. Plast. Reconstr. Surg. 2006, 117, 6S–11S. [Google Scholar] [CrossRef] [Green Version]
- Molan, P.; Rhodes, T. Honey: A biologic wound dressing. Wounds 2015, 27, 141–151. [Google Scholar]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Chindera, K.; Mahato, M.; Sharma, A.K.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edward-Jones, V.T.J.; Nichols, E.; Westgate, S.; Spruce, P. Should antimicrobial dressings be classified according to their activity and be subject to stewardship like antibiotics? Wounds 2019, 15, 20–23. [Google Scholar]
- MarketsandMarkets. Wound Dressings Market by Type (Traditional, Advanced (Alginate, Collagen, Hydrogel, Foam, Hydrocolloid, Film)), Wound Type (Traumatic, Surgical, Diabetic Foot, Venous Leg Ulcer & Burns), End User (Hospital, Ascs, Homecare)—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/wound-dressings-market-123903496.html (accessed on 26 November 2022).
- Kunjikuttan, R.V.P.; Jayasree, A.; Biswas, R.; Jayakumar, R. Recent developments in drug-eluting dressings for the treatment of chronic wounds. Exp. Opin. Drug Deliv. 2016, 13, 1645–1647. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.R.; Wang, Y. Drug delivery systems for wound healing. Curr. Pharm. Biotechnol. 2015, 16, 621–629. [Google Scholar] [CrossRef]
- Settipalli, S. A Robust Market Rich with Opportunities: Advanced Wound Dressings. Available online: https://www.pm360online.com/a-robust-market-rich-with-opportunities-advanced-wound-dressings/ (accessed on 12 December 2022).
- East, G.C.; McIntyre, J.E.; Shao, J. Polybiguanides: Synthesis and characterization of polybiguanides containing hexamethylene groups. Polymer 1997, 38, 3973–3984. [Google Scholar] [CrossRef]
- Küsters, M.; Beyer, S.; Kutscher, S.; Schlesinger, H.; Gerhartz, M. Rapid, simple and stability-indicating determination of polyhexamethylene biguanide in liquid and gel-like dosage forms by liquid chromatography with diode-array detection. J. Pharm. Anal. 2013, 3, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Zhang, J.; Feng, X.; Du, Z.; Jiang, Y.; Shi, Y.; Yang, G.; Tan, L. Polyhexamethylene biguanide chemically modified cotton with desirable hemostatic, inflammation-reducing, intrinsic antibacterial property for infected wound healing. Chin. Chem. Lett. 2022, 33, 2975–2981. [Google Scholar] [CrossRef]
- Gupta, B.S.; Edwards, J.V. Textile materials and structures for topical management of wounds. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 55–104. [Google Scholar]
- Moore, G.K. Aspects of nonwoven bonding—The development and potential of hydroentanglement. TAPPI J. 1988, 71, 112–114. [Google Scholar]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Ashjaran, A. Properties and applications of bacterial cellulose as a biological non-woven fabric. Asian J. Chem. 2013, 25, 783–788. [Google Scholar] [CrossRef]
- Parikh, D.V.; Calamari, T.A.; Sawhney, A.P.S.; Sachinvala, N.D.; Goynes, W.R.; Hemstreet, J.M.; Von Hoven, T. Woven and nonwoven medical/surgical materials. Int. Nonwovens J. 1999, os-8, 1558925099OS-1558800117. [Google Scholar] [CrossRef] [Green Version]
- Kesting, R.E. Phase inversion membranes. ACS Symp. Ser. 1985, 269, 131–164. [Google Scholar]
- Strathmann, H. Production of microporous media by phase inversion processes. ACS Symp. Ser. 1985, 269, 165–195. [Google Scholar]
- Bernauer, U.; Bodin, L.; Celleno, L.; Chaudhry, Q.; Coenraads, P.-J.; Dusinska, M.; Duus-Johansen, J.; Ezendam, J.; Gaffet, E.; Galli, C.L.; et al. SCCS Opinion on Polyaminopropyl Biguanide (PHMB)—Submission III, SCCS/1581/16, Preliminary Version of 23 December 2016; Scientific Committee on Consumer Safety—European Commission: Luxembourg, 2016. [Google Scholar]
- Gao, Y.; Cranston, R. Recent advances in antimicrobial treatments of textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar]
- Magina, S.; Santos, M.D.; Ferra, J.; Cruz, P.; Portugal, I.; Evtuguin, D. High pressure laminates with antimicrobial properties. Materials 2016, 9, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chivu, A.; Chindera, K.; Mendes, G.; An, A.; Davidson, B.; Good, L.; Song, W. Cellular gene delivery via poly(hexamethylene biguanide)/pDNA self-assembled nanoparticles. Eur. J. Pharm. Biopharm. 2021, 158, 62–71. [Google Scholar] [CrossRef]
- Bromberg, L.; Raduyk, S.; Hatton, T.A. Functional magnetic nanoparticles for biodefense and biological threat monitoring and surveillance. Anal. Chem. 2009, 81, 5637–5645. [Google Scholar] [CrossRef]
- Ren, L.; Chen, J.; Lu, Q.; Han, J.; Wu, H. Anti-biofouling nanofiltration membrane constructed by in-situ photo-grafting bactericidal and hydrophilic polymers. J. Membr. Sci. 2021, 617, 118658. [Google Scholar] [CrossRef]
- Chen, Y.D.; Shu, C.; Duan, Z.H.; Xu, J.J.; Li, X.J.; Chen, F.; Luo, Q.J.; Li, X.D. Synthesis and characterization of an anti-caries and remineralizing fluorine-containing cationic polymer PHMB-F. Biomater. Sci. 2021, 9, 2009–2019. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Chiou, J.-C.; Chen, W.-X.; Yu, J.-L.; Kan, C.-W. A salt-free, zero-discharge and dyebath-recyclable circular coloration technology based on cationic polyelectrolyte complex for cotton fabric dyeing. Cellulose 2022, 29, 1249–1262. [Google Scholar] [CrossRef]
- Britz, J.; Meyer, W.H.; Wegner, G. Poly(alkylene biguanides) as proton conductors for high-temperature pemfcs. Adv. Mater. 2010, 22, E72–E76. [Google Scholar] [CrossRef]
- Tanış, E. New optoelectronic material based on biguanide for orange and yellow organic light emitting diode: A combined experimental and theoretical study. J. Mol. Liq. 2022, 358, 119161. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Butt, M.A.; Khonina, S.N. Carbon dioxide gas sensor based on polyhexamethylene biguanide polymer deposited on silicon nano-cylinders metasurface. Sensors 2021, 21, 378. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, L.; Song, D.; Yu, J.; Liu, Q.; Liu, J.; Chen, R.; Sun, G.; Wang, J. Functionalized GO-doped double network antibacterial hydrogels for efficient uranium extraction from seawater. Desalination 2022, 540, 115993. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, M.; Ge, D.; Dong, Y.; Bai, L.; Han, Y.; Zhu, N. Polyhexamethylene biguanidine used as a new type sewage sludge conditioning agent: Effect on sludge dewaterability and mechanism. J. Environ. Manag. 2022, 315, 115146. [Google Scholar] [CrossRef]
- Moore, K.; Gray, D. Using PHMB antimicrobial to prevent wound infection. Wounds 2007, 3, 96–102. [Google Scholar]
- Landelle, C.; von Dach, E.; Haustein, T.; Agostinho, A.; Renzi, G.; Renzoni, A.; Pittet, D.; Schrenzel, J.; François, P.; Harbarth, S. Randomized, placebo-controlled, double-blind clinical trial to evaluate the efficacy of polyhexanide for topical decolonization of MRSA carriers. J. Antimicrob. Chemother. 2015, 71, 531–538. [Google Scholar] [CrossRef]
- Renzoni, A.; Von Dach, E.; Landelle, C.; Diene, S.M.; Manzano, C.; Gonzales, R.; Abdelhady, W.; Randall, C.P.; Bonetti, E.J.; Baud, D.; et al. Impact of exposure of methicillin-resistant Staphylococcus aureus to polyhexanide in vitro and in vivo. Antimicrob. Agents Chemother. 2017, 61, e00272-17. [Google Scholar] [CrossRef] [Green Version]
- Hübner, N.O.; Kramer, A. Review on the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Skin. Pharmacol. Physiol. 2010, 23 (Suppl. S1), 17–27. [Google Scholar] [CrossRef]
- Larkin, D.F.; Kilvington, S.; Dart, J.K. Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology 1992, 99, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Valluri, S.; Fleming, T.P.; Laycock, K.A.; Tarle, I.S.; Goldberg, M.A.; Garcia-Ferrer, F.J.; Essary, L.R.; Pepose, J.S. In vitro and in vivo effects of polyhexamethylene biguanide against Herpes simplex virus infection. Cornea 1997, 16, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Krebs, F.C.; Miller, S.R.; Ferguson, M.L.; Labib, M.; Rando, R.F.; Wigdahl, B. Polybiguanides, particularly polyethylene hexamethylene biguanide, have activity against human immunodeficiency virus type 1. Biomed. Pharmacother. 2005, 59, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.; Maillard, J.-Y.; Denyer, S.P.; McGeechan, P. Polyhexamethylene biguanide exposure leads to viral aggregation. J. Appl. Microbiol. 2010, 108, 1880–1888. [Google Scholar] [CrossRef]
- Shoukat, K.; Pilling, S.; Rout, S.; Bradbury, J.; Humphreys, P.N. A systematic comparison of antimicrobial wound dressings using a planktonic cell and an immobilized cell model. J. Appl. Microbiol. 2015, 119, 1552–1560. [Google Scholar] [CrossRef] [Green Version]
- Müller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [Green Version]
- European Chemicals Agency (ECHA). Opinion Proposing Harmonised Classification and Labelling at Community Level of Polyhexamethylene Biguanide or Poly(Hexamethylene) Biguanide Hydrochloride or PHMB; ECHA—Committee for Risk Assessment: Helsinki, Finland, 2011. [Google Scholar]
- Sukakul, T.; Dahlin, J.; Pontén, A.; Antelmi, A.; Bruze, M.; Hamnerius, N.; Hauksson, I.; Isaksson, M.; Lejding, T.; Svedman, C. Contact allergy to polyhexamethylene biguanide (polyaminopropyl biguanide). Contact Dermat. 2020, 84, 326–331. [Google Scholar] [CrossRef]
- Fabry, W.; Kock, H.J. In-vitro activity of polyhexanide alone and in combination with antibiotics against Staphylococcus aureus. J. Hosp. Infect. 2014, 86, 68–72. [Google Scholar] [CrossRef]
- Koburger, T.; Hübner, N.-O.; Braun, M.; Siebert, J.; Kramer, A. Standardized comparison of antiseptic efficacy of triclosan, PVP—Iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemother. 2010, 65, 1712–1719. [Google Scholar] [CrossRef] [Green Version]
- Fabry, W.H.K.; Kock, H.J.; Vahlensieck, W. Activity of the antiseptic polyhexanide against gram-negative bacteria. Microb. Drug. Resist. 2014, 20, 138–143. [Google Scholar] [CrossRef]
- Kaehn, K. Polihexanide: A safe and highly effective biocide. Skin. Pharmacol. Physiol. 2010, 23, 7–16. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, L.P.; Hassan, K.Z.; Brittan, H.; Johnson, N.; Collins, A.N. Characterization of the biocide polyhexamethylene biguanide by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Appl. Polym. Sci. 2006, 102, 4928–4936. [Google Scholar] [CrossRef]
- De Paula, G.F.; Netto, G.I.; Mattoso, L.H.C. Physical and chemical characterization of poly(hexamethylene biguanide) hydrochloride. Polymers 2011, 3, 928–941. [Google Scholar] [CrossRef]
- Blackburn, R.S.; Harvey, A.; Kettle, L.L.; Payne, J.D.; Russell, S.J. Sorption of poly(hexamethylenebiguanide) on cellulose: Mechanism of binding and molecular recognition. Langmuir 2006, 22, 5636–5644. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Ledwith, A.; Bamford, C.H.; Hann, R.A. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim. Biophys. Acta Biomembr. 1984, 769, 57–66. [Google Scholar] [CrossRef]
- Glukhov, E.; Stark, M.; Burrows, L.L.; Deber, C.M. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J. Biol. Chem. 2005, 280, 33960–33967. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.; Eberlein, T.; Müller, G.; Dissemond, J.; Assadian, O. Re-evaluation of polihexanide use in wound antisepsis in order to clarify ambiguities of two animal studies. J. Wound Care 2019, 28, 246–255. [Google Scholar] [CrossRef]
- Zaki, A.M.; Troisi, A.; Carbone, P. Unexpected like-charge self-assembly of a biguanide-based antimicrobial polyelectrolyte. J. Chem. Phys. Lett. 2016, 7, 3730–3735. [Google Scholar] [CrossRef]
- Wilson, K.S.; von Hippel, P.H. Transcription termination at intrinsic terminators: The role of the RNA hairpin. Proc. Natl. Acad. Sci. USA 1995, 92, 8793–8797. [Google Scholar] [CrossRef] [Green Version]
- Mason, P.E.; Neilson, G.W.; Enderby, J.E.; Saboungi, M.-L.; Dempsey, C.E.; MacKerell, A.D.; Brady, J.W. The structure of aqueous guanidinium chloride solutions. J. Am. Chem. Soc. 2004, 126, 11462–11470. [Google Scholar] [CrossRef]
- Brown, C.C. Antimicrobial Fabric for Surgical Drape. European Patent EP0136900b1, 28 September 1984. [Google Scholar]
- Barrett, S. Wound-bed preparation: A vital step in the healing process. Brit. J. Nurs. 2017, 26, S24–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagelstein, S.M.; Ivins, N. Treating recalcitrant venous leg ulcers using a PHMB impregnated dressing: A case study evaluation. Wounds 2013, 9, 84–90. [Google Scholar]
- Motta, G.J.; Trigilia, D. The effect of an antimicrobial drain sponge dressing on specific bacterial isolates at tracheostomy sites. Ostomy Wound Manag. 2005, 51, 60–62, 64–66. [Google Scholar]
- Kirker, K.R.; Fisher, S.T.; James, G.A.; McGhee, D.; Shah, C.B. Efficacy of polyhexamethylene biguanide-containing antimicrobial foam dressing against MRSA relative to standard foam dressing. Wounds 2009, 21, 229–233. [Google Scholar]
- Johnson, S.; Leak, K. Evaluating a dressing impregnated with polyhexamethylene biguanide. Wounds 2011, 7, 20–25. [Google Scholar]
- Sibbald, R.G.; Coutts, P.; Woo, K.Y. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing—Clinical trial results. Adv. Skin Wound Care 2011, 24, 78–84. [Google Scholar] [CrossRef]
- Lee, W.R.; Tobias, K.M.; Bemis, D.A.; Rohrbach, B.W. In vitro efficacy of a polyhexamethylene biguanide-impregnated gauze dressing against bacteria found in veterinary patients. Vet. Surg. 2004, 33, 404–411. [Google Scholar] [CrossRef]
- Shah, C. Polyhexamethylene biguanide (PHMB) treated wound dressings and vancomycin-resistant enterococci (VRE). Manag. Infect. Control 2007, 7, 26–34. [Google Scholar]
- Mueller, S.W.; Krebsbach, L.E. Impact of an antimicrobial-impregnated gauze dressing on surgical site infections including methicillin-resistant Staphylococcus aureus infections. Am. J. Infect. Control 2008, 36, 651–655. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Serralta, V.; Davis, S.; Orr, R.; Eaglstein, W.; Mertz, P.M. The effect of an antimicrobial gauze dressing impregnated with 0.2-percent polyhexamethylene biguanide as a barrier to prevent Pseudomonas aeruginosa wound invasion. Wounds 2002, 14, 169–176. [Google Scholar]
- Motta, G.J.; Milne, C.T.; Corbett, L.Q. Impact of antimicrobial gauze on bacterial colonies in wounds that require packing. Ostomy Wound Manag. 2004, 50, 48–62. [Google Scholar]
- Davis, S.; Mertz, P.M.; Cazzaniga, A.; Serralta, V.; Orr, R.; Eaglstein, W. The use of new antimicrobial gauze dressings: Effects on the rate of epithelialization of partial-thickness wounds. Wounds 2002, 14, 252–256. [Google Scholar]
- Wright, J.B.; Lam, K.; Olson, M.E.; Burrell, R.E. Is antimicrobial efficacy sufficient? A question concerning the benefits of new dressings. Wounds 2003, 15, 133–142. [Google Scholar]
- Fumarola, S.; Butcher, M.; Cooper, P.; Gray, D.; Russell, F.; Stringfellow, S.; Bertram, M.; Duguid, K.; Pirie, G.; Shand, S. A clinical audit of Suprasorb® X + PHMB. Wounds 2010, 6, 78–87. [Google Scholar]
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.C. HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen. 2009, 17, 730–738. [Google Scholar] [CrossRef]
- Piatkowski, A.; Drummer, N.; Andriessen, A.; Ulrich, D.; Pallua, N. Randomized controlled single center study comparing a polyhexanide containing bio-cellulose dressing with silver sulfadiazine cream in partial-thickness dermal burns. Burns 2011, 37, 800–804. [Google Scholar] [CrossRef]
- Eberlein, T.; Haemmerle, G.; Signer, M.; Gruber Moesenbacher, U.; Traber, J.; Mittlboeck, M.; Abel, M.; Strohal, R. Comparison of PHMB-containing dressing and silver dressings in patients with critically colonised or locally infected wounds. J. Wound Care 2012, 21, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Muangman, P.; Nitimonton, S.; Aramwit, P. Comparative clinical study of Bactigras and Telfa AMD for skin graft donor-site dressing. Int. J. Mol. Sci. 2011, 12, 5031–5038. [Google Scholar] [CrossRef]
- Forder, R.W.D. The management of a neuropathic diabetic foot ulcer using ActivHeal® PHMB foam. Diabet. Foot J. 2016, 19, 205–209. [Google Scholar]
- WoundSource. Woundsource: The Kestrel Wound Product Sourcebook. Available online: https://www.woundsource.com/ (accessed on 10 August 2021).
- Rembe, J.D.; Fromm-Dornieden, C.; Böhm, J.; Stuermer, E.K. Influence of human acute wound fluid on the antibacterial efficacy of different antiseptic polyurethane foam dressings: An in vitro analysis. Wound Repair Regen. 2018, 26, 27–35. [Google Scholar] [CrossRef]
- Bain, M.A.; Thibodeaux, K.T.; Speyrer, M.S.; Carlson, E.; Koullias, G.J. Effect of native type I collagen with polyhexamethylene biguanide antimicrobial on wounds: Interim registry results. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2251. [Google Scholar] [CrossRef]
- Kingsley, A.; Tadej, M.; Colbourn, A.; Kerr, A.; Aslan, C.B. Suprasorb® X + PHMB: Antimicrobial and hydrobalance action in a new wound dressing. Wounds 2009, 5, 72–77. [Google Scholar]
- Jiang, B.; Larson, J.C.; Drapala, P.W.; Pérez-Luna, V.H.; Kang-Mieler, J.J.; Brey, E.M. Investigation of lysine acrylate containing poly(N-isopropylacrylamide) hydrogels as wound dressings in normal and infected wounds. J. Biomed. Mater. Res. Appl. Biomater. 2012, 100, 668–676. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J. Biomed. Mater. Res. Appl. Biomater. 2012, 100, 1556–1565. [Google Scholar] [CrossRef]
- Dilamian, M.; Montazer, M.; Masoumi, J. Antimicrobial electrospun membranes of chitosan/poly(ethylene oxide) incorporating poly(hexamethylene biguanide) hydrochloride. Carbohydr. Polym. 2013, 94, 364–371. [Google Scholar] [CrossRef]
- Basmaji, P.; de Olyveira, G.M.; dos Santos, M.L.; Guastaldi, A.C. Novel antimicrobial peptides bacterial cellulose obtained by symbioses culture between polyhexanide biguanide (PHMB) and green tea. J. Biomater. Tissue Eng. 2014, 4, 59–64. [Google Scholar] [CrossRef]
- Llorens, E.; Calderon, S.; del Valle, L.J.; Puiggali, J. Polybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties. Mater. Sci. Eng. Mater. Biol. Appl. 2015, 50, 74–84. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Amornsudthiwat, P.; Pienpinijtham, P.; Aramwit, P. Interaction and effectiveness of antimicrobials along with healing-promoting agents in a novel biocellulose wound dressing. Mater. Sci. Eng. Mater. Biol. Appl. 2015, 55, 95–104. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: In vitro, in vivo and clinical studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef]
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.C. Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine. J. Mater. Sci. Mater. Med. 2015, 26, 245. [Google Scholar] [CrossRef]
- Ampawong, S.; Aramwit, P. A study of long-term stability and antimicrobial activity of chlorhexidine, polyhexamethylene biguanide, and silver nanoparticle incorporated in sericin-based wound dressing. J. Biomater. Sci. Polym. Ed. 2017, 28, 1286–1302. [Google Scholar] [CrossRef]
- Knafl, D.; Thalhammer, F.; Vossen, M.G. In-vitro release pharmacokinetics of amikacin, teicoplanin and polyhexanide in a platelet rich fibrin-layer (PRF)—A laboratory evaluation of a modern, autologous wound treatment. PLoS ONE 2017, 12, e0181090. [Google Scholar] [CrossRef] [Green Version]
- Bueno, C.Z.; Moraes, A.M. Influence of the incorporation of the antimicrobial agent polyhexamethylene biguanide on the properties of dense and porous chitosan-alginate membranes. Mater. Sci. Eng. Mater. Biol. Appl. 2018, 93, 671–678. [Google Scholar] [CrossRef]
- Lefebvre, E.; Lembre, P.; Picard, J.; El-Guermah, L.; Seyer, D.; Garde, V.L. Ephemeral biogels to control anti-biofilm agent delivery: From conception to the construction of an active dressing. Mater. Sci. Eng. Mater. Biol. Appl. 2018, 82, 210–216. [Google Scholar] [CrossRef]
- de Mattos, I.B.; Holzer, J.C.J.; Tuca, A.C.; Groeber-Becker, F.; Funk, M.; Popp, D.; Mautner, S.; Birngruber, T.; Kamolz, L.P. Uptake of PHMB in a bacterial nanocellulose-based wound dressing: A feasible clinical procedure. Burns 2019, 45, 898–904. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Xie, Y.; Yang, Y.; Zheng, Y.; Meng, H.; He, W.; Qiao, K. Highly transparent, highly flexible composite membrane with multiple antimicrobial effects used for promoting wound healing. Carbohydr. Polym. 2019, 222, 114985. [Google Scholar] [CrossRef]
- Worsley, A.; Vassileva, K.; Tsui, J.; Song, W.; Good, L. Polyhexamethylene biguanide:polyurethane blend nanofibrous membranes for wound infection control. Polymers 2019, 11, 915. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Chen, Z.L.; Xiang, Y.; Tang, T.; Zhou, H.; Hong, X.D.; Fan, H.; Zhang, X.D.; Luo, P.F.; Ma, B.; et al. Development of a PHMB hydrogel-modified wound scaffold dressing with antibacterial activity. Wound Repair Regen. 2020, 28, 480–492. [Google Scholar] [CrossRef]
- Liang, A.; Zhang, M.; Luo, H.; Niu, L.; Feng, Y.; Li, M. Porous poly(hexamethylene biguanide) hydrochloride loaded silk fibroin sponges with antibacterial function. Materials 2020, 13, 285. [Google Scholar] [CrossRef] [Green Version]
- Massarelli, E.; Silva, D.; Pimenta, A.F.R.; Fernandes, A.I.; Mata, J.L.G.; Armes, H.; Salema-Oom, M.; Saramago, B.; Serro, A.P. Polyvinyl alcohol/chitosan wound dressings loaded with antiseptics. Int. J. Pharm. 2021, 593, 120110. [Google Scholar] [CrossRef]
- Asadi, L.; Mokhtari, J.; Abbasi, M. An alginate-PHMB-AgNPs based wound dressing polyamide nanocomposite with improved antibacterial and hemostatic properties. J. Mater. Sci. Mater. Med. 2021, 32, 7. [Google Scholar] [CrossRef]
- Dydak, K.; Junka, A.; Dydak, A.; Brożyna, M.; Paleczny, J.; Fijalkowski, K.; Kubielas, G.; Aniołek, O.; Bartoszewicz, M. In vitro efficacy of bacterial cellulose dressings chemisorbed with antiseptics against biofilm formed by pathogens isolated from chronic wounds. Int. J. Mol. Sci. 2021, 22, 3996. [Google Scholar] [CrossRef]
- Montemezzo, M.; Ferrari, M.D.; Kerstner, E.; Santos, V.D.; Victorazzi Lain, V.; Wollheim, C.; Frozza, C.O.d.S.; Roesch-Ely, M.; Baldo, G.; Brandalise, R.N. PHMB-loaded PDMS and its antimicrobial properties for biomedical applications. J. Biomater. Appl. 2021, 36, 252–263. [Google Scholar] [CrossRef]
- Ahani, E.; Montazer, M.; Mianehro, A.; Samadi, N.; Toliyat, T.; Mahmoudi Rad, M. Preparation of long-lasting antibacterial wound dressing through diffusion of cationic-liposome-encapsulated polyhexamethylene biguanide. React. Funct. Polym. 2021, 169, 105092. [Google Scholar] [CrossRef]
- Chanabodeechalermrung, B.; Chaiwarit, T.; Sommano, S.R.; Rachtanapun, P.; Kantrong, N.; Chittasupho, C.; Jantrawut, P. Dual crosslinked ion-based bacterial cellulose composite hydrogel containing polyhexamethylene biguanide. Membranes 2022, 12, 825. [Google Scholar] [CrossRef]
- Harrington, R.E.; Guda, T.; Lambert, B.; Martin, J. Sterilization and disinfection of biomaterials for medical devices. In Biomaterials Science. An Introduction to Materials in Medicine, 4th ed.; Wagner, W.R., Zhang, G., Sakiyama-Elbert, S.E., Yaszemski, M.J., Eds.; Academic Press: San Diego, CA, USA, 2020; pp. 1431–1446. [Google Scholar]
- Petersen, S.; Hussner, J.; Reske, T.; Grabow, N.; Senz, V.; Begunk, R.; Arbeiter, D.; Kroemer, H.K.; Schmitz, K.-P.; Meyer zu Schwabedissen, H.E.; et al. In vitro study of dual drug-eluting stents with locally focused sirolimus and atorvastatin release. J. Mater. Sci. Mater. Med. 2013, 24, 2589–2600. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Thomas, S.; Uzun, M. Testing dressings and wound management materials. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2019; pp. 23–54. [Google Scholar]
- Medical Engineering Technologies Ltd (MET). Wound Dressing Performance Testing. Available online: https://met.uk.com/medical-device-testing/wound-dressings-performance (accessed on 21 September 2022).
- The Surgical Materials Testing Laboratory (SMTL). Wound Dressing Testing at SMTL. Available online: http://www.smtl.co.uk/testing-services/54-wound-dressings-testing-services/57-dressings-testing.html (accessed on 21 September 2022).
- EN 13726-1:2002; Test Methods for Primary Wound Dressings. Part 1—Aspects of Absorbency. European Committee for Standardization (CEN): Brussels, Belgium, 2002.
- EN 13726-2:2002; Test Methods for Primary Wound Dressings. Part 2—Moisture Vapour Transmission Rate of Permeable Film Dressings. European Committee for Standardization (CEN): Brussels, Belgium, 2002.
- EN 13726-3:2003; Test Methods for Primary Wound Dressings. Part 3—Waterproofness. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
- EN 13726-4:2002; Test Methods for Primary Wound Dressings. Part 4—Conformability. European Committee for Standardization (CEN): Brussels, Belgium, 2002.
- EN 13726-5:2000; Test Methods for Primary Wound Dressings. Part 5—Bacterial Barrier Properties. European Committee for Standardization (CEN): Brussels, Belgium, 2000.
- EN 13726-6:2003; Test Methods for Primary Wound Dressings. Part 6—Odour Control. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
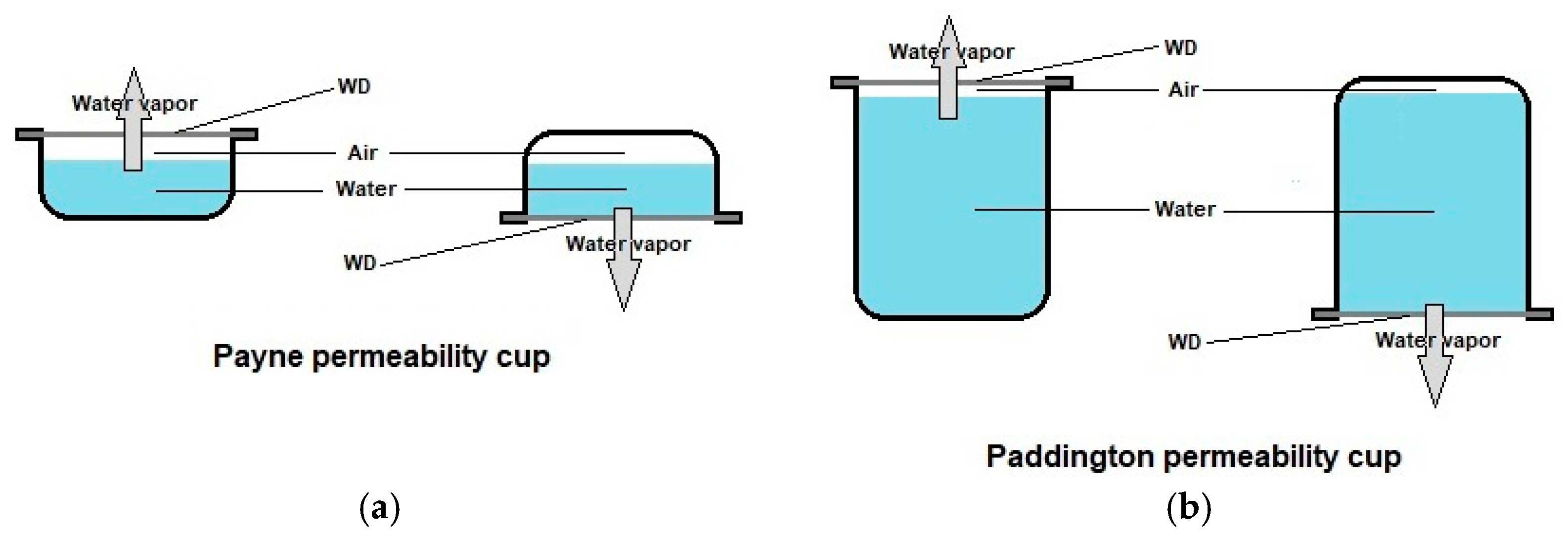
- Bainbridge, P.; Browning, P.; Bernatchez, S.F.; Blaser, C.; Hitschmann, G. Comparing test methods for moisture-vapor transmission rate (MVTR) for vascular access transparent semipermeable dressings. J. Vasc. Access 2021, 11297298211050485. [Google Scholar] [CrossRef]
- Minsart, M.; Van Vlierberghe, S.; Dubruel, P.; Mignon, A. Commercial wound dressings for the treatment of exuding wounds: An in-depth physico-chemical comparative study. Burns Trauma 2022, 10, tkac024. [Google Scholar] [CrossRef]
- Ruiz-Cardona, L.; Sanzgiri, Y.D.; Benedetti, L.M.; Stella, V.J.; Topp, E.M. Application of benzyl hyaluronate membranes as potential wound dressings: Evaluation of water vapour and gas permeabilities. Biomaterials 1996, 17, 1639–1643. [Google Scholar] [CrossRef]
- Wu, P.; Fisher, A.C.; Foo, P.P.; Queen, D.; Gaylor, J.D.S. In vitro assessment of water vapour transmission of synthetic wound dressings. Biomaterials 1995, 16, 171–175. [Google Scholar] [CrossRef] [PubMed]
- ASTM E96-90; Standard Methods for Water Vapor Transmission of Materials. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 1990.
- Haryanto, H.; Arisandi, D.; Suriadi, S.; Imran, I.; Ogai, K.; Sanada, H.; Okuwa, M.; Sugama, J. Relationship between maceration and wound healing on diabetic foot ulcers in indonesia: A prospective study. Int. Wound J. 2017, 14, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Knighton, D.R.; Silver, I.A.; Hunt, T.K. Regulation of wound-healing angiogenesis—Effect of oxygen gradients and inspired oxygen concentration. Surgery 1981, 90, 262–270. [Google Scholar] [PubMed]
- Pandit, A.S.; Feldman, D.S. Effect of oxygen treatment and dressing oxygen permeability on wound healing. Wound Repair Regen. 1994, 2, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Sirvio, L.M.; Grussing, D.M. The effect of gas permeability of film dressings on wound environment and healing. J. Investig. Dermatol. 1989, 93, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Said, H.K.; Hijjawi, J.; Roy, N.; Mogford, J.; Mustoe, T. Transdermal sustained-delivery oxygen improves epithelial healing in a rabbit ear wound model. Arch. Surg. 2005, 140, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Kessler, L.; Bilbault, P.; Ortéga, F.; Grasso, C.; Passemard, R.; Stephan, D.; Pinget, M.; Schneider, F. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: A prospective randomized study. Diabetes Care 2003, 26, 2378–2382. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Hu, H. Spacer fabric-based exuding wound dressing—Part II: Comparison with commercial wound dressings. Text. Res. J. 2017, 87, 1481–1493. [Google Scholar] [CrossRef]
- GB/T 19789-2005; Packaging Materials. Plastic Film and Sheet Oxygen Permeability Test. Coulometric Method. Standardization Administration of China (SAC): Beijing, China, 2005.
- ISO 15105-2:2003; Plastics—Film and Sheeting—Determination of Gas-Transmission Rate. Part 2: Equal-Pressure Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2003.
- ASTM D737-04; Standard Test Method for Air Permeability of Textile Fabrics. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2004.
- Layek, B.; Rahman Nirzhor, S.S.; Rathi, S.; Kandimalla, K.K.; Wiedmann, T.S.; Prabha, S. Design, development, and characterization of imiquimod-loaded chitosan films for topical delivery. AAPS PharmSciTech 2019, 20, 58. [Google Scholar] [CrossRef]
- Sarisuta, N.; Parrott, E.L. Relationship of dissolution rate to viscosity of polymeric solutions. J. Pharm. Sci. 1982, 71, 1375–1380. [Google Scholar] [CrossRef]
- Jalil, R.; Ferdous, A.J. Effect of viscosity increasing agent and electrolyte concentration on the release rate of theophylline from a HPMC based sustained release capsules. Drug Dev. Ind. Pharm. 1993, 19, 2637–2643. [Google Scholar] [CrossRef]
- Zheng, D.W.; Li, J.L.; Li, C.; Xu, Z.S.; Cheng, S.X.; Zhang, X.Z. Viscosity enhanced release (VER) effect in nanoporous drug delivery systems: Phenomenon and mechanism. J. Mater. Chem. 2015, 3, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- US Food & Drug Administration (FDA). Guidance for Industry. Extended Release Oral Dosage Forms: Development, Evaluation, and 1654 Application of In vitro/In vivo Correlations; Center for Drug Evaluation and Research (CDER): Rockville, MD, USA, 1997. [Google Scholar]
- Shen, J.; Burgess, D.J. In vitro–in vivo correlation for complex non-oral drug products: Where do we stand? J. Control. Release 2015, 219, 644–651. [Google Scholar] [CrossRef] [Green Version]
- US Food & Drug Administration (FDA). Regulatory Information. Available online: https://www.fda.gov/regulatory-information (accessed on 29 September 2022).
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8 (Suppl. S3), 4–9. [Google Scholar] [CrossRef]
- World Union of Wound Healing Societies (WUWHS). Consensus Document. Wound Exudate: Effective Assessment and Management; Wounds International: London, UK, 2019. [Google Scholar]
- Trengove, N.J.; Langton, S.R.; Stacey, M.C. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen. 1996, 4, 234–239. [Google Scholar] [CrossRef]
- Gethin, G.T.; Cowman, S.; Conroy, R.M. The impact of manuka honey dressings on the surface pH of chronic wounds. Int. Wound J. 2008, 5, 185–194, Retraction in Int. Wound J. 2014, 11, 342–342. [Google Scholar] [CrossRef]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Dini, V.; Salvo, P.; Janowska, A.; Di Francesco, F.; Barbini, A.; Romanelli, M. Correlation between wound temperature obtained with an infrared camera and clinical wound bed score in venous leg ulcers. Wounds 2015, 27, 274–278. [Google Scholar]
- Hellgren, L.; Vincent, J. Degradation and liquefaction effect of streptokinase-streptodornase and stabilized trypsin on necroses, crusts of fibrinoid, purulent exudate and clotted blood from leg ulcers. J. Int. Med. Res. 1977, 5, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Lamke, L.O.; Nilsson, G.E.; Reithner, H.L. The evaporative water loss from burns and the water-vapour permeability of grafts and artificial membranes used in the treatment of burns. Burns 1977, 3, 159–165. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A. Monitoring neuropathic ulcer healing with infrared dermal thermometry. J. Foot Ankle Surg. 1996, 35, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.; Ivory, J.D.; Sezgin, D.; Muller, H.; O’Connor, G.; Vellinga, A. What is the “normal” wound bed temperature? A scoping review and new hypothesis. Wound Repair Regen. 2021, 29, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Vowden, P.; Bond, E.; Meuleneire, F. Managing high viscosity exudate. Wounds 2015, 11, 56–60. [Google Scholar]
- Merck KGaA. PBS Tablets—Calbiochem. Available online: https://www.merckmillipore.com/PT/en/product/PBS-Tablets-Calbiochem,EMD_BIO-524650#documentation (accessed on 12 January 2022).
- Lutz, J.B.; Zehrer, C.L.; Solfest, S.E.; Walters, S.A. A new in vivo test method to compare wound dressing fluid handling characteristics and wear time. Ostomy Wound Manag. 2011, 57, 28–36. [Google Scholar]
- Tateishi, T.; Hyodo, K.; Kondo, K.; Miura, K. Simulator test of artificial joints. Mater. Sci. Eng. 1994, 1, 121–125. [Google Scholar] [CrossRef]
- Bowler, P.G.; Jones, S.A.; Walker, M.; Parsons, D. Microbicidal properties of a silver-containing Hydrofiber® dressing against a variety of burn wound pathogens. J. Burn Care Rehabil. 2004, 25, 192–196. [Google Scholar] [CrossRef]
- Kadam, S.; Madhusoodhanan, V.; Dhekane, R.; Bhide, D.; Ugale, R.; Tikhole, U.; Kaushik, K.S. Milieu matters: An in vitro wound milieu to recapitulate key features of, and probe new insights into, mixed-species bacterial biofilms. Biofilm 2021, 3, 100047. [Google Scholar] [CrossRef]
- European Pharmacopoeia 6.0. Dissolution Test for Solid Dosage Forms (01/2008:20903); European Directorate for the Quality of Medicines & Health Care, Council of Europe: Strasbourg, France, 2008; pp. 266–275. [Google Scholar]
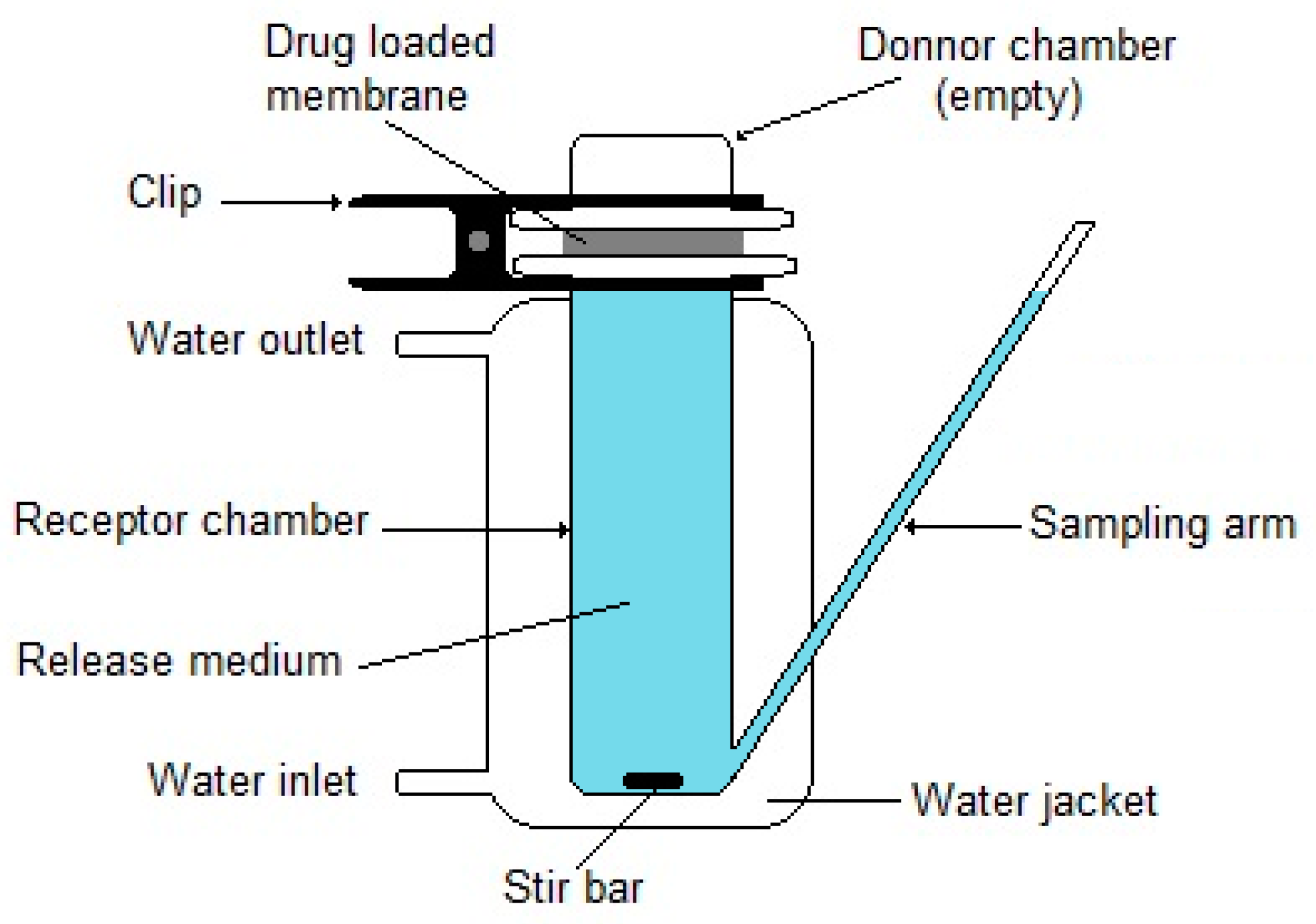
- Tieppo, A.; Boggs, A.C.; Pourjavad, P.; Byrne, M.E. Analysis of release kinetics of ocular therapeutics from drug releasing contact lenses: Best methods and practices to advance the field. Cont. Lens Anterior Eye 2014, 37, 305–313. [Google Scholar] [CrossRef]
- Steffansen, B.; Herping, S.P.K. Novel wound models for characterizing ibuprofen release from foam dressings. Int. J. Pharm. 2008, 364, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Dealey, C.; Cameron, J.; Arrowsmith, M. A study comparing two objective methods of quantifying the production of wound exudate. J. Wound Care 2006, 15, 149–153. [Google Scholar] [CrossRef]
- Slack, S.M. Properties of biological fluids. In Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: London, UK, 2020; pp. 1519–1524. [Google Scholar]
- Thomas, S.T. Testing dressings and wound management materials. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 20–47. [Google Scholar]
- Mogrovejo-Valdivia, A.; Maton, M.; Garcia-Fernandez, M.J.; Tabary, N.; Chai, F.; Neut, C.; Martel, B.; Blanchemain, N. In vitro microbiological and drug release of silver/ibuprofen loaded wound dressing designed for the treatment of chronically infected painful wounds. Antibiotics 2021, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Franz, T.J. Percutaneous absorption on the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef]
- US Pharmacopoeia. <1724> Semisolid Drug Products—Performance Tests; The United States Pharmacopeial Convention: Rockville, MD, USA, 2014. [Google Scholar]
- Pawar, H.V.; Tetteh, J.; Boateng, J.S. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloids Surf. Biointerfaces 2013, 102, 102–110. [Google Scholar] [CrossRef]
- Addicks, W.J.; Flynn, G.L.; Weiner, N. Validation of a flow-through diffusion cell for use in transdermal research. Pharm. Res. 1987, 4, 337–341. [Google Scholar] [CrossRef]
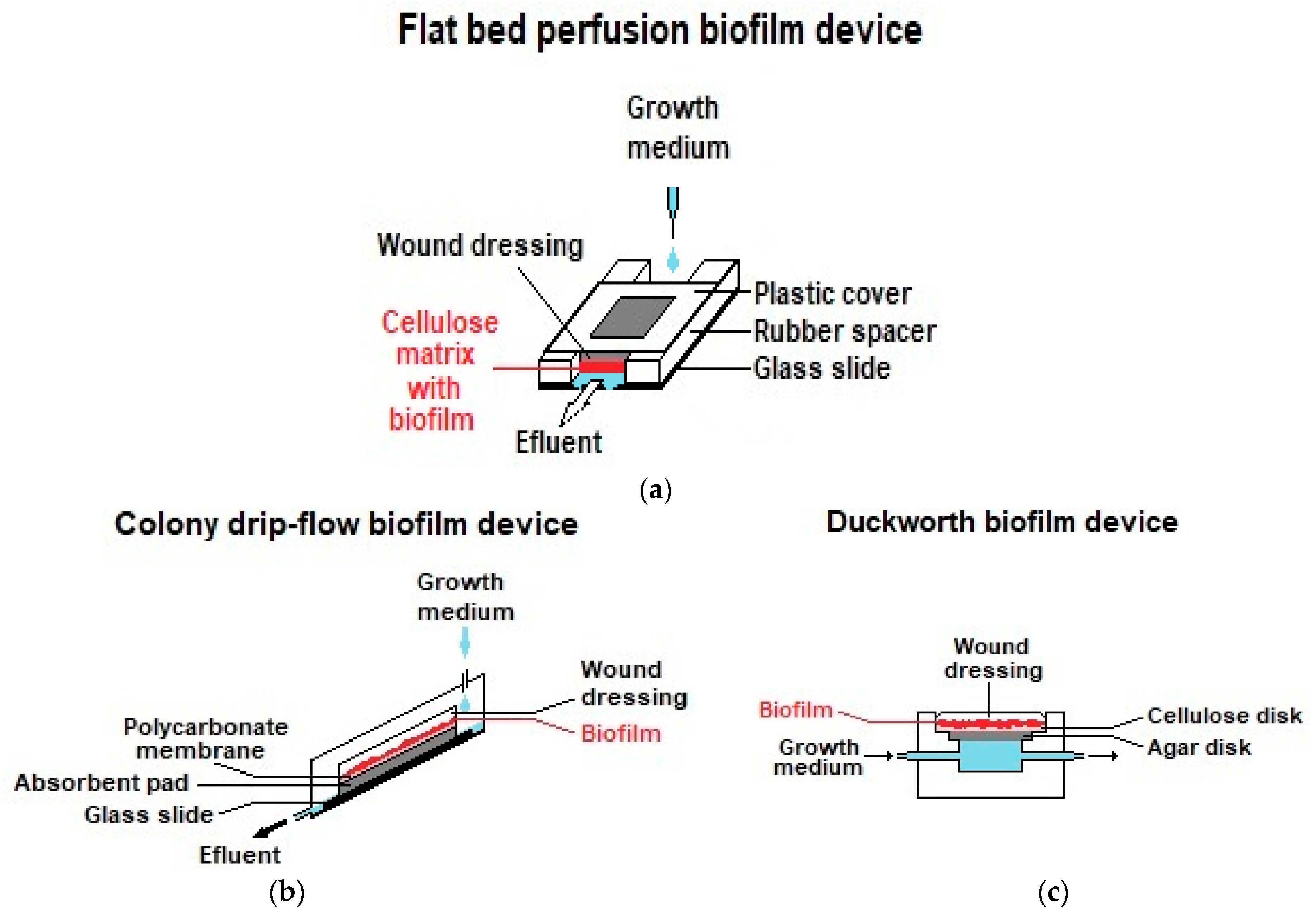
- Thorn, R.M.S.; Greenman, J. A novel in vitro flat-bed perfusion biofilm model for determining the potential antimicrobial efficacy of topical wound treatments. J. Appl. Microbiol. 2009, 107, 2070–2079. [Google Scholar] [CrossRef]
- Lipp, C.; Kirker, K.; Agostinho, A.; James, G.; Stewart, P. Testing wound dressings using an in vitro wound model. J. Wound Care 2010, 19, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, P.F.; Rowlands, R.S.; Barbour, M.E.; Maddocks, S.E. A novel flow-system to establish experimental biofilms for modelling chronic wound infection and testing the efficacy of wound dressings. Microbiol. Res. 2018, 215, 141–147. [Google Scholar] [CrossRef]
- Thomas, S.; Fram, P.; Philips, P. The Importance of Compression on Dressing Performance. Available online: www.worldwidewounds.com/2007/November/Thomas-Fram-Phillips/Thomas-Fram-Phillips-Compression-WRAP.html (accessed on 5 September 2021).
- Aramwit, P.; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci. Biotechnol. Biochem. 2007, 71, 2473–2477. [Google Scholar] [CrossRef] [Green Version]
- Hurd, T.; Zuiliani, N.; Posnett, J. Evaluation of the impact of restructuring wound management practices in a community care provider in Niagara, Canada. Int. Wound J. 2008, 5, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Haynes, J.; Bielby, A.; Searle, R. Putting patients first: Reducing the human and economic costs of wounds. Wounds 2011, 7, 47–55. [Google Scholar]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2015, 12, 47–52. [Google Scholar] [CrossRef]
- Puca, V.; Marulli, R.Z.; Grande, R.; Vitale, I.; Niro, A.; Molinaro, G.; Prezioso, S.; Muraro, R.; Di Giovanni, P. Microbial species isolated from infected wounds and antimicrobial resistance analysis: Data emerging from a three-years retrospective study. Antibiotics 2021, 10, 1162. [Google Scholar] [CrossRef]
- Kalan, L.; Grice, E.A. Fungi in the wound microbiome. Adv. Wound Care 2018, 7, 247–255. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- ASTM E2922-15; Standard Guide for the Use of Standard Test Methods and Practices for Evaluating Antibacterial Activity on Textiles. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2015.
- EN 17854:2022; Antimicrobial Wound Dressings—Requirements and Test Method. European Committee for Standardization (CEN): Brussels, Belgium, 2022.
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: An overview of the evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Nedelea, A.-G.; Plant, R.L.; Robins, L.I.; Maddocks, S.E. Testing the efficacy of topical antimicrobial treatments using a two- and five-species chronic wound biofilm model. J. Appl. Microbiol. 2022, 132, 715–724. [Google Scholar] [CrossRef]
- Seth, A.K.; Geringer, M.R.; Hong, S.J.; Leung, K.P.; Mustoe, T.A.; Galiano, R.D. In vivo modeling of biofilm-infected wounds: A review. J. Surg. Res. 2012, 178, 330–338. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices. Part 1: Evaluation and Testing within a Risk Management Process. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization (ISO): Geneva, Switzerland, 2009.
- Ortega-Llamas, L.; Quiñones-Vico, M.I.; García-Valdivia, M.; Fernández-González, A.; Ubago-Rodríguez, A.; Sanabria-de la Torre, R.; Arias-Santiago, S. Cytotoxicity and wound closure evaluation in skin cell lines after treatment with common antiseptics for clinical use. Cells 2022, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-23:2021; Biological Evaluation of Medical Devices. Part 23: Tests for Irritation. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- Luepke, N.P.; Kemper, F.H. The HET-CAM test: An alternative to the draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Parnell, L.K.S.; Volk, S.W. The evolution of animal models in wound healing research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.; Davis, S.C. Relevance of animal models for wound healing. Wounds 2008, 20, 3–8. [Google Scholar] [PubMed]
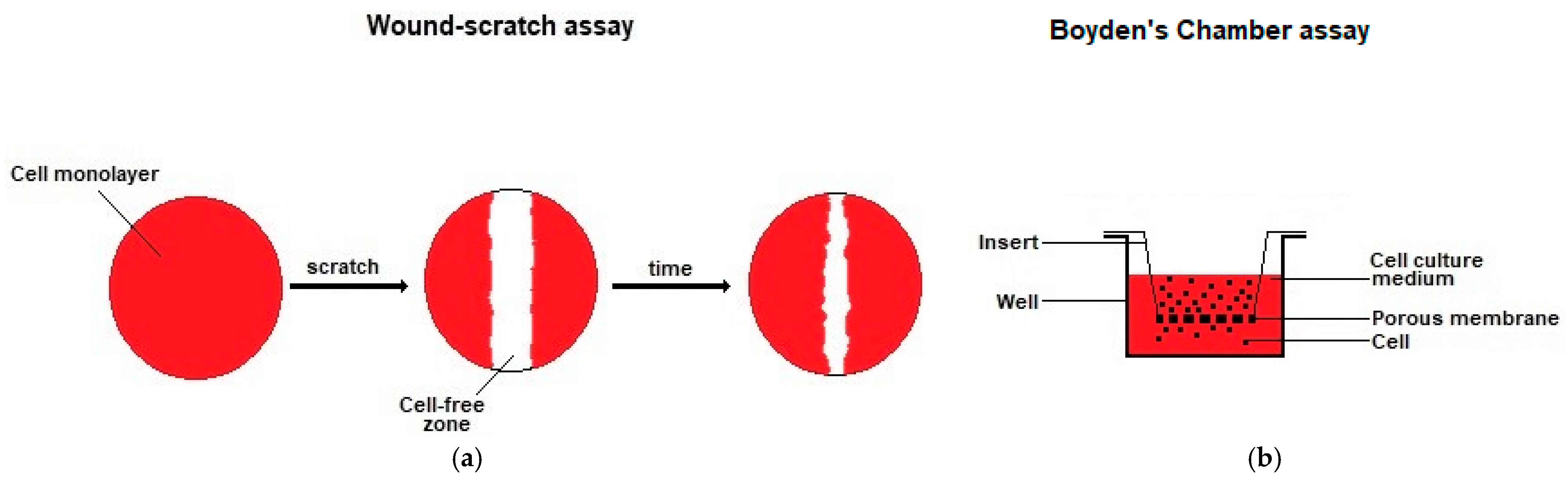
- Martinotti, S.; Ranzato, E. Scratch wound healing assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar]
- Chen, H.C. Boyden chamber assay. Methods Mol. Biol. 2005, 294, 15–22. [Google Scholar]
- Chen, Z.J.; Yang, J.P.; Wu, B.M.; Tawil, B. A novel three-dimensional wound healing model. J. Dev. Biol. 2014, 2, 198–209. [Google Scholar] [CrossRef]
- Wiegand, C.; Abel, M.; Hipler, U.-C.; Elsner, P. Effect of non-adhering dressings on promotion of fibroblast proliferation and wound healing in vitro. Sci. Rep. 2019, 9, 4320. [Google Scholar] [CrossRef] [Green Version]
- Ngo, Q.; Anand, P.; Wang, Y.; Ananthanarayanan, A.; Gong, P.; Newby, C.S.; McMillian, M.M. Developing in vitro assays to quantitatively evaluate the interactions of dressings with wounds. Wound Repair Regen. 2019, 27, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]
- EU Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu (accessed on 20 November 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov (accessed on 20 November 2022).
- Japanese Primary Registries Network. Available online: https://rctportal.niph.go.jp/en (accessed on 20 November 2022).
- Chinese Clinical Trial Registry. Available online: https://www.chictr.org.cn (accessed on 20 November 2022).
- International Clinical Trials Registry Platform. Available online: https://trialsearch.who.int (accessed on 20 November 2022).
- Google Patents. Available online: https://patents.google.com (accessed on 20 November 2022).
- Ni, Y.; Qian, Z.; Yin, Y.; Yuan, W.; Wu, F.; Jin, T. Polyvinyl alcohol/chitosan/polyhexamethylene biguanide phase separation system: A potential topical antibacterial formulation with enhanced antimicrobial effect. Molecules 2020, 25, 1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baier, G.; Cavallaro, A.; Vasilev, K.; Mailander, V.; Musyanovych, A.; Landfester, K. Enzyme responsive hyaluronic acid nanocapsules containing polyhexanide and their exposure to bacteria to prevent infection. Biomacromolecules 2013, 14, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Grutzner, V.; Unger, R.E.; Baier, G.; Choritz, L.; Freese, C.; Bose, T.; Landfester, K.; Kirkpatrick, C.J. Enzyme-responsive nanocomposites for wound infection prophylaxis in burn management: In vitro evaluation of their compatibility with healing processes. Int. J. Nanomed. 2015, 10, 4111–4124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abri, S.; Ghatpande, A.A.; Ress, J.; Barton, H.A.; Leipzig, N.D. Polyionic complexed antibacterial heparin–chitosan particles for antibiotic delivery. ACS Appl. Biol. Mater. 2019, 2, 5848–5858. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Akhtar, N.; Ghauri, M.A.; Rajoka, M.I.; Khalid, Z.M.; Hussain, I. Polyhexamethylene biguanide functionalized cationic silver nanoparticles for enhanced antimicrobial activity. Nanoscale Res. Lett. 2012, 7, 267. [Google Scholar] [CrossRef] [Green Version]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsa-Kortelainen, S.; Pinornaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytonen, V.P. 3D-printable bioactivated nanocellulose-alginate hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef]
- Yu, I.; Kaonis, S.; Chen, R. A study on degradation behavior of 3D printed gellan gum scaffolds. Procedia CIRP 2017, 65, 78–83. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart bandages: The future of wound care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef]
| Bacteria | MIC (µg/mL) | MBC (µg/mL) | Ref. |
|---|---|---|---|
| S. aureus | 1 | 2 | [53] |
| 0.5 | 1 | [54] | |
| MRSA | 2 | 2 | [53] |
| E. coli | 0.5 | 1 | [54] |
| 2 | 2 | [55] | |
| P. aeruginosa | 2 | 2 | [54] |
| 8 | 8 | [55] |
| Commercial Name | Format | Material | PHMB Loading Mode/[PHMB] | Duration of Antimicrobial Activity | Sources |
|---|---|---|---|---|---|
| ActivHeal® PHMB | Foam, pad | Polyurethane | Impregnation/nd | 7 days | Advanced Medical Solutions, Ltd., Winsford, UK; [85] |
| CelluDress-PHMB | Pad | Polyester + viscose | nd 1 | nd | Medicareplus International Ltd., Wembley, UK; [68] |
| CurityTM AMD Antimicrobial Woven Sponges | Sponge, strip | Cotton | Impregnation/0.2% | Up to 3 days | Cardinal Health, Dublin, OH, USA; [86] |
| DracoFoam PHMB | Foam | Polyurethane | nd | Up to 7 days | Dr. Ausbüttel & Co. GmbH, Dortmund, Germany; [87] |
| ExcilonTM AMD | Sponge, gauze | Polyester + rayon | Impregnation/0.2% | Up to 3 days | Cardinal Health, Dublin, OH, USA; [86] |
| Fitostimoline® Plus Gauze | Gauze | nd | nd 2 | nd | Farmaceutici Damor S.p.A., Napoli, Italy |
| Gemcore360°TM PHMB Foam Border Dressing | Foam | nd | Impregnation/nd | Up to 7 days | GEMCO Medical, Hudson, OH, USA; [86] |
| Gemcore360°TM PHMB Non-Adhesive Foam Dressing | |||||
| Kendall™ AMD | Foam, disc | Polyurethane | Impregnation/0.5% | Up to 7 days | Cardinal Health, Dublin, OH, USA; [86] |
| Kerlix™ AMD | Gauze, sponge | Cotton | Impregnation/0.2% | Up to 3 days | |
| McKesson PHMB Hydrophilic Foam Dressing | Foam | Polyurethane | Impregnation/0.8–1% | Up to 7 days | McKesson Medical-Surgical, Irving, TX, USA; [86] |
| PuraPly® AM | Sheet, disc | Crosslinked ECM | nd | nd | Organogenesis Inc., Canton, MA, USA; [88] |
| Sterilux® AMD Antimicrobial Gauze | Gauze, sponge | Cotton | nd/0.1% PHMB (+0.02% BKC) | Up to 7 days | Hartmann USA, Inc., Rock Hill, SC, USA; [86] |
| Suprasorb® X + PHMB | Sheet | Bacterial cellulose | nd/0.3% | nd | Lohmann & Rauscher GmbH & Co. KG, Neuwied, Germany; [89] |
| Suprasorb® P + PHMB | Foam | Polyurethane | nd | ||
| Telfa™ AMD | Pad, island | Cotton | Impregnation/0.2% | Up to 3 days | Cardinal Health, Dublin, OH, USA; [86] |
| TielleTM PHMB | Foam | Polyurethane | Impregnation/nd | nd | 3M/KCI, St. Paul, MN, USA |
| ID | Matrix Composition | Membrane Preparation | Drug-Loading Method and Drug-Loading Yield | Ref. |
|---|---|---|---|---|
| PRM1 | PNIPAAm-based copolymer 1 | Free-radical polymerization. | -Soaking, overnight, RT. [PHMB]: 0.1 and 1% w/v. Agitation not mentioned. -Yield: nd. | [90] |
| PRM2a | PEsUR/CA | Non-weaving: electrospinning of a PEsUR/CA/PHMB solution in DMF/THF. | -Addition. [PHMB]: 1 wt% relative to polymer mass. -Yield: nd. | [91] |
| PRM2b | Non-weaving: co-electrospinning of CA/PHMB and PEsUR/PHMB solutions in DMF/THF. | |||
| PRM3 | Chitosan/ PEO | Non-weaving: electrospinning of a chitosan/PEO/PHMB solution in acetic acid, followed by crosslinking with glutaraldehyde in a vapor phase. | -Addition. [PHMB]: 0.15 and 0.3% w/v relative to solution volume. 2 -Yield: nd. | [92] |
| PRM4 | Bacterial cellulose | Non-weaving: bacterial cellulose produced by G. xylinus 3 in PHMB-containing growth medium. | -Bacteria grown in culture medium supplemented with [PHMB] = 0.2–0.4 wt% relative to solution mass. -Yield: nd. | [93] |
| PRM5 | PLA | Non-weaving: electrospinning of a PLA/PHMB solution in chloroform/acetone/formic acid. | -Addition. [PHMB]: 0.02–0.25% w/v relative to solution volume. -Yield: nd. | [94] |
| PRM6 | Bacterial cellulose | Non-weaving: bacterial cellulose produced by A. xylinum. 3 | -Impregnation. [PHMB]: 0.3% w/v. Additionally impregnated with SS 1%. No further conditions mentioned. -Yield: nd. | [95,96] |
| PRM7 | Non-weaving: bacterial cellulose produced by K. xylinus. | -Soaking for 48 h, 20 °C, with shaking. [PHMB]: 1% w/v. -Yield: 8.9 µg/mg of sample. | [97] | |
| PRM8 | SS/PVA/Gly | Phase inversion, thermally induced phase separation: freeze-drying of a SS/PVA/Gly solution, followed by immersion in a Gly solution and drying. | -Soaking for 20 min. [PHMB]: 0.2% w/v. No further conditions mentioned. -Terminal sterilization by gamma radiation. -Yield: nd. | [98] |
| PRM9 | PRF/silicone | Weaving or non-weaving: 4 silicone gauze spray-coated with a PRF/PHMB/trypsin solution. | -Addition. [PHMB]: nd. -Yield: nd. | [99] |
| PRM10 | Chitosan/ alginate | Phase inversion: solvent evaporation of a chitosan/alginate/PHMB/Pluronic F68 solution, followed by crosslinking with CaCl2. | -Addition. [PHMB]: 1% and 10 wt% relative to polymer mass. -Yield: 7–73 µg PHMB/mg of sample. | [100] |
| PRM11a | Gelatin | Phase inversion, crosslinking: transglutaminase-induced crosslinking of gelatin solutions with PHMB and EDTA. | -Addition. [PHMB]: 0.4% w/v relative to solution volume. -Yield: nd. | [101] |
| PRM11b | Phase inversion, crosslinking: temporary transglutaminase-induced crosslinking of gelatin solutions that contain PHMB, EDTA and a protease. | |||
| PRM12 | Bacterial cellulose | Non-weaving: commercial bacterial cellulose WD. | -Impregnation, 2 h, RT. [PHMB]: 0.04 and 1% w/v PHMB (+0.1% UDAPB). -Yield: 0.024% (0.04% PHMB sample); 0.076% (0.1% PHMB sample). | [102] |
| PRM13 | Non-weaving: commercial bacterial cellulose. | -Soaking for 24 h. [PHMB]: 0.1–0.5% w/v (contained PEG). No further conditions mentioned. -Yield: nd. | [103] | |
| PRM14 | PEtUR | Non-weaving: electrospinning of a solution of a commercial PEtUR in TFE that contains PHMB. | -Addition. [PHMB]: 5–35% wt% relative to polymer mass. -Yield: 0.12–0.81 mg of PHMB. | [104] |
| PRM15 | Gelatin | Phase inversion, crosslinking: gelatin solution crosslinked with glutaraldehyde, followed by addition of Gly and solvent evaporation. Crosslinked gelatin membrane placed on a collagen layer and covered with a silicone layer. | -Soaking for 48 h. [PHMB]: 0.25–2% w/v. No further conditions mentioned. -Yield: nd. | [105] |
| PRM16 | Reg-SF | Phase inversion, thermally induced phase separation: freeze-drying of a reg-SF/Gly/PHMB aqueous solution. | -Addition. [PHMB]: 0.5–10 wt% relative to polymer mass. -Terminal sterilization by gamma radiation. -Yield: nd. | [106] |
| PRM17 | PVA/chitosan | Phase inversion, thermally induced phase separation: freeze-drying after freeze–thaw cycling of PVA/chitosan aqueous solutions. | -Soaking for 24 h, at 36 °C, with shaking. [PHMB]: 0.5% w/v. -Sterilization by autoclaving before drug loading. -Yield: 13–23 µg PHMB/mg of dry sample. | [107] |
| PRM18 | PAm/alginate/ AgNP | Weaving: commercial woven PAm treated with an oxygen plasma and dip coated with an alginate solution that contains AgNPs and PHMB, followed by crosslinking with CaCl2. | -Addition. [PHMB]: 0.04–0.2% w/v. -Yield: nd. | [108] |
| PRM19 | Cotton/alginate/ AgNP | Weaving or non-weaving: 4 commercial cotton gauze dip-coated with an alginate solution that contains AgNPs and PHMB, followed by crosslinking with CaCl2. | ||
| PRM20 | Bacterial cellulose | Non-weaving: bacterial cellulose produced by A. xylinum. 3 | -Impregnation, overnight, 4 °C. [PHMB]: 0.1% w/v (+0.1% UDAPB). -Yield: nd. | [109] |
| PRM21 | PDMS-based elastomer | Phase inversion, crosslinking: reaction between polysiloxane oligomers with and without vinyl groups. | -Addition. [PHMB]: 0.1–0.5 wt% relative to solution mass. -Yield: nd. | [110] |
| PRM22a | Wool | Weaving: commercial woven wool fabric treated with a non-ionic surfactant and a protease. | -Soaking overnight, 70 °C. [PHMB]: 0.2–5% w/v. Agitation not mentioned. -Yield: nd. | [111] |
| PRM22b | -Soaking overnight, 70 °C. [PHMB]: 0.2–5% w/v, encapsulated in cationic nanoliposomes. Agitation not mentioned. -Yield: nd. | |||
| PRM23a | Bacterial cellulose/ alginate | Non-weaving: commercial bacterial cellulose/alginate/PEG solution crosslinked with CaCl2, followed by freeze-drying. | -Soaking, 24 h. [PHMB]: nc. No further conditions mentioned. -Yield: ca. 100% of PHMB in the soaking solution. | [112] |
| PRM23b | Bacterial cellulose/pectin | Non-weaving: commercial bacterial cellulose/pectin/PEG solution crosslinked with CaCl2, followed by freeze-drying. | -Soaking, 24 h. [PHMB]: nc. No further conditions mentioned. -Yield: 98% of PHMB in the soaking solution. | |
| PRM23c | Bacterial cellulose/ alginate/pectin | Non-weaving: commercial bacterial cellulose/alginate/pectin/PEG solution crosslinked with CaCl2, followed by freeze-drying. |
| Biological Characterization | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Physical Characterization | Biocompatibility | ||||||||
| ID | Absorptive Capacity | MVTR | Air or Oxygen Permeability | Drug Release | Antibacterial Activity | Antifungal Activity | Cytotoxi-city | Other Biological Effects | Wound Healing |
| PRM1 | 1 | ||||||||
| PRM2a | |||||||||
| PRM2b | |||||||||
| PRM3 | |||||||||
| PRM4 | |||||||||
| PRM5 | |||||||||
| PRM6 | |||||||||
| PRM7 | |||||||||
| PRM8 | |||||||||
| PRM9 | |||||||||
| PRM10 | |||||||||
| PRM11a | |||||||||
| PRM11b | |||||||||
| PRM12 | |||||||||
| PRM13 | 2 | ||||||||
| PRM14 | |||||||||
| PRM15 | 3 | ||||||||
| PRM16 | |||||||||
| PRM17 | |||||||||
| PRM18 | |||||||||
| PRM19 | |||||||||
| PRM20 | |||||||||
| PRM21 | |||||||||
| PRM22a | |||||||||
| PRM22b | |||||||||
| PRM23a | |||||||||
| PRM23b | |||||||||
| PRM23c | |||||||||
| Standard | Title | Comment | Ref. |
|---|---|---|---|
| EN 13726-1:2002 | Test methods for primary wound dressings. Part 1: Aspects of absorbency | Provides information concerning the evaluation of the quantity of fluid that a wound dressing can absorb and retain when compressed. | [119] |
| EN 13726-2:2002 | Test methods for primary wound dressings. Part 2: Moisture vapor transmission rate of permeable film dressings | Provides information concerning the evaluation of fluid-handling properties of the wound dressing, which determine the degree of hydration of the wound and surrounding tissues. | [120] |
| EN 13726-3:2003 | Test methods for primary wound dressings. Part 3: Waterproofness | Provides information concerning the evaluation of the ability to prevent strike-through of blood or other fluids. | [121] |
| EN 13726-4:2003 | Test methods for primary wound dressings. Part 4: Conformability | Provides information concerning how comfortable a wound dressing is, measuring resistance to stretching and ability to return to its original shape after stress. | [122] |
| EN 13726-5:2000 1 | Test methods for primary wound dressings. Part 5: Bacterial barrier properties | Provides information concerning the evaluation of the antimicrobial properties of wound dressings. | [123] |
| EN 13726-6:2003 | Test methods for primary wound dressings. Part 6: Odor control | Provides information concerning the evaluation of the efficacy in absorbing odor. | [124] |
| ID | Physical Properties 2 | Ref. |
|---|---|---|
| PRM1 |
| [90] |
| PRM2a |
| [91] |
| PRM2b |
| |
| PRM13 |
| [103] |
| PRM15 |
| [105] |
| PRM17 |
| [107] |
| PRM18 |
| [108] |
| PRM19 |
| |
| PRM23a |
| [112] |
| PRM23b |
| |
| PRM23c |
|
| ID | Drug-Release Assay Conditions (Assay, Release Medium and Sampling Mode) | Type of Release and Duration | [PHMB]max Released |
|---|---|---|---|
| PRM2a | -Batch assay. -Distilled water (40 mL), 37 °C, with shaking. -Sampling not detailed. | -Initial burst release. -ca. 1 h. | np |
| PRM2b | |||
| PRM3 | -Batch assay. -Distilled water (10 mL), 37 °C, with shaking (30 rpm). -2 mL aliquots; replenishment with new medium. | -Initial burst release. -ca. 24 h (for loading with 0.3% PHMB). | np |
| PRM5 | -Batch assay. -50 mL PBS, 37 °C, with shaking (200 rpm). -Aliquots of undisclosed volume; replenishment with new medium. | -Initial burst release. -0.5 h (for loading with 0.25% PHMB). | np |
| PRM6 | -Batch assay. -PBS (3 mL), pH 7.4, at 37 °C; no agitation mentioned. -Sampling not detailed. | -Initial burst release. -ca. 12 h. -Membrane also released SS. | ca. 0.016% w/v |
| PRM7 | -Batch assay. -Salt solution 2 (20 mL), pH 6.6, 32 °C, with shaking (70 rpm). -0.5 mL aliquots; no replenishment. | -Sustained release. -ca. 24 h. | np |
| PRM8 | -Batch assay. -PBS (volume not mentioned), pH 7.4, 37 °C, with stirring. -Sampling: 1.5 mL; replenishment with new medium. | -Bimodal release: 1st burst release for ca. 3 h; 2nd release after 24 h, for 48 h. -Membrane also released SS. | np |
| PRM10 | -Batch assay. -PBS (60 mL), pH 7.4, 37 °C, with shaking (100 rpm). -1 mL aliquots; replenishment with new medium. | -Initial burst release. -4 h (for loading with 1% PHMB) to 11 h (for loading with 10% PHMB). | ca. 0.1–5 µg PHMB/mg of sample |
| PRM11a | -Single-face release in a cuvette. 3 -50 mM TRIS (2 mL), pH 7.4, 37 °C, with unspecified agitation. -Sampling not clear. | -Initial burst release. -ca. 5 h. | ca. 0.002% w/v |
| PRM11b | -Bimodal release: 1st burst release for ca. 15 h; 2nd release after ca. 30 h for ca. 20 h. | ca. 0.04% w/v | |
| PRM13 | -Batch assay. -SBF. No further conditions mentioned. -Sampling not detailed. | -Bimodal release: 1st slow release for ca. 15 h; 2nd release for an extra 20 h (for loading with 0.1% PHMB) or 45 h (for loading with 0.2% PHMB) -Highest duration: ca. 60 h (for loading with 0.2% PHMB). | ca. 0.009 (0.1% sample); ca. 0.017% (0.2% sample) |
| PRM14 | -Batch assay. -PBS, RT. No agitation. No other conditions mentioned. -Sampling not detailed. | -Initial burst release followed by sustained release. -ca. 5 days (for loading with 5 and 15 wt% PHMB); 48 h for loading with 25 wt% PHMB); 1 h for loading with 35 wt% PHMB. | From 0.02 to 0.2 mg of PHMB |
| PRM16 | -Batch assay. -PBS (10 mL), pH 7.4, 37 °C, shaking (60 rpm). -2 mL aliquots; replenishment with new medium. | -Initial burst release, followed by sustained release. -ca. 1 day for loading with 1 %wt PHMB; ca. 4 days, for loading with 5 wt% PHMB; ca. 20 days for loading with 10 wt% PHMB. | np |
| PRM17 | -Franz diffusion cell assay. -PBS (volume not mentioned), 34 °C, with stirring. -0.2 mL aliquots; replenishment with new medium. | -Sustained release. -48 h. | 4–5 µg PHMB/mg dry sample. |
| PRM21 | -Batch assay. -SBF (1 mL). No further conditions mentioned. -Sampling: 0.3 mL sample; no further details. | -Burst release for loading with 0.3% PHMB; duration: ca. 24 h. -Bimodal release for loading with 0.1 and 0.5 wt% PHMB: 1st release for ca. 24 h; 2nd release after 24 h, for another 24 h. | ca. 0.02%–0.05% w/v |
| PRM22a | -Batch assay. -PBS. No further details. -Sampling: not mentioned. | -Initial burst release. -Duration: ca. 48 h (for loading with 5% PHMB). | np |
| PRM22b | -Small initial burst release, followed by sustained release. -Duration: ca. 5 days (for loading with 5% PHMB in cationic nanoliposomes). | ||
| PRM23a | -Batch assay. -PBS (50 mL), pH 7.4, 37 °C, with stirring (50 rpm). -5 mL aliquots; replenishment with new medium. | -Initial burst release. -Duration: 4 h. | np |
| ID | PHMB Loading | Antimicrobial Activity Evaluation (Test Organism, Assay and Results) |
|---|---|---|
| PRM1 | Soaking. [PHMB]: 0.1 or 1% w/v | -E. coli -Test bacteria inoculated into aliquots collected from a PBS solution 1, 2, 3, 4, 5 and 6 h after the immersion of the membrane, followed by overnight incubation at 37 °C. ■ Very significant reduction in bacterial numbers at all times tested, peaking at 2 h. Note: This PRM was also assayed for infection control, employing a rat full-thickness excisional wound model infected with P. aeruginosa, at days 4, 8 and 12 post-surgery. Decreases in surface bacterial population were significantly more pronounced for the treated groups (unloaded and loaded membranes) than for the untreated control for samples collected at days 8 and 12. No differences in deep tissue bacterial population between treated and untreated groups for all time points. |
| PRM2a | Addition. [PHMB]: 1 wt% | -E. coli -Test bacteria inoculated into PRM, followed by 5 h incubation at 37 °C. ■ High antibacterial activity; almost complete bacterial elimination. |
| PRM2b | -E. coli -Test bacteria inoculated into PRM, followed by 5 h incubation at 37 °C. ■ High antibacterial activity; almost complete bacterial elimination. | |
| PRM3 | Addition. [PHMB]: 0.15 or 0.3% w/v | -S. aureus and E. coli -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 24 h incubation at 36 °C. ■ High, [PHMB]-dependent antibacterial activity for both test bacteria. |
| PRM5 | Addition. [PHMB]: 0.02, 0.075, 0.15 or 0.25% w/v | -E. coli and M. luteus -Test bacteria inoculated into PRM, followed by 48 h incubation at 37 °C. ■ Total growth inhibition of both bacteria for PRMs loaded with [PHMB] ≥ 0.15%. PRMs loaded with 0.02 and 0.075% w/v PHMB did not kill bacteria, even after 45 h. |
| PRM6 | Impregnation. [PHMB]: 0.3% w/v | -B. subtilis, S. aureus, MRSA, E. coli, A. baumannii, and P. aeruginosa -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 24 h incubation at 37 °C. -Test bacteria inoculated into PRM, followed by 24 h incubation at 37 °C. ■ High antibacterial activity against all bacteria in both assays. ■ Agar disc diffusion assay: antibacterial activity was superior to a commercial CHX-based AMD (Bactigras®); comparable to a commercial silver-based AMD (Acticoat®) for Gram-negative bacteria, but inferior against P. aeruginosa; comparable to a commercial PRWD (Suprasorb® X + PHMB), but inferior against B. subtilis. |
| PRM7 | Soaking. [PHMB]: 1% w/v | -S. aureus -Test bacteria inoculated into PRM and into PRM extracts prepared in CB medium (0.02–20 mg membrane/mL DMEM; 24 h at 37 °C), followed by 24 h incubation at 37 °C. ■ High antibacterial activity observed for PRM and for extraction ratios > 0.02 mg membrane/mL medium. |
| PRM8 | Soaking. [PHMB]: 0.2% w/v | -E. coli, A. baumannii, P. aeruginosa, B. subtilis, S. aureus and MRSA -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 24 h incubation at 37 °C. ■ Active against all bacteria, except P. aeruginosa. Activity comparable to that of an equivalent membrane loaded with PVP-I. ■ Activity against E. coli, A. baumannii, B. subtilis and S. aureus, but not against MRSA, lost upon storage of PRM at 30 °C for 1–6 months. A commercial WD (Allevyn®) maintained its antibacterial activity for 6 months of storage. |
| PRM9 | Addition. [PHMB]: np | -MSSA, P. aeruginosa and K. pneumoniae -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by repeated 24 h incubations for 7 days (temperature not mentioned). ■ Antibacterial activity lasted for 24 h only. |
| PRM10 | Addition. [PHMB]: 1 or 10 wt% | -S. aureus and P. aeruginosa -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 48 h incubation at 35 °C. -Test bacteria inoculated into PRM on an agar plate, followed by 48 h incubation at 35 °C. ■ Antibacterial activity only for [PHMB] = 10 wt% PHMB. No activity against P. aeruginosa. Bacterial permeation through the PRM occurred. No bacterial growth on the PRM surface. |
| PRM11a | Addition. [PHMB]: 0.4% w/v | -P. aeruginosa and S. aureus as colonies on agar plates and as single species biofilms -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 48 h incubation at 37 °C. -PRM applied to single-species biofilms on glass coverslips, followed by 24 h incubation at 37 °C. ■ Agar disc diffusion assay: high antibacterial activity observed; not due to PHMB but to another PRM component (EDTA). ■ Biofilm assay: high reduction in the number of viable bacteria after 24 h. Lower activity against S. aureus. |
| PRM11b | Addition. [PHMB]: 0.4% w/v | -P. aeruginosa e S. aureus as colonies on agar plates and as single- and as multi-species biofilms -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 48 h incubation at 37 °C. -PRM applied to biofilms on glass coverslips, followed by 24 h incubation at 37 °C. ■ Agar disc diffusion assay: high antibacterial activity observed; not due to PHMB but to another PRM component (EDTA). ■ Biofilm assay: reduction in the number of viable bacteria in single- and multi-species biofilms, after 24 h; less active against single-species biofilms of S. aureus. Total elimination of all single- and mixed-species biofilms after 48 h. |
| PRM12 | Impregnation. [PHMB]: 0.04% or 0.1% (+0.1% UDAPB) | -S. aureus -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by overnight incubation at 37 °C. ■ Antibacterial activity comparable to that of a commercial PRWD (Suprasorb® X + PHMB) for PRMs loaded with both [PHMB], although the PRWD was loaded with a [PHMB] that was 7.5- or 3-times higher, respectively. |
| PRM13 | Soaking. [PHMB]: 0.1 or 0.2% w/v | -S. aureus and E. coli -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 24 h incubation at 37 °C. -Test bacteria inoculated into PRM, followed by 24 h incubation at 37 °C. -Fungal growth assay: sample storage in a humid environment. ■ Agar disc diffusion assay: high activity against both bacteria; higher activity for loading with 0.2% w/v PHMB. ■ Inoculation assay: almost no bacteria survived on the PRM’s surface. ■ Fungal growth assay: no fungal growth for at least 6 weeks. |
| PRM14 | Addition. [PHMB]: 5, 15, 25 or 35% wt% relative to polymer mass | -S. aureus -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by overnight incubation at 37 °C. -Test bacteria inoculated into PRM, followed by overnight incubation at 37 °C. ■ Agar disc diffusion assay: antibacterial activity increased with [PHMB], with highest activities for [PHMB] ≥ 15%. Superior to a commercial silver-based AMD (Actisorb® Silver 220), that did not show antibacterial activity. ■ Inoculation assay: no surviving bacteria after 24 h for [PHMB] ≥ 15%. Superior to Actisorb® Silver 220 that showed minimal antibacterial activity. |
| PRM15 | Soaking. [PHMB]: 0.1, 0.5, 1 or 2% w/v | -K. pneumoniae, A. baumannii, and S. aureus -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by repeated 24 h incubations for 9 days at 35 °C. ■ Antibacterial activity up to 3–5 days for PRMs loaded with 0.1 and 0.5% PHMB and up to 7–8 days for PRMs loaded with 1 and 2% PHMB. Note: This PRM ([PHMB]: 1% w/v) was also assayed for infection control in a rat full-thickness excisional wound model infected with A. baumannii., at days 3, 7, 14 and 21 post-surgery. A decrease in wound infection was observed during the earlier treatment stage. After 21 days, bacteria were still present, but in significantly lower numbers than in the control group (unloaded membrane); however, 3 out of 10 animals still showed infection symptoms. |
| PRM16 | Addition. [PHMB]: 0.5, 1, 2, 5, 10 wt% relative to polymer mass | -S. aureus and E. coli -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 24 h incubation at 37 °C. ■ Antibacterial activity for PRMs loaded with [PHMB] ≥ 2 wt%, with high activity for [PHMB] of 5 and 10 wt%. |
| PRM17 | Soaking. [PHMB]: 0.5% w/v | -S. aureus and S. epidermidis -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 24 h incubation at 37 °C. ■ Antibacterial activity against both bacteria. |
| PRM18 | Addition. [PHMB]: 0.04, 0.08, 0.12, 0.16 or 0.2% w/v | -S. aureus -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 24 h incubation at 36 °C. ■ Antibacterial activity for all [PHMB]. Antibacterial activity also due to AgNPs. Optimal [PHMB]: 0.12% w/v. ■ PRM19 with higher antibacterial activity than PRM18. |
| PRM19 | ||
| PRM20 | Impregnation. [PHMB]: 0.1% w/v (+0.1% w/v UDAPB) | -S. aureus, S. epidermidis, E. faecium, E. coli, K. pneumoniae, E. cloacae, P. aeruginosa, A. baumannii, and the fungal species C. albicans as biofilm cultures -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by overnight incubation at 37 °C. -PRM contacted with a biofilm on agar discs, followed by 24 h incubation at 37 °C. ■ Agar disc diffusion assay: inhibition of growth of all bacterial strains, in contrast to a commercial silver-based AMD (Aquacel® Ag). Less effective for most bacterial strains than equivalent membranes loaded with PVP-I (7.5%) and CHX (0.5%). ■ Contact with biofilms assay: reduced bacterial growth of all tested bacterial strains in AE and TSB media, with the exception of a single strain of E. epidermidis in TSB. Better performance than Aquacel® Ag, but slightly inferior performance than equivalent membranes loaded with OCT and PVP-I. Activity depended on the growth medium employed, being higher in the AE medium. |
| PRM21 | Addition. [PHMB]: 0.1, 0.3 or 0.5 wt% | -P. aeruginosa, A. baumannii, S. aureus, S. epidermidis, S. pyogenes, B. subtilis and C. albicans -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by 18 h incubation at 37 °C. -Test bacteria inoculated into PRM, followed by 24 h incubation at 36 °C. ■ Agar disc diffusion assay: antibacterial activity with almost all [PHMB] concentrations against almost all species. The exceptions were the absence of activity against P. aeruginosa and A. baumannii for all [PHMB] and against B. subtilis for 0.1 and 0.5 wt% PHMB. ■ Inoculation assay: inhibition of biofilm formation of all bacterial species increased with [PHMB], being higher for PRMs loaded with 0.5 wt% PHMB, except B. subtilis, which was not inhibited. |
| PRM22a | Soaking. [PHMB]: 0.2, 0.5, 1, 2, 3 or 5% w/v | -E. coli and S. aureus -Test bacteria inoculated into PRM, followed by 1 or 3 h incubation (temperature not mentioned). ■ High antibacterial activity for PRMs loaded with [PHMB] ≥ 0.5%. |
| PRM22b | Soaking. [PHMB]: 0.2, 0.5, 1, 2, 3 or 5% w/v (in NLs) | -E. coli and S. aureus -Test bacteria inoculated into PRM, followed by 1 or 3 h incubation (temperature not mentioned). ■ High antibacterial activity for PRMs loaded with [PHMB] ≥ 0.5%. |
| PRM23a | Soaking. [PHMB]: nc | -S. aureus and P. aeruginosa -PRM applied to an agar plate with test bacteria (agar disc diffusion assay), followed by overnight incubation at 37 °C. ■ High antibacterial activity against both bacteria. |
| ID | PHMB Loading | Cytotoxicity Evaluation (Sample Tested, Cell Model, Contact Type, Contact Duration, Endpoint, Assay and Results) |
|---|---|---|
| PRM2b | Addition. [PHMB]: 1 wt% relative to polymer mass | -Membrane. -Rat skin fibroblasts. -Cells seeded on membrane, followed by 3-day incubation. -Percentage of live cells (to total number of cells) and cell morphology (microscopy). ■ No cytotoxicity. ■ No cytotoxicity for unloaded membrane. |
| PRM5 | Addition. [PHMB]: 0.02, 0.075, 0.15 and 0.25% (PHMB weight to formulation volume) | -Membrane. -MDCK epithelial canine kidney cells and MRC-5 human embryo lung fibroblasts. -Cells seeded on membrane, followed by 4-day incubation (cell viability). -Cell viability, as assessed by oxidoreductase activity (MTT assay). ■ Cytotoxicity for 0.25% w/v PHMB against both cell lines. ■ No cytotoxicity for unloaded membrane. |
| PRM6 | Impregnation. [PHMB]: 0.3% w/v | -Membrane extracts prepared in cell culture medium (1 cm2 membrane/3 mL DMEM; 24 h). -L929 mouse subcutaneous fibroblasts. -Extracts added to cells, followed by 24 h incubation. -Cell viability, as assessed by oxidoreductase activity (MTT assay). ■ No cytotoxicity. ■ No cytotoxicity for Bactigras®, a commercial CHX-based AMD, tested under the same conditions. |
| PRM7 | Soaking. [PHMB]: 1% w/v | -Membrane extracts prepared in cell culture medium (0.02–20 mg membrane/mL DMEM; 24 h at 37 °C). -HaCaT human epidermal keratinocytes. -Extracts added to cells, followed by 1, 24 and 48 h incubations. -Cell proliferation, as assessed by ATP levels (ATPLiteTM-M assay). ■ Incubation time- and extraction ratio-dependent effects. Cytotoxicity at 24 h for extraction ratios ≥ 2 mg membrane/mL DMEM. ■ No cytotoxicity for unloaded membrane. |
| PRM14 | Addition. [PHMB]: 5, 15, 25 and 35 wt% relative to polymer mass | -Membrane. -HaCaT human epidermal keratinocytes. -Membrane placed in direct contact with the cell monolayer, followed by 24 and 48 h incubations. -Cell viability, as assessed by oxidoreductase activity (alamarBlueTM assay). ■ Cytotoxicity for 15, 25 and 35 wt% PHMB for both time points. ■ No cytotoxicity for unloaded membrane. ■ Cytotoxicity for Actisorb®, a commercial silver-based AMD, tested under the same conditions. |
| PRM15 | Soaking. [PHMB]: 1% w/v | -Membrane. -Human skin fibroblasts (CI 0159) and human vascular endothelial cells (CI 0478). -Cells seeded on membrane, followed by 3-, 7-, 14- and 21-day incubations. -Cell viability, as assessed by oxidoreductase activity (MTT assay). ■ No cytotoxicity. ■ No cytotoxicity for unloaded membrane. |
| PRM17 | Soaking. [PHMB]: 0.5% w/v | -Membrane. -NIH 3T3 mouse embryo fibroblasts. -Membrane placed on hanging inserts above cells (indirect contact), followed by 24 h incubation. -Cell viability, as assessed by oxidoreductase activity (MTT assay). ■ No cytotoxicity. |
| PRM21 | Addition. [PHMB]: 0.1, 0.3 or 0.5 wt% relative to solution mass | -Membrane extracts prepared in cell culture medium (4 cm2 membrane/2 mL DMEM; 24 h at 37 °C). -L929 mouse subcutaneous fibroblasts and HaCaT human epidermal keratinocytes. -Extracts added to cells, followed by 3-day incubation. -Cell viability, as assessed by oxidoreductase activity (MTT assay). ■ [PHMB]-dependent decrease in cell viability for both cell lines. Cytotoxicity for 0.5 wt% PHMB, but only against HaCaT cells. ■ No cytotoxicity for unloaded membrane extract. |
| PRM22a | Soaking. [PHMB]: 1 or 5% w/v | -Membrane extracts prepared in cell culture medium (6.5 cm2 membrane/2 mL DMEM; 24 h). -Primary human skin fibroblasts. -Extracts added to cells, followed by 24 h incubation. -Cell viability, as assessed by oxidoreductase activity (MTT assay). ■ Mild cytotoxicity for both PHMB concentrations. ■ No cytotoxicity for unloaded membrane extract. |
| PRM22b | Soaking. [PHMB]: 1 or 5% w/v, encapsulated in nanoliposomes | -Membrane extracts prepared in cell culture medium (6.5 cm2 membrane/2 mL DMEM; 24 h). -Primary human skin fibroblasts. -Extracts added to cells, followed by 24 h incubation. -Cell viability, as assessed by oxidoreductase activity (MTT assay). ■ Subcytotoxicity (cell viabilities of ca. 70% for both PHMB concentrations). ■ No cytotoxicity for unloaded membrane extract. |
| PRM23a | Soaking. [PHMB]: nc | -Membrane extracts prepared in cell culture medium (2 × 2 × 2 mm3 in DMEM; 48 h). -HaCaT human epidermal keratinocytes. -Extracts added to cells, followed by 24 h incubation. -Cell viability, as assessed by oxidoreductase activity (MTT assay). ■ No cytotoxicity. ■ No cytotoxicity for unloaded membrane extract. |
| ID | Other Biological Studies |
|---|---|
| PRM6 | -Subcutaneous implantation in rats, followed for 28 days. ■ Lower inflammatory response than with a CHX-based commercial AMD. -Sensitization evaluation in healthy volunteers, by a patch test, with 3 patches applied consecutively for 9 days. ■ Non-sensitizing. |
| PRM15 | -Intradermal stimulation in rabbits, by injection of aliquots from the drug-release study. Observed for 72 h. ■ Minimal intradermal stimulation. -Sensitization in guinea pigs, by a patch test, for 21 days. ■ No sensitization. -Subcutaneous implantation in rats, for 21 days. ■ Good healing. No infection. No necrosis. |
| PRM17 | -HET-CAM irritation test with 5 min of contact. ■ No irritation. |
| PRM18 | -Hemostatic capacity qualitatively evaluated through visual inspection of blood added to the WDs. ■ Alginate increased blood-clotting rate. |
| ID | Wound-Healing Evaluation (Model, Controls, Duration, Parameters Evaluated and Results) |
|---|---|
| PRM1 | -Model: rat full-thickness excisional wound model. -Controls: untreated wound and wound treated with unloaded membrane. -Assay duration: 12 days. -Parameter evaluated: wound size. -Results: only the sample loaded with 0.1% PHMB was evaluated. Decreased wound size for treatment and unloaded membrane groups relative to the untreated control for all time points studied. After 12 days, most wounds were closed. |
| PRM2b | -Model: rat burn wound model. -Controls: untreated wound and wound treated with unloaded membrane. -Assay duration: 28 days. -Parameter evaluated: observation of the wound and histological evaluation. -Results: the best formulation showed an almost regenerated epidermal layer, while control groups showed an ongoing inflammatory response. |
| PRM4 | -Case study: two human diabetic toe amputation wounds. -Results: complete wound healing in 4 and in 60 days. |
| PRM6 | -Model: rat full-thickness excisional wound model. -Controls: wound treated with a CHX-based AMD. -Assay duration: 21 days. -Parameters evaluated: wound size and area covered by collagen, after staining. -Results: after 14 days, higher reduction in wound size and higher collagen formation than with a commercial CHX-based AMD. After 21 days, wound almost fully closed, in contrast to wound treated with the CXH-based AMD treatment. -In vitro evaluation: wound-scratch assay. ■ Assay: wound-scratch. ■ Model: L929 mouse fibroblasts. ■ Sample: extracts in cell culture medium (DMEM). ■ Controls: extract of commercial CHX-based AMD and DMEM. ■ Assay duration: 72 h. ■ Result: cell migration comparable to the DMEM control and to the commercial CHX-based AMD. -In vitro evaluation: Boyden’s chamber assay. ■ Assay: Boyden’s chamber. ■ Model: L929 mouse fibroblasts. ■ Sample: extracts in DMEM. ■ Controls: extract of commercial CHX-based AMD in DMEM and DMEM. ■ Assay duration: 24 h. ■ Results: cell migration comparable to the DMEM control and to the commercial CHX-based AMD. |
| PRM13 | -Model: rat incisional wound model -Controls: untreated wound and wound treated with 2 commercial WDs (not AMDs). -Assay duration: 4 weeks, with weekly changes. -Parameter evaluated: wound area. -Results: only the sample loaded with 0.2% PHMB was evaluated. Faster decrease in wound size for treatment with the PRM compared to the untreated group and to the 2 groups treated with the 2 commercial WDs, being the only one that showed a fully closed wound after 4 weeks. |
| PRM15 | -Model: rat full-thickness excisional wound model, with sample sutured to the wound. -Controls: treatment with unloaded membrane. -Assay duration: over a 21-day period. -Parameters evaluated: angiogenesis and inflammatory indicators. -Results: only the 1% PHMB sample was evaluated. No effect on angiogenesis or on inflammatory indicators. No effect on neovascularization or wound-repair capacity. |
| PRM17 | -Case study: dog with contact ulcers on rear limbs. -Results: complete healing after 3 weeks of treatment, in contrast to wound treated with a non-medicated commercial WD. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guiomar, A.J.; Urbano, A.M. Polyhexanide-Releasing Membranes for Antimicrobial Wound Dressings: A Critical Review. Membranes 2022, 12, 1281. https://doi.org/10.3390/membranes12121281
Guiomar AJ, Urbano AM. Polyhexanide-Releasing Membranes for Antimicrobial Wound Dressings: A Critical Review. Membranes. 2022; 12(12):1281. https://doi.org/10.3390/membranes12121281
Chicago/Turabian StyleGuiomar, António Jorge, and Ana M. Urbano. 2022. "Polyhexanide-Releasing Membranes for Antimicrobial Wound Dressings: A Critical Review" Membranes 12, no. 12: 1281. https://doi.org/10.3390/membranes12121281





